

Environmental Medicine: Integrating a Missing Element into Medical Education (1995)
Chapter: case study 19: lead toxicity.

1 Lead Toxicity
This monograph is one in a series of self-instructional publications designed to increase the primary care provider’s knowledge of hazardous substances in the environment and to aid in the evaluation of potentially exposed patients. See page 27 for more information about continuing medical education credits and continuing education units.

U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES
Public Health Service
Agency for Toxic Substances and Disease Registry
A hyperactive 5-year-old with disturbed hearing and hypochromic anemia
A 5-year-old boy is brought to your office by his mother, who is concerned that her child is hyperactive. At a parent-teacher conference last week, the kindergarten teacher said that the boy seems impulsive and has trouble concentrating, and recommended evaluation by a physician as well as by the school psychologist. The mother states that he has always seemed restless and easily distracted, but that these first 6 months in kindergarten have been especially trying.
Family history reveals that the boy lives with his sister, mother, and maternal grandparents in an older suburb of your community. The child’s monthly weekend visits to his father’s house are working out fine. However, he seems to be fighting more with his sister, who has an attention-deficit disorder and is repeating first grade. Since the mother moved in with her parents after her divorce 4 years ago, she has worked with the grandfather in an automobile radiator repair shop, where her children often come to play after school. She was just laid off, however, and expressed worry about increasing financial dependence on her parents. She also worries that the grandfather, who has gout and complains increasingly of abdominal pain, may become even more irritable when he learns that she is pregnant. Her third child is due in 4 months.
On chart review, you see that the boy was last seen in your clinic for his preschool physical 1 year ago, results of which were normal. A note describes a very active 4-year-old who could dress himself without help but could not correctly name the primary colors. His vision was normal, but hearing acuity was below normal, and speech and language were slightly delayed. Immunizations are up to date.
Further history on that visit indicated adequate diet, with no previous pica. Spun hematocrit was diminished at 30%. Peripheral blood smear showed hypochromia and microcytosis. There was no evidence of blood loss, and stool examination was negative for occult blood. The diagnosis was “mild iron deficiency anemia,” and iron therapy was prescribed. The family failed to keep several follow-up appointments, but the child did apparently complete the prescribed 3-month course of iron supplements. He receives no medications at this time and has no known allergies.
On physical examination today, you note that the boy is in the tenth percentile for height and weight. His attention span is very short, making him appear restless, and he has difficulty following simple instructions. Except for language and social skills, he has reached most important developmental milestones.
(a) What should be included in this boy’s problem list?
_________________________________________________________________
(b) List several possible causes for the anemia.
(c) What tests would you order to confirm or rule out your diagnosis?
Answers are incorporated in Challenge answers (11) through (14) on page 25.
Who’s at Risk
❑ Young children have a great potential for lead exposure and are especially susceptible to its toxic effects.
❑ Since blood lead readily crosses the placenta, lead poses a substantial threat to the developing fetus.
❑ Workers may bring lead dust home on skin and clothes and unknowingly expose family members.
By and large, children show a greater sensitivity to lead’s effects than adults do. The incomplete development of the blood-brain barrier in very young children (up to 36 months of age) increases the risk of lead’s entry into the developing nervous system, which can result in prolonged neurobehavioral disorders. Children absorb and retain more lead in proportion to their weight than do adults. Young children also show a greater prevalence of iron deficiency, a condition that can increase gastrointestinal absorption of lead.
No economic or racial subgroup of children is free from the risk of having blood lead levels high enough to cause adverse health effects. In 1984, approximately 17% of children in the United States were estimated to be at risk of lead poisoning. Sizable numbers of children from families with incomes well above the poverty line have been reported to have elevated blood lead levels. The prevalence of elevated levels, nevertheless, remains highest among inner-city, underprivileged children who live in deteriorating pre-1970s housing containing leaded-paint surfaces. Lead in paint and lead in soil and dust are the principal sources of exposure.
The percentage of African-American children affected by lead is disproportionate to their number in the U.S. population. In 1984 African-American children constituted 46% of the children at risk. The family income categories of these children show that the higher percentage is related to economic factors. African-American children are over represented in the poor and low-income groups as well as in inner-city areas. Other minorities are similarly affected; 15% of Mexican-Americans and 20% of Puerto Rican-Americans exceed a blood lead cutoff of 15 µg/dL As blood lead levels in the general population are declining because of restrictions on leaded gasoline use, race and income will become better indicators of the likelihood of exposure to leaded paint and, consequently, elevated blood lead levels.
Since lead readily crosses the placenta, the fetus is at risk. Fetal exposure can cause potentially adverse neurologic effects in utero and during postnatal development. According to the Public Health Service, in 1984, more than 400,000 fetuses were exposed to lead through maternal blood lead concentrations associated with early developmental effects.
More than 1 million workers in over 100 different occupations may be exposed to lead. In lead-related industries, workers not only may inhale lead dust and lead oxide fumes, but may eat, drink, and smoke in or near contaminated areas, increasing the probability of lead ingestion. If showers and changes of clothing are not provided, workers can bring lead dust home on their skin, shoes, and clothing, thus inadvertently exposing family members.
Exposure Pathways
❑ The primary sources of environmental exposure to lead are leaded paint, auto emissions, and drinking water.
Lead is a naturally occurring element that has been used almost since the beginning of civilization. Because of the many industrial activities that have brought about its wide distribution, lead is ubiquitous in the environment today. All humans have lead in their bodies, primarily as a result of exposure to manmade sources.
Today, the major environmental sources of metallic lead and its salts are paint, auto exhaust, food, and water. For children, the most important pathways are ingestion of chips from lead-painted surfaces, inhalation of lead from automobile emissions, food from lead-soldered cans, drinking water from lead-soldered plumbing, and medications in the form of folk remedies.
❑ A wide variety of workers, hobbyists, and substance abusers may encounter potentially high levels of lead. Certain folk remedies may also cause lead poisoning.
Automobile emissions have been an important source of lead exposure for urban residents, particularly in areas with congested traffic. Although inhalation of lead from gasoline is no longer considered a public health problem, the lead from dust in automobile emissions has been deposited in the soil. Children playing near roads and freeways may come in contact with contaminated soil.
The lead content of paint was not regulated until 1977. Many older structures, residential and commercial, have leaded paint that is peeling, flaking, and chipping. Children can ingest loose paint as a result of pica (compulsive eating of nonfood items) and through mouthing of items contaminated with lead from paint, dust, and soil. High levels of lead in soil and house dust have been associated with increased blood lead levels in children.
❑ Lead enters the body primarily through ingestion and inhalation.
Food may contain lead from the environment or from containers. Agricultural vehicles are not required to use unleaded gasoline; consequently, lead can be deposited on and retained by crops, particularly leafy vegetables. Acidic foods have been found to leach lead from lead solder in cans and lead glazes used in making pottery and ceramicware. Water from leaded pipes, soldered plumbing, or water coolers is another potential source of lead exposure. Stationary or point sources of lead include mines and smelters.
Several folk remedies used in this country have been shown to contain large amounts of lead. Two Mexican folk remedies are azarcon and greta, which are used to treat “empacho,” a colic-like illness. Azarcon and greta are also known as liga, Maria Luisa, alarcon, coral, and rueda . Lead-containing remedies and cosmetics used by some Asian communities are chuifong tokuwan, pay-looah, ghasard, bali goli, and kandu . Middle Eastern remedies and cosmetics include alkohl, kohl, surma, saoott, and cebagin .
In addition to these environmental sources, many occupations, hobbies, and other activities result in potential exposures to high levels of lead and can put the entire family at risk of lead poisoning. Sources of lead exposure are listed below. Lead-glazed pottery, particularly if it is imported, is a potential source of exposure that is often overlooked. Even “safe” ceramicware can become harmful; dishwashing may chip or wear off the protective glaze and expose lead-containing pigments.
Inorganic lead enters the body primarily through inhalation and ingestion and does not undergo biologic transformation. In contrast, organic lead, found primarily in gasoline as tetraethyl lead, enters the body through inhalation and skin contact and is metabolized in the liver. In 1976 and in 1984, federal regulation drastically reduced the amount of lead in gasoline, and today organic lead in gasoline is not as great an environmental concern in the United States as it is in other countries, where it remains a serious hazard.
Sources of lead exposure
Occupational
Plumbers, pipe fitters
Lead miners
Auto repairers
Glass manufacturers
Shipbuilders
Plastic manufacturers
Lead smelters and refiners
Police officers
Steel welders or cutters
Construction workers
Rubber product manufacturers
Gas station attendants
Battery manufacturers
Bridge reconstruction workers
Firing range instructors
Environmental
Lead-containing paint
Soil/dust near lead industries, roadways, lead-painted homes
Plumbing leachate
Ceramicware
Leaded gasoline
Hobbies and Related Activities
Glazed pottery making
Target shooting at firing ranges
Lead soldering (e.g., electronics)
Preparing lead shot, fishing sinkers
Stained-glass making
Car or boat repair
Home remodeling
Substance Use
Folk remedies
“Health foods”
Moonshine whiskey
Gasoline “huffing”
Biologic Fate
❑ Once in the bloodstream, lead is primarily distributed among three compartments—blood, soft tissue, and mineralizing tissue. The bones and teeth of adults contain more than 95% of total lead in the body.
❑ In times of stress, the body can mobilize lead stores, thereby increasing the level of lead in the blood.
❑ The body accumulates lead over a lifetime and normally releases it very slowly.
In the human body, inorganic lead is not metabolized but is directly absorbed, distributed, and excreted. The rate at which lead is absorbed depends on its chemical and physical form and on the physiologic characteristics of the exposed person (e.g., nutritional status and age). Inhaled lead deposited in the lower respiratory tract is completely absorbed. The amount of lead absorbed from the GI tract of adults is typically 10% to 15% of the ingested quantity; for pregnant women and children, the amount absorbed can increase to as much as 50%. The quantity absorbed increases significantly under fasting conditions and with iron or calcium deficiency.
Once in the blood, lead is distributed primarily among three compartments—blood, soft tissue (kidney, bone marrow, liver, and brain), and mineralizing tissue (bones and teeth). Mineralizing tissue contains about 95% of the total body burden of lead in adults.
The lead in mineralizing tissues accumulates in subcompartments that differ in the rate at which lead is resorbed. In bone, there is both a labile component, which readily exchanges lead with the blood, and an inert pool. The lead in the inert pool poses a special risk because it is a potential endogenous source of lead. When the body is under physiologic stress such as pregnancy, lactation, or chronic disease, this normally inert lead can be mobilized, increasing the lead level in blood. Because of these mobile lead stores, significant drops in a person’s blood lead level can take several months or sometimes years, even after complete removal from the source of lead exposure.
Of the lead in the blood, 99% is associated with erythrocytes; the remaining 1% is in the plasma, where it is available for transport to the tissues. The blood lead not retained is either excreted by the kidneys or through biliary clearance into the gastrointestinal tract. In single-exposure studies with adults, lead has a half-life, in blood, of approximately 25 days; in soft tissue, about 40 days; and in the non-labile portion of bone, more than 25 years. Consequently, after a single exposure a person’s blood lead level may begin to return to normal; the total body burden, however, may still be elevated.
For lead poisoning to develop, major acute exposures to lead need not occur. The body accumulates this metal over a lifetime and releases it slowly, so even small doses, over time, can cause lead poisoning. It is the total body burden of lead that is related to the risk of adverse effects.
Physiologic Effects
❑ Lead affects primarily the peripheral and central nervous systems, the blood cells, and metabolism of vitamin D and calcium. Lead also causes reproductive toxicity.
Whether lead enters the body through inhalation or ingestion, the biologic effects are the same; there is interference with normal cell function and with a number of physiologic processes. The lowest observable blood lead levels associated with specific health effects in chronically exposed children and adults are shown in Figure 1 .
Neurologic Effects
❑ Neurologic deficits, as well as other effects caused by lead poisoning, may be irreversible.
The most sensitive target of lead poisoning is the nervous system. In children, neurologic deficits have been documented at exposure levels once thought to cause no harmful effects. In addition to the lack of a precise threshold, childhood lead toxicity may have permanent effects. One study showed that damage to the central nervous system (CNS) that occurred as a result of lead exposure at age 2 resulted in continued deficits in neurologic development, such as
❑ Effects in children generally occur at lower blood lead levels than in adults.
❑ The developing nervous system in children can be affected adversely at blood lead levels of less than 10 µg/dL.
Figure 1 . Effects of inorganic lead on children and adults— lowest observable adverse effect levels

Adapted from ATSDR, Toxicological Profile for Lead (1989)
lower IQ scores and cognitive deficits, at age 5. In another study that measured total body burden, primary school children with high tooth lead levels but with no known history of lead poisoning had larger deficits in psychometric intelligence scores, speech and language processing, attention, and classroom performance than children
with lower levels of lead. A 1990 follow-up report of children with elevated lead levels in their teeth noted a sevenfold increase in the odds of failure to graduate from high school, lower class standing, greater absenteeism, more reading disabilities, and deficits in vocabulary, fine motor skills, reaction time, and hand-eye coordination 11 years later. The reported effects are more likely caused by the enduring toxicity of lead than by recent excessive exposures because the blood lead levels found in the young adults were low (less than 10 micrograms per deciliter [µg/dL]).
Hearing acuity, particularly at higher frequencies, has been found to decrease with increasing blood lead levels. Hearing loss may contribute to the apparent learning disabilities or poor classroom behavior exhibited by children with lead intoxication.
Adults also experience CNS effects at relatively low blood lead levels, manifested by subtle behavioral changes, fatigue, and impaired concentration. Peripheral nervous system damage, primarily motor, is seen mainly in adults. Peripheral neuropathy with mild slowing of nerve conduction velocity has been reported in asymptomatic lead workers. Lead neuropathy is believed to be a motor neuron, anterior horn cell disease with peripheral dying-back of the axons. Frank wrist drop occurs only as a late sign of lead intoxication.
Hematologic Effects
❑ Lead inhibits several enzymes that are critical to the synthesis of heme.
❑ Lead poisoning in children only rarely results in anemia.
Lead inhibits the body’s ability to make hemoglobin by interfering with several enzymatic steps in the heme pathway. Ferrochelatase, which catalyzes the insertion of iron into protoporphyrin IX, is quite sensitive to lead. A decrease in the activity of this enzyme results in an increase of the substrate, erythrocyte protoporphyrin (EP), in the red blood cells. Recent data indicate that the EP level, which has been used to screen for lead toxicity in the past, is not sufficiently sensitive at lower levels of blood lead and is therefore not as use ful a screening test for lead poisoning as previously thought. (See Laboratory Evaluation for further discussion of EP testing.)
Lead can induce two types of anemia. Acute high-level lead poisoning has been associated with hemolytic anemia. In chronic lead poisoning, lead induces anemia by both interfering with erythropoiesis and by diminishing red blood cell survival. It should be emphasized, however, that anemia is not an early manifestation of lead poisoning and is evident only when the blood lead level is significantly elevated for prolonged periods.
Endocrine Effects
❑ Lead interferes with a hormonal form of vitamin D, which affects multiple processes in the body, including cell maturation and skeletal growth.
A strong inverse correlation exists between blood lead levels and levels of vitamin D. Because the vitamin D-endocrine system is responsible in large part for the maintenance of extra- and intracellular calcium homeostasis, it is likely that lead impairs cell growth and maturation and tooth and bone development.
Renal Effects
❑ Lead-induced chronic renal insufficiency may result in gout.
A direct effect on the kidney of long-term lead exposure is nephropathy. Impairment of proximal tubular function manifests in aminoaciduria, glycosuria, and hyperphosphaturia (a Fanconi-like syndrome). There is also evidence of an association between lead exposure and hypertension, an effect that maybe mediated through renal mechanisms. Gout may develop as a result of lead-induced hyperuricemia, with selective decreases in the fractional excretion of uric acid before a decline in creatinine clearance. Renal failure accounts for 10% of deaths in patients with gout.
Reproductive and Developmental Effects
❑ Maternal lead stores readily cross the placenta, placing the fetus at risk.
An increased frequency of miscarriages and stillbirths among women working in the lead trades was reported as early as the turn of the century. Although the data concerning exposure levels are incomplete, these effects were probably a result of far greater exposures than are currently found in lead industries. Reliable dose-effect data for reproductive effects in women are still lacking today.
Increasing evidence indicates that lead not only affects the viability of the fetus, but development as well. Developmental consequences of prenatal exposure to low levels of lead include reduced birth weight and premature birth. Lead is an animal teratogen; however, most studies in humans have failed to show a relationship between lead levels and congenital malformations.
The effects of lead on the male reproductive system in humans have not been well characterized. The available data support a tentative conclusion that testicular effects, including reduced sperm counts and motility, may result from chronic exposure to lead.
Carcinogenic Effects
❑ EPA’s Science Advisory Board has recommended that lead be considered a probable human carcinogen.
Case reports have implicated lead as a potential renal carcinogen in humans, but the association remains uncertain. Soluble salts, such as lead acetate and lead phosphate, have been reported to cause kidney tumors in rats.
Clinical Evaluation
History and physical examination.
❑ The first signs of lead poisoning in children are often subtle neurobehavioral problems that adversely affect classroom behavior and social interaction.
❑ Speech or hearing impairments, or both, are not uncommon in lead-exposed children.
Medical evaluation of a patient with suspected lead exposure includes a full workup and medical history. Clues to potential exposure are often obtained by discussing the following with the family:
occupational history of all home occupants
family history, including use of unusual medicines
location, age, and physical condition of residence, school, day-care center, etc.
home remodeling activities
condition of household pets
hobbies of all family members
use of imported or glazed ceramics
drinking water source and type of pipe
nutritional status
proximity to industrial facilities and hazardous waste sites
The physical examination should include special attention to the hematologic, cardiovascular, gastrointestinal, and renal systems. The nervous system, including behavioral changes, should be carefully evaluated. A purplish line on the gums (lead line) is rarely seen today, but if present, usually indicates severe and prolonged lead poisoning.
For children, hearing, speech, and other developmental milestones should be carefully evaluated and documented. In certain geographic areas, iron deficiency is common in children 9 to 24 months of age. Since iron and calcium deficiencies are known to enhance the absorption of lead and to aggravate pica, it is especially important to assess the nutritional status of young children.
Signs and Symptoms
❑ Most persons with lead toxicity are not overtly symptomatic.
Because of differences in individual susceptibility, symptoms of lead intoxication and their onset may vary. With increasing exposure, the severity of symptoms can be expected to increase. Those symptoms most often associated with varying degrees of lead toxicity are listed below. In symptomatic lead intoxication, blood lead levels generally range from 35 to 50 µg/dL in children and 40 to 60 µg/dL in adults. Severe toxicity is frequently found in association with blood lead levels of 70 µg/dL or more in children and 100 µg/dL or more in adults.
Continuum of signs and symptoms associated with lead toxicity
Mild Toxicity
Myalgia or paresthesia
Mild fatigue
Irritability
Occasional abdominal discomfort
Moderate Toxicity
General fatigue
Difficulty concentrating
Muscular exhaustibility
Diffuse abdominal pain
Weight loss
Constipation
Severe Toxicity
Paresis or paralysis
Encephalopathy-may abruptly lead to seizures, changes in consciousness, coma, and death
Lead line (blue-black) on gingival tissue
Colic (intermittent, severe abdominal cramps)
Some of the hematologic signs of lead poisoning mimic other diseases or conditions. In the differential diagnosis of microcytic anemia, lead poisoning can usually be ruled out by obtaining a venous blood lead concentration; if the blood lead level is less than 25 µg/dL, the anemia usually reflects iron deficiency or hemoglobinopathy. Two rare diseases, acute intermittent porphyria and coproporphyria, also result in heme abnormalities similar to those of lead poisoning.
Other effects of lead poisoning can be misleading. Patients exhibiting neurologic signs due to lead poisoning have been treated only for peripheral neuropathy or carpal tunnel syndrome, delaying treatment for lead intoxication. Failure to correctly diagnose lead-induced gastrointestinal distress has led to inappropriate abdominal surgery.
Laboratory Evaluation
If pica or accidental ingestion of lead-containing objects (such as curtain weights or fishing sinkers) is suspected, an abdominal radiograph should be taken. Hair analysis is not usually an appropriate assay for lead toxicity because no correlation has been found between the amount of lead in the hair and the exposure level. The probability of environmental lead contamination of a laboratory specimen and inconsistent sample preparation make the results of hair analysis difficult to interpret. Suggested laboratory tests to evaluate lead intoxication include the following:
CBC with peripheral smear
Blood lead level
Erythrocyte protoporphyrin level
BUN and creatinine level
❑ Basophilic stippling is not always seen in lead-poisoned patients.
CBC with Peripheral Smear . In a lead-poisoned patient, the hematocrit and hemoglobin values may be slightly to moderately low. The differential and total white count may appear normal. The peripheral smear may be either normochromic and normocytic or hypochromic and microcytic. Basophilic stippling is usually seen only in patients who have been significantly poisoned for a prolonged period. Eosinophilia may appear in patients with lead toxicity but does not show a clear dose-response effect.
❑ The best screening and diagnostic test for lead poisoning is a blood lead level.
Blood Lead Level. A blood lead level is the most useful screening and diagnostic test for lead exposure. A blood lead level reflects lead’s dynamic equilibrium between absorption, excretion, and deposition in soft- and hard-tissue compartments. For chronic exposures, blood lead levels often underrepresent the total body burden; nevertheless, it is the most widely accepted and commonly used measure of lead exposure. Blood lead levels respond relatively rapidly to abrupt or intermittent changes in lead intake (for example, ingestion of lead paint chips by children) and, within a limited range, bear a linear relationship to those intake levels.
Lead is most harmful to children under 6 years of age. Every child who has a developmental delay, behavioral disorder, or speech impairment, or who may have been lead-exposed, should be considered for a blood lead test. Equally important, siblings, housemates, and playmates of children with suspected lead toxicity probably have similar exposures to lead and should be promptly screened. For occupationally exposed adults, consult the federal lead standard for the mandated type and frequency of lead screening (p. 20, Workplace, Air).
Today, the average blood lead level in the U.S. population is below 10 µg/dL, down from an average of 16 µg/dL (in the 1970s), the level before the legislated removal of lead from gasoline. A blood lead level of 10 µg/dL is about 3 times higher than the average level found in some remote populations.
The levels defining lead poisoning have been progressively declining. (See Biologic Guidelines in Standards and Regulations.) Currently, the consensus level of concern for children is 10 to 14 µg/dL (see Table 1 ). Effects on stature have been reported to begin at levels as low as 4 µg/dL, the present limit for accurate blood lead measurement. Taken together, effects occur over a wide range of blood lead concentrations, with no indication of a threshold. No safe level has yet been found for children. Even in adults, effects are being discovered at lower and lower levels as more sensitive analyses and measures are developed.
❑ Using an EP or ZPP assay to screen children for lead poisoning is not as useful as once thought.
EP and ZPP Levels . Until recently, the test of choice for screening asymptomatic children and other populations at risk was erythrocyte protoporphyrin (EP), commonly assayed as zinc protoporphyrin (ZPP). An elevated level of protoporphyrin in the blood is a result of accumulation secondary to enzyme dysfunction in the erythrocytes. It reaches a steady state in the blood only after the entire population of circulating erythrocytes has turned over, about 120 days. Consequently, it lags behind blood lead levels and is an indirect measure of long-term lead exposure.
Table 1 . Interpretation of blood lead test results and follow-up activities: class of child based on blood lead concentration
The major disadvantage of using EP (ZPP) testing as a method for lead screening is that it is not sensitive at the lower levels of lead poisoning. Data from the second National Health and Nutrition Examination Survey (NHANES II) indicate that 58% of 118 children with blood lead levels above 30 µg/dL had EP levels within normal limits. This finding shows that a significant number of children with lead toxicity would be missed by reliance on EP (ZPP) testing alone as the screening tool. An EP (ZPP) level is still useful in screening patients for iron deficiency anemia.
Normal values of ZPP are usually below 35 µg/dL. Hyperbilirubinernia (jaundice) will cause falsely elevated readings when the hematofluorometer is used. EP is elevated in iron deficiency anemia and in sickle cell and other hemolytic anemias. In erythropoietic protoporphyria, an extremely rare disease, EP is markedly elevated (usually above 300 µg/dL).
❑ Renal function may be impaired in lead-exposed persons.
BUN, Creatinine, and Urinalysis . These parameters may reveal only late, significant effects of lead on renal function. Renal function in adults can also be assessed by measuring the fractional excretion of uric acid (normal range 5% to 10%; less than 5% in saturnine gout; greater than 10% in Fanconi syndrome).
Treatment and Management
❑ All therapeutic chelating agents have potentially adverse side effects and should be used cautiously.
❑ The type of therapy required will normally depend on the patient’s blood lead level. Asymptomatic patients with blood lead levels below 25 µg/dL usually require only separation from the source of exposure.
It is not sufficient to provide treatment only; the patient and lead source must be permanently separated. After diagnosing lead poisoning, the physician should call upon the resources of the local health authority to determine the lead source (e.g., home, workplace). If the lead poisoning is caused by leaded paint in the home, the patient and all other family members should be rehoused until the home has undergone safe and satisfactory lead abatement. Family members and other persons likely to have been exposed should be tested for lead poisoning. Steps should be taken to identify and correct dietary deficiencies, particularly of calcium and iron, and to educate family members on the preventable hazards of lead.
The most reliable index of exposure is a measurement of blood lead concentration. In those asymptomatic children having blood lead levels below 25 µg/dL, treatment is probably not indicated, and removal from the source is the most important action. Patient followup to confirm a decreasing blood lead level is needed, however.
❑ Children with blood lead levels of 45 µg/dL or greater should be referred for appropriate chelation therapy immediately.
The Centers for Disease Control (CDC) recommends that children with blood lead levels of 45 µg/dL or greater should be referred for appropriate chelation therapy immediately. Some practitioners routinely treat children with blood lead levels between 25 and 44 µg/dL with chelation therapy and some do not use chelating agents for children with blood lead levels in this range. Other practitioners base this decision on the results of a provocative EDTA test. Only very minimal data exist about chelation therapy for children with blood lead levels below 25 µg/dL, and such children should not be chelated except in the context of approved clinical trials.
❑ The EDTA challenge test will indicate the extent of lead stores in the body. Some practitioners use this test when deciding whether to institute chelation therapy for a patient with a blood lead level between 25 and 44 µg/dL.
Several drugs (see Table 2 ) are used in the treatment of lead poisoning. These drugs, capable of binding or chelating lead, deplete the soft and hard (skeletal) tissues of lead and thus reduce its acute toxicity. All drugs have potential side effects and must be used with caution. In rare cases, the chelating agent, calcium disodium ethylenediaminetetraaceate acid (CaNa 2 EDTA) has caused proteinuria, microscopic hematuria, proximal tubule damage, hypercalcemia, and fever. Before instituting this therapy or using the chelation challenge test, the patient should be hospitalized and a physician experienced in chelation should be consulted. Such physicians can be identified by contacting an accredited regional poison control center, university medical center, or state or local health department.
Table 2 . Chelating agents used in treating children who have lead poisoning
Standards and Regulations
The number of federal standards and regulations reflect the extent to which lead is considered a public health problem. In some cases, the lead levels are mandated; in others, they are only recommended standards ( Table 3 ).
Table 3 . Summary of standards and regulations for lead
Biologic Guidelines
❑ CDC lowered the recommended action level for lead poisoning in children in 1991.
Lead levels that in the past were considered safe are now considered hazardous. As new information has emerged about the neurologic, reproductive, and possible hypertensive toxicity of lead, and as more sensitive parameters are developed, the levels defining lead poisoning have been progressively lowered. Between 1986 and 1988, several studies demonstrated neurobehavioral impairment in lead-exposed children with blood lead levels as low as 10 to 14 µg/dL. As more data become available, the definition of lead toxicity level will likely continue to be lowered ( Figure 2 ).
Figure 2 . CDC’s action level for blood lead in children has steadily declined.

*Emphasis is on primary prevention efforts (i.e., elimination of lead hazards before children are poisoned).
❑ Several states require primary care physicians to report cases of lead poisoning.
Physician Reporting Requirements. Several states require primary care physicians and persons in charge of screening programs to report both presumptive and confirmed cases of lead toxicity to the appropriate health agency so that abatement of the lead source, education of the patient, and remediation steps can be undertaken. In many states, laboratories performing blood lead or EP (ZPP) tests are also required to report abnormal results to the appropriate health agency.
❑ OSHA has set required standards for the amount of lead allowed in workroom air at 50 µg/m 3 averaged over an 8-hour workday.
The federal lead standard specifies the permissible exposure limit (PEL) of lead in the workplace, the frequency and extent of medical monitoring, and other responsibilities of the employer. The Occupational Safety and Health Administration (OSHA) has set a PEL of lead in workroom air at 50 µg/m 3 averaged over an 8-hour workday for workers in general industry. For those exposed to air concentrations at or above the action level of 30 µg/m 3 for more than 30 days per year, OSHA mandates periodic determination of blood lead levels. If a blood lead level is found to be greater than 40 µg/dL, the worker must be notified in writing and provided with medical examination. If a worker’s blood lead level reaches 60 µg/dL (or averages 50 µg/dL or more), the employer is obligated to remove the employee from excessive exposure, with maintenance of seniority and pay, until the employee’s blood lead level falls below 40 µg/dL (29 CFR §1910.1025). A copy of the lead standard can be obtained by calling your regional office of OSHA.
Environment
❑ EPA will probably lower its present ambient air standard for lead.
Occupational exposure limits are generally set to accommodate 8-hour workdays and healthy persons; they bear little relation to environmental limits, which are set to protect the most susceptible persons in the general population. EPA requires that the concentration of lead in air the general public may breathe shall not exceed 1.5 µg/m 3 averaged over a calendar quarter. This standard will probably be lowered. To reduce the amount of lead released into the environment, EPA regulations now limit the level of lead in unleaded gasoline to 0.05 grams per gallon.
Drinking Water
❑ EPA’s proposed goal for lead in drinking water after treatment is zero.
EPA estimates that about 20% of the U.S. population (including 3.8 million children) consumes drinking water with lead levels above 20 µg/dL. EPA is required to set drinking water standards with two levels of protection. The primary standards define contaminant levels in drinking water as levels above which the water source requires treatment. These maximum contaminant levels (MCLs) are limits enforceable by law and are set as close as possible to the maximum contaminant level goals (MCLGs), the levels determined to be safe by toxicologic and biomedical considerations, independent of feasibility. EPA has promulgated a final rule for lead in drinking water: this rule does not establish an MCL; the MCLG is zero and the action level is set at 15 µg/L. If more than 10% of targeted tap water samples exceed the action level, certain actions are required of water system administrators. For further information, call the U.S. EPA Safe Drinking Water Hotline toll-free at 1–800–426–4791.
The use of lead solder and other lead-containing materials in connecting household plumbing to public water supplies was banned by EPA as of June 1988. Many older structures, however, still have lead pipe or lead-soldered plumbing internally, which may substantially increase the lead content of water at the tap. Regulations controlling the lead content of drinking-water coolers in schools went into effect in 1989.
❑ Most lead in food comes from solder in cans or glazes on ceramicware.
Regulating lead contamination in foods is the responsibility of the Food and Drug Administration (FDA). FDA has set a goal of less than 100 µg/day as the total lead intake by children 1 to 5 years of age. Lead in food and beverages is encountered by virtually this entire age group in the United States.
According to a 1988 ATSDR report, FDA has estimated that about 20% of all dietary lead comes from canned food; about two-thirds of that amount results from lead solder in cans. The number of food cans that are lead-soldered continues to decline. In 1979, over 90% of all food cans were lead soldered; in 1986, this figure was 20%, or less than about 2 million cans. It is important to note that imported canned foods are not included in these figures and may still contain lead. Imported glazed ceramics and lead-containing pottery are also potential sources of dangerously high levels of lead.
❑ Today, paint intended for residential use is limited to 0.06% lead content.
Since 1977, the Consumer Product Safety Commission has limited the lead in most paints to 0.06% (600 ppm by dry weight). Paint for bridges and marine use may contain greater amounts of lead.
Suggested Reading List
Cullen MR, Robins JM, Eskenazi B. Adult inorganic lead intoxication: presentation of 31 new cases and a review of recent advances in the literature. Medicine (Baltimore) 1983;62:221–47.
Gerber GB, Leonard IA, Jacquet P. Toxicity, mutagenicity and teratogenicity of lead. Mutat Res 1980;76:115–41.
Kehoe RA. Occupational lead poisoning: clinical types. J Occup Med 1972;14:298–300.
Piomelli S, Needleman HL, Rosen JF. Lead poisoning. American Academy of Pediatrics Update (audiotape available) 1988;9(4):1–9.
Putnam RD. Review of toxicology of inorganic lead. Am Ind Hyg Assoc J 1986;47:700–3.
Chelation Therapy
Chisolm JJ Jr, Kaplan E. Lead poisoning in childhood—comprehensive management and prevention. J Pediatr 1968;73(6):942–50.
Chisolm JJ Jr. The use of chelating agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr 1968;73(1):1–38.
Markowitz ME, Rosen JF. Assessment of lead stores in children: validation of an 8-hour CaNa 2 EDTA provocative test. J Pediatr 1984;104(3):337–41.
Markowitz ME, Rosen JF. Need for the lead mobilization test in children with lead poisoning. J Pediatr 1991;119(2):305–10.
Piomelli S, Rosen JF, Chisolm JJ Jr, Graef JW. Management of childhood lead poisoning. J Pediatr 1984;105(4):523– 32.
Rosen JF, Markowitz ME, Bijur PE, et al. Sequential measurements of bone lead content by L X-ray fluorescence in CaNa 2 EDTA-treated lead-toxic children. Environ Health Perspect 1991;93:271–7.
Rosen JF, Markowitz ME, Bijur PE, et al. Sequential measurements of bone lead content by L X-ray fluorescence in CaNa 2 EDTA-treated lead-toxic children [published erratum appears in Environ Health Perspect 1991;92:181]. Environ Health Perspect 1991;91:57–62.
Neurobehavioral Development
Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N Eng J Med 1987;316:1037–43.
Needleman HL, Gunnor C, Leviton A, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med 1979;300:689–95.
Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to lead in childhood. An 11-year follow-up report. N Engl J Med 1990;322:83–8.
Schwartz J, Otto D. Blood lead, hearing thresholds, and neurobehavioral development in children and youth. Arch Environ Health 1987;42:153–9.
Moore MR, Goldberg A, Yeung-Laiwah AC. Lead effects on the heme biosynthetic pathway. Ann NY Acad Sci 1985;191–202.
Nephropathy
Lurakis MF, Pitone JM. Occupational lead exposure, acute intoxication, and chronic nephropathy: report of a case and review of the literature. J Am Osteopath Assoc 1984;83:361–6.
Reproductive Effects
Mitchell JW, ed. Occupational medicine forum: lead toxicity and reproduction. J Occup Med 1987;29:397–9.
Uzych L. Teratogenesis and mutagenesis associated with the exposure of human males to lead: a review. Yale J Biol Med 1985;58:9–17.
Kunkel DB. The toxic emergency. Emergency Medicine 1986;18(Mar):207–17.
Marcus WL. Lead health effects in drinking water. Toxicol Ind Health 1986;2:363–400.
Related Government Documents
Agency for Toxic Substances and Disease Registry. The nature and extent of lead poisoning in children in the United States: A report to Congress. Atlanta: US Department of Health and Human Services, Public Health Service, 1988. DHHS report no. 99–2966.
Agency for Toxic Substances and Disease Registry. Toxicological profile for lead—draft. Atlanta: US Department of Health and Human Services, Public Health Service, 1992.
Centers for Disease Control. Preventing lead poisoning in young children: a statement by the Centers for Disease Control, January 1985. Atlanta: US Department of Health and Human Services, Public Health Service, 1985. DHHS report no. 99–2230. Revised October 1991.
Centers for Disease Control. Criteria for a recommended standard: occupational exposure to inorganic lead revised criteria. Atlanta: US Department of Health, Education, and Welfare, Public Health Service, 1978. Report no. (NIOSH) 78–158.
Centers for Disease Control. Lead poisoning following ingestion of homemade beverage stored in a ceramic jug-New York. Atlanta: US Department of Health and Human Services. MMWR 1989;38(21):379–80.
Centers for Disease Control. Occupational and environmental lead poisoning associated with battery repair shops-Jamaica. Atlanta: US Department of Health and Human Services. MMWR 1989;38(27):474–81.
Centers for Disease Control. Cadmium and lead exposure associated with pharmaceuticals imported from Asia-Texas. Atlanta: US Department of Health and Human Services. MMWR 1989;38(35):612–4.
Centers for Disease Control. Surveillance for occupational lead exposure-United States, 1987. Atlanta: US Department of Health and Human Services. MMWR 1989;38(37):642–6.
Centers for Disease Control. Lead poisoning in bridge demolition workers-Massachusetts. Atlanta: US Department of Health and Human Services. MMWR 1989;38(40):687–94.
Environmental Protection Agency. Air quality criteria for lead, Vol 2. Research Triangle Park, North Carolina: US Environmental Protection Agency, Office of Health and Environmental Assessment. Report no. EPA-600/ 8–83/028bF.
Environmental Protection Agency. Maximum Contaminant Level Goals and National Primary Drinking Water Regulations for Lead and Copper. Federal Register 1991;56:26460, 26477.
Office of the Federal Register. Code of federal regulations; occupational safety and health standards. Appendix C-Medical surveillance guidelines. Washington, DC: Office of the Federal Register, National Archives and Records Administration, 1988. (29 CFR §1910.1025).
Answers to Pretest and Challenge Questions
Pretest questions are found on page 1. Challenge questions begin on page 3.
All members of the family are at risk; they should be promptly evaluated and, if necessary, treated. The mother’s unborn child is also at risk. Workers in the radiator repair shop and their families, and any of the children’s playmates who have accompanied them to the repair shop after school, should also be screened.
The boy’s mother is 5 months pregnant. Since the placenta presents no barrier to lead, the fetus’ blood lead level is likely to be similar to that of the mother. It is during the initial weeks of pregnancy that the neurologic system of the conceptus is formed; therefore, damage to the fetus may have already occurred. The mother is no longer working at the repair shop, but you should alert her and the family to the possibility of continued lead exposure via the grandfather, who may be bringing lead dust home on his skin, shoes, or clothes.
Two of the obvious sources of lead suggested in the case study are leaded paint at home (paint flakes, household dust, and soil) and fumes and dust from solder at the radiator repair shop. You should determine if the boy ever had pica (a compulsive eating of nonfood items, to be distinguished from normal hand-to-mouth behavior of children). Pica is more common in children aged 2 to 5, so it is unlikely that this is a present behavior. Exposure to high levels of lead at the radiator repair shop is very possible, and you need to ascertain the type and length of the boy’s play at the shop.
To evaluate less obvious, but possible, sources of lead exposure, you might inquire about the proximity of the child’s home and play areas to freeways, hazardous waste sites, and industry. The occupations of all adults in the household are important; children of lead-exposed workers have been shown to have higher lead levels than control groups. Do any of the boy’s associates or does the father have hobbies involving lead, such as those mentioned on page 4? You might also inquire whether the home is undergoing remodeling, whether any home or folk remedies are used, if glazed ceramicware is used for food, or if there are lead or lead-soldered pipes in the house that could contaminate the drinking water.
If a child does not have pica and there is nothing to suggest that a lead-containing object has recently been ingested, an abdominal X ray will likely be negative. On long-bone radiograms, opacities in the metaphysial plates may be seen after 4–8 weeks or more of lead exposure. These “lead lines” (which are due to dense zones of calcium and not deposited lead) are more likely to be found in larger bones (e.g., radius and tibia) than in smaller bones (e.g., ulna and fibula). Lead lines seen in the smaller bones may be indicative of a longer exposure, usually several months. Radiographs are helpful only in the rare circumstances that they are positive. Negative X rays do not rule out lead poisoning.
Even with complete removal from the source of exposure, the blood lead level will drop only gradually because, without chelation, lead is only slowly excreted. In addition, even as it is excreted, it may be replaced by lead currently stored in bones and teeth.
This rebound phenomenon is due to the mobilization of lead from the body’s stores in bones and teeth.
The major effects of lead on the human body are damage to the neurologic, hematologic, renal, and reproductive systems.
Because of an incompletely developed blood-brain barrier, children under 36 months of age are particularly susceptible to neurologic damage at very low blood lead levels. Since children (to age 7) are more sensitive to lead’s effects, most adverse effects of lead are often manifested at lower blood lead levels in children than in adults.
History suggests delayed language ability, slightly impaired hearing, short stature, possible attention deficit disorder, and anemia. The child is also experiencing passive exposure to his mother’s cigarette smoke and family disruption related to his parents’ divorce.
Three of the most common causes of microcytic anemia are iron deficiency, hemoglobinopathy, and lead poisoning. In lead-poisoned patients, anemia is usually evident only when the blood lead level is significantly elevated for prolonged periods. It manifests in only a relatively small number of children with chronic lead poisoning. It is possible for a patient to be both lead-poisoned and to have anemia due to some other cause. The relative rarity of nutritional iron deficiency in this boy’s age group and the absence of evidence for blood loss suggest consideration of other etiologies to explain the anemia.
An elevated ZPP level is most often due to iron deficiency anemia, hemolytic anemias, or lead poisoning. A rare disease that may cause the ZPP level to be markedly elevated is erythropoietic protoporphyria.
To confirm lead poisoning, the best test is a venous blood lead level. If the blood lead level is below 25 µg/dl, then a serum ferritin level and other iron studies can be used to determine if iron deficiency anemia exists.
With an elevated blood lead level of 50 µg/dL, the conclusion is that the boy is lead-poisoned. In this case, the child should be referred for appropriate chelation therapy immediately. It is important to immediately identify and eliminate all sources of lead exposure for both the boy and his family. Environmental evaluation, intervention, and remediation should begin immediately. All household members should be screened for lead exposure (See Table 1 , page 15). Adequate diet for the family should be stressed.
You should consult with a physician experienced in treating lead-poisoned patients. To identify such physicians, contact your state or local health department, a university medical center, or a certified regional poison control center.
Knowing the subgroups at greatest risk of lead exposure, you should take every opportunity to educate these subpopulations, your colleagues, and the community about the hazards of lead poisoning and the steps to prevent its occurrence. Those children and members of the community whom you suspect may be in danger of lead poisoning should be promptly screened.
In certain states, public health authorities must be notified if a patient’s blood lead level and ZPP level exceed certain limits. In any case, you should contact your state or local health department so all sources of lead in the home can be identified and abated. You should also notify OSHA so the radiator repair shop can be brought, if required, into compliance with the federal lead standard. A NIOSH health hazard evaluation could also be requested. The reason for notifying these agencies is to prevent lead exposure in others.
The federal lead standard mandates that a worker with a blood lead level of 60 µg/dl or higher (or an average of 50 µg/dL)undergo medical removal from the lead hazard and be reassigned with retention of job seniority and pay. In addition to referring her for obstetrical evaluation, you should recommend that the mother talk to her employer, employee representative, and OSHA to clarify her work status under the lead standard and possible reinstatement procedures.
Sources of Information
More information on the adverse effects of lead and the treatment and management of lead-exposed persons can be obtained from ATSDR, your state and local health departments, and university medical centers. Case Studies in Environmental Medicine: Lead Toxicity is one of a series. For other publications in this series, please use the order form on the back cover. For clinical inquiries, contact ATSDR, Division of Health Education, Office of the Director, at (404) 639–6204.
People are increasingly concerned about potential environmental health hazards and often ask their physicians questions such as: "Is the tap water safe to drink?" "Is it safe to live near power lines?" Unfortunately, physicians often lack the information and training related to environmental health risks needed to answer such questions. This book discusses six competency based learning objectives for all medical school students, discusses the relevance of environmental health to specific courses and clerkships, and demonstrates how to integrate environmental health into the curriculum through published case studies, some of which are included in one of the book's three appendices. Also included is a guide on where to obtain additional information for treatment, referral, and follow-up for diseases with possible environmental and/or occupational origins.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Lead poisoning
On this page, risk factors, complications.
Lead poisoning occurs when lead builds up in the body, often over months or years. Even small amounts of lead can cause serious health problems. Children younger than 6 years are especially vulnerable to lead poisoning, which can severely affect mental and physical development. At very high levels, lead poisoning can be fatal.
Lead-based paint and lead-contaminated dust in older buildings are common sources of lead poisoning in children. Other sources include contaminated air, water and soil. Adults who work with batteries, do home renovations or work in auto repair shops also might be exposed to lead.
There is treatment for lead poisoning, but taking some simple precautions can help protect you and your family from lead exposure before harm is done.
Initially, lead poisoning can be hard to detect — even people who seem healthy can have high blood levels of lead. Signs and symptoms usually don't appear until dangerous amounts have accumulated.
Lead poisoning symptoms in children
Signs and symptoms of lead poisoning in children include:
- Developmental delay
- Learning difficulties
- Irritability
- Loss of appetite
- Weight loss
- Sluggishness and fatigue
- Abdominal pain
- Constipation
- Hearing loss
- Eating things, such as paint chips, that aren't food (pica)
Lead poisoning symptoms in newborns
Babies exposed to lead before birth might:
- Be born prematurely
- Have lower birth weight
- Have slowed growth
Lead poisoning symptoms in adults
Although children are primarily at risk, lead poisoning is also dangerous for adults. Signs and symptoms in adults might include:
- High blood pressure
- Joint and muscle pain
- Difficulties with memory or concentration
- Mood disorders
- Reduced sperm count and abnormal sperm
- Miscarriage, stillbirth or premature birth in pregnant women
From Mayo Clinic to your inbox
Lead is a metal that occurs naturally in the earth's crust, but human activity — mining, burning fossil fuels and manufacturing — has caused it to become more widespread. Lead was also once used in paint and gasoline and is still used in batteries, solder, pipes, pottery, roofing materials and some cosmetics.
Lead in paint
Lead-based paints for homes, children's toys and household furniture have been banned in the United States since 1978. But lead-based paint is still on walls and woodwork in many older homes and apartments. Most lead poisoning in children results from eating chips of deteriorating lead-based paint.
Water pipes and imported canned goods
Lead pipes, brass plumbing fixtures and copper pipes soldered with lead can release lead particles into tap water. Lead solder in food cans, banned in the United States, is still used in some countries.
Other sources of lead exposure
Lead sometimes can also be found in:
- Soil. Lead particles from leaded gasoline or paint settle on soil and can last years. Lead-contaminated soil is still a major problem around highways and in some urban settings. Some soil close to walls of older houses contains lead.
- Household dust. Household dust can contain lead from lead paint chips or from contaminated soil brought in from outside.
- Pottery. Glazes found on some ceramics, china and porcelain can contain lead that can leach into food served or stored in the pottery.
- Toys. Lead is sometimes found in toys and other products produced abroad.
- Cosmetics. Tiro, an eye cosmetic from Nigeria, has been linked to lead poisoning. Kohl is another eye makeup that may contain lead.
- Herbal or folk remedies. Lead poisoning has been linked to greta and azarcon, traditional Hispanic medicines, as well as some from India, China and other countries.
- Mexican candy. Tamarind, an ingredient used in some candies made in Mexico, might contain lead.
- Lead bullets. Time spent at firing ranges can lead to exposure.
- Occupations. People are exposed to lead and can bring it home on their clothes when they work in auto repair, mining, pipe fitting, battery manufacturing, painting, construction and certain other fields.
Factors that may increase your risk of lead poisoning include:
- Age. Infants and young children are more likely to be exposed to lead than are older children. They might chew paint that flakes off walls and woodwork, and their hands can be contaminated with lead dust. Young children also absorb lead more easily, and it's more harmful for them than it is for adults and older children.
- Living in an older home. Although the use of lead-based paints has been banned since the 1970s, older homes and buildings often retain remnants of this paint. People renovating an older home are at even higher risk.
- Certain hobbies. Making stained glass and some jewelry requires the use of lead solder. Refinishing old furniture might put you in contact with layers of lead paint.
- Living in developing countries. Developing countries often have less strict rules regarding exposure to lead than do developed countries. American families who adopt a child from another country might want to have the child's blood tested for lead poisoning. Immigrant and refugee children also should be tested.
Lead can harm an unborn child. If you're pregnant or planning a pregnancy, be especially careful to avoid exposure to lead.
Exposure to even low levels of lead can cause damage over time, especially in children. The greatest risk is to brain development, where irreversible damage can occur. Higher levels can damage the kidneys and nervous system in both children and adults. Very high lead levels may cause seizures, unconsciousness and death.
Simple measures can help protect you and your family from lead poisoning:
- Wash hands and toys. To help reduce hand-to-mouth transfer of contaminated dust or soil, wash your children's hands after outdoor play, before eating and at bedtime. Wash their toys regularly.
- Clean dusty surfaces. Clean your floors with a wet mop and wipe furniture, windowsills and other dusty surfaces with a damp cloth.
- Remove shoes before entering the house. This will help keep lead-based soil outside.
- Run cold water. If you have older plumbing containing lead pipes or fittings, run your cold water for at least a minute before using. Don't use hot tap water to make baby formula or for cooking.
- Prevent children from playing on soil. Provide them with a sandbox that's covered when not in use. Plant grass or cover bare soil with mulch.
- Eat a healthy diet. Regular meals and good nutrition might help lower lead absorption. Children especially need enough calcium, vitamin C and iron in their diets to help keep lead from being absorbed.
- Keep your home well maintained. If your home has lead-based paint, check regularly for peeling paint and fix problems promptly. Try not to sand, which generates dust particles that contain lead.
Jan 21, 2022
- Sample JA. Childhood lead poisoning: Clinical manifestations and diagnosis. https://www.uptodate.com/contents/search. Accessed Nov. 10, 2021.
- Lead FAQs. Centers for Disease Control and Prevention. https://www.cdc.gov/nceh/lead/faqs/lead-faqs.htm. Accessed Nov. 11, 2021.
- Sample JA. Childhood lead poisoning: Management. https://www.uptodate.com/contents/search. Accessed Nov. 10, 2021.
- Markowitz M. Lead poisoning: An update. Pediatrics in Review. 2021; doi:10.1542/pir.2020-0026.
- Lead poisoning. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health. Accessed Nov. 11, 2021.
- Goldman RH, et al. Lead exposure and poisoning in adults. https://www.uptodate.com/contents/search. Accessed Nov. 10, 2021.
- Protect your family from sources of lead. U.S. Environmental Protection Agency. https://www.epa.gov/lead/protect-your-family-sources-lead. Accessed Nov. 11, 2021.
- Sample JA. Childhood lead poisoning: Exposure and prevention. https://www.uptodate.com/contents/search. Accessed Nov. 10, 2021.
- What are possible health effects from lead exposure? Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/csem/leadtoxicity/physiological_effects.html. Accessed Nov. 11, 2021.
- Ferri FF. Lead poisoning. In: Ferri's Clinical Advisor 2022. Elsevier; 2022. https://www.clinicalkey.com. Accessed Nov. 22, 2021.
- Diseases & Conditions
- Lead poisoning symptoms & causes
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.48(9); 2019 Sep

Effects of lead from ammunition on birds and other wildlife: A review and update
Deborah j. pain.
1 Department of Zoology, University of Cambridge, David Attenborough Building, Pembroke Street, Cambridge, CB2 3QZ UK
2 Wildfowl & Wetlands Trust, Slimbridge, Gloucestershire GL2 7BT UK

Rafael Mateo
3 Toxicología de Fauna Silvestre, Instituto de Investigación en Recursos Cinegéticos (IREC), CSIC-UCLM-JCCM, Ronda de Toledo 12, 13005 Ciudad Real, Spain
Rhys E. Green
Poisoning of wild birds following ingestion of lead from ammunition has long been recognised and considerable recent research has focused on terrestrial birds, including raptors and scavengers. This paper builds upon previous reviews and finds that both the number of taxa affected and geographical spread of cases has increased. Some lead may also be absorbed from embedded ammunition fragments in injured birds which risk sub-lethal and welfare effects. Some papers suggest inter-specific differences in sensitivity to lead, although it is difficult to disentangle these from other factors that influence effect severity. Sub-lethal effects have been found at lower blood lead concentrations than previously reported, suggesting that previous effect-level ‘thresholds’ should be abandoned or revised. Lead poisoning is estimated to kill a million wildfowl a year in Europe and cause sub-lethal poisoning in another ≥ 3 million. Modelling and correlative studies have supported the potential for population-level effects of lead poisoning in wildfowl, terrestrial birds, raptors and scavengers.
Introduction
Lead toxicity to humans has been known for centuries, attracting considerable attention as a public health issue in the late twentieth century when longitudinal studies highlighted irreversible effects of low-level chronic exposure to lead on children’s IQ. Introduced legislation subsequently controlled or eliminated many uses of lead (e.g. in petrol and paint) to reduce exposure (Stroud 2015 ). Lead poisoning of wildlife from ammunition (gunshot or bullets) has been recognised for over a century (Calvert 1876 ). Birds suffer lead poisoning following direct ingestion (i) of spent lead gunshot from the environment or (ii) of ammunition or ammunition-fragments embedded in their food. (i) is widespread among wildfowl and terrestrial game birds, especially those with a muscular gizzard that eat grit to help grind their food; (ii) among raptors and scavenging birds that eat birds and mammals shot by people, or their discarded remains. An extensive literature links avian lead poisoning to ammunition sources. This includes experimental evidence of dose-dependent effects and field evidence of source, pathway and effects including: ammunition fragments in the alimentary tract of dead and living birds (through post-mortem and x-radiography examinations); ammunition fragments in regurgitated pellets (primarily of raptors); temporal and spatial correlations between elevated tissue lead levels in birds and hunting activities; spatial analyses of elevated tissue lead concentrations in relation to potential sources of exposure to spent ammunition and lead isotopic studies to match tissue lead concentrations with sources. Studies provide overwhelming support for ammunition-derived lead being the major contributor to elevated tissue lead concentrations in wild birds. However, while substantial progress has been made at reducing human exposure to lead from a variety of sources, progress with reducing wildlife exposure to lead from ammunition has been patchy and sometimes ineffective. Lead gunshot has been banned and replaced with non-toxic alternative ammunition types in some places or for some uses (e.g. for all wildfowl hunting in the USA from 1991/92 and all shooting in Denmark from 1996). Many reviews, workshops and conferences have considered this subject in recent decades. Of particular significance are the proceedings of: an International Waterfowl and Wetlands Research Bureau Workshop (Pain 1992 ); a conference in the USA convened by The Peregrine Fund addressing the implications of lead from spent ammunition for both wildlife and human health (Watson et al. 2009 ); a symposium held at Oxford University in the UK (Delahay and Spray 2015 ) and the publication of the final report of the Lead Ammunition Group (LAG 2015 ), set up to advise the UK government on risks from lead ammunition and mitigation options. A recent proposal for a European Union-wide restriction on the use of lead gunshot for shooting in and over wetlands also includes a detailed evidence review (ECHA 2017 ). Two scientific consensus reports draw attention to the overwhelming support given by environmental and public health scientists to evidence about the toxic effects on humans and wildlife of lead from ammunition and the need to prevent them (Group of Scientists 2013 , 2014 ). In the following review we do not attempt to repeat previous reviews. Instead, we summarise their key conclusions and update them with results from the substantial literature published during the last 5 years. We evaluate whether the evidence underlying previous conclusions has been reinforced or refuted and highlight areas of research where understanding has been significantly advanced.
A literature search using ‘Web of Science’ was conducted for the period 2013–2018 using the following search terms: Lead, ammunition; lead, ammunition, poisoning; bird/reptile/amphibian/mammal/invertebrate/fish and lead and poisoning; bird/reptile/amphibian/mammal/invertebrate/fish and lead and ammunition/bullet/shot; lead, shot, poisoning; lead, bullet, poisoning; lead, game (sorted by animal and poisoning); game; wildlife, lead, poisoning; fishing, lead, poisoning; angling, lead, poisoning; wildfowl, lead, poisoning; waterfowl, lead, poisoning; blood, lead, bird; scavenger, lead, poisoning; vulture, lead, poisoning; plant, lead, ammunition. Preliminary searches of other databases were subsequently undertaken (e.g. Google Scholar and simple ‘Google’ online searches) but it soon became evident that the vast majority of relevant literature had been identified by Web of Science. References that the authors considered added to previous reviews, expanded information or contradicted the conclusions of previous reviews are included below. For brevity, material that simply confirmed the conclusions of previous reviews, or dealt with methodological issues not directly relevant to current knowledge of the effects of lead from ammunition on wildlife, generally has not been included. The literature search was initially conducted in summer 2018 and updated at the end of December 2018.
Effects of lead on wildlife
Lead is a toxic non-essential metal that has no compensatory beneficial effects in living organisms. It is an accumulative metabolic poison that is non-specific, affecting a wide range of physiological and biochemical systems including the haematopoietic, vascular, nervous, renal, immune and reproductive systems. It causes adverse effects on behaviour and survival (Eisler 1988 ; ATSDR 2007 ; EFSA 2010 ). After absorption by a vertebrate animal, inorganic lead effects are independent of its source. Birds are the most studied and probably the most affected taxon with respect to poisoning from the ingestion of lead from ammunition. However, the toxic effects of lead are broadly similar in all vertebrates and well known from numerous experimental and field studies (reviewed in Eisler 1988 ; Pattee and Pain 2003 ; Franson and Pain 2011 ). Clinical signs of poisoning in birds are often associated with chronic extended exposure at a level that is not initially likely to cause immediate failure of biological function or death, although death may result. Signs include anaemia, lethargy, muscle wastage and loss of fat reserves, green diarrhoea staining the vent, wing droop, loss of balance and coordination and other neurological signs such as leg paralysis or convulsions (e.g. Wobester 1997 ; Friend and Franson 1999 ; Pattee and Pain 2003 ). In contrast, after acute exposure to high levels of lead, birds die rapidly without such signs.
After ingestion of ammunition or ammunition fragments by birds, lead may be eliminated rapidly from the alimentary tract with little lead absorption, retained until completely eroded, solubilised and absorbed, or show any intermediate outcome. Absorbed lead is transported in the bloodstream and deposited rapidly into soft tissues, primarily the liver and kidney, but also into bone and the growing feathers of birds. Lead in bone is retained for long periods and accumulates during an animal’s lifetime, whereas lead in soft tissues has a much shorter half-life (weeks to months). Blood lead (PbB) remains elevated for weeks or months after exposure. Previously suggested ‘thresholds’ to guide the interpretation of tissue lead concentrations are given in Table 1 . The physiological effects of lead in birds have been widely reviewed (e.g. Pain and Green 2015 ). An individual bird’s susceptibility to lead poisoning is influenced by many biological and environmental factors and the sensitivity to lead seems, to some degree, to vary between species. In addition to the direct impacts of lead on welfare and survival, indirect effects are likely to occur. These may include increased susceptibility to infectious diseases, parasite infestations (via lead’s immunosuppressive effects), and increased susceptibility to death from a range of other causes, such as collision with power lines (Kelly and Kelly ( 2005 ), Ecke et al. ( 2017 )—via its effects on muscular strength and coordination) and being shot (e.g. shown by Bellrose 1959 ; reviewed in Pain and Green 2015 ).
Table 1
Suggested interpretation of tissue lead concentrations in Anseriformes, Falconiformes and Accipitriformes
Adapted from Franson and Pain ( 2011 ) Table 16.1
a Anseriformes
b Falconiformes and Accipitriformes (previously grouped under Falconiformes in Franson and Pain ( 2011 ))
c Lead concentrations in bone reflect lifetime accumulation and concentrations may be similar in cases of short-term acute exposure and long-term chronic exposure
It is currently considered that there are no identified “no observed adverse effect levels” (NOAEL) or “predicted no effect concentrations” (PNEC) for lead in humans (EFSA 2010 ) and the same is thus likely for other vertebrates. Hence, the use of acceptable thresholds for exposure to lead involves acceptance of some level of avoidable harm.
Pathways of exposure
Movement of lead derived from spent ammunition into animals via water, soil and plants.
The deposition of lead ammunition into the environment can result in elevated soil and water concentrations in relation to the amount deposited and environmental conditions. Wild animals can be exposed to ammunition-derived lead from water or the ingestion of contaminated soil, or via plants or lower organisms that have taken up such lead (reviewed in LAG 2015 ; Pain and Green 2015 ). Comparatively little information exists on wildlife effects via these pathways relative to the direct ingestion of lead ammunition or fragments. Recent studies on non-avian taxa support uptake of and effects of ammunition-derived lead via these pathways. One recent study (Rodríguez-Seijo et al. 2017 ) found high lead levels in soils and the whole bodies of the lumbricid worm Eisenia andrei associated with an abandoned shooting range in northwest Spain. High contents of lead and Polycyclic Aromatic Hydrocarbons (PAH) in soil samples and in E. andrei , were associated with a reduction in the number of juveniles produced [although this was from both PAH and Pb combined], whereas, Vibrio fischeri, Raphidocelis subcapitata and Daphnia magna displayed a slight toxic response to the soil leachates tested.
Mariussen et al. ( 2017 ) studied the accumulation of lead (Pb) in brown trout ( Salmo trutta ) from Lake Kyrtjønn within an abandoned shooting range in Norway, compared to a nearby reference site (both lakes were acidic). Brown trout from Lake Kyrtjønn had significantly elevated lead in bone and other tissues and significantly inhibited ALAD activity in the blood compared to those from the reference site. Trout eggs were placed in stream outlets from both lakes and lead concentrations were significantly elevated in eggs and alevins from Lake Kyrtjønn compare to the reference lake. The authors concluded that adult brown trout, fertilised eggs and alevins, may be subjected to increased stress due to chronic exposure to Pb.
Direct ingestion of spent lead ammunition from the environment
Lead gunshot has been used for centuries. An estimated 600–700 million cartridges containing lead gunshot (18 000–21 000 tonnes of lead; c. 200 thousand million individual gunshot) are used annually in Europe for hunting. Some of it instantly kills the animals at which it is fired, but a proportion of them are wounded. The viscera of killed animals of some species, such as deer, are discarded in the environment. Only a small proportion of gunshot strike their targets, and so the remaining spent ammunition is dispersed widely into the environment (ECHA 2017 ). Under most environmental conditions, metallic lead is relatively stable. Over time, ammunition lead deposited on the soil or in water will degrade through a process of erosion and chemical reaction and lead compounds with different solubilities may form on its surface. Soil lead concentrations generally increase, especially in areas of high deposition and/or when soil conditions, such as low pH, facilitate this. Degradation of lead shot is slow, probably taking tens to hundreds of years. Thus a “historical legacy” of gunshot remains available to wildlife, increasing over time where shooting with lead gunshot continues. Gunshot generally sinks slowly through most types of soil and mud and may be available to feeding birds for many years, although a high proportion of gunshot ingested is that most recently deposited. Pain et al. ( 2015 ) review this in relation to soil types and management practices.
Gunshot densities are higher in areas of intense or regular shooting. In wetlands, densities may range from just a few to several hundred gunshot/m 2 (Fig. 1 ) but thousands/m 2 can be found in some situations like target shooting areas (e.g. O’Halloran et al. 1988 ).

Densities of lead shot in wetlands due to waterfowl hunting and sport shooting. Modified from Descalzo and Mateo ( 2018 ), updated from Mateo ( 2009 ). Shot densities are from individual or multiple sites in each country and from depths ranging from 5 to 30 cm. Data from Table 2 of Descalzo and Mateo ( 2018 ), updated from Mateo ( 2009 )
There is evidence of direct ingestion of spent ammunition from soil or mud by many species of birds including wildfowl, some other water birds and game birds across Europe, North America and other countries where studies have been conducted. A substantial body of evidence spanning more than half a century exists documenting this pathway (e.g. Mateo 2009 ; Pain and Green 2015 ). Reported levels of gunshot ingestion, in terms of the proportions of killed or trapped birds with shot in the alimentary tract vary among wildfowl species according to their feeding habits. Species that feed on seeds and hard-bodied benthic animals, such as molluscs, tend to ingest larger particles of grit to assist in breaking up their food, whereas species that graze plant leaves ingest small grit particles. For a given wildfowl species, or for species with comparable feeding ecology, the proportion of birds with ingested shot tends to be higher in Europe than North America (Fig. 2 ). Few recent papers add to the literature on this pathway, presumably because the literature is already vast and few questions remain unanswered. Also, legislation has been introduced in some places to limit exposure—although this is incomplete and has met with variable success (see papers is Delahay and Spray 2015 ). The few recent studies on this pathway support previous conclusions of exposure where a pathway exists, and further document the wide geographical extent of the problem and range of species affected.

Prevalence of Pb shot ingestion in waterfowl species from North America ( n = 171 697) and Europe ( n = 75 761). Modified from Mateo ( 2009 ). American wigeon ( Anas americana ), Eurasian wigeon ( Anas penelope ), gadwall ( Anas strepera ), green-winged teal ( Anas carolinensis ), common teal ( Anas crecca ), mallard ( Anas platyrhynchos ), northern pintail ( Anas acuta ), northern shoveler ( Anas clypeata ), common pochard ( Aythya ferina ), redhead ( Aythya americana )
Recent European studies have reported little or no evidence of gunshot ingestion in geese feeding in areas with no hunting or low gunshot densities (Aloupi et al. 2015 ; Mateo et al. 2016 for studies in Greece and Bulgaria respectively). Runia and Solem ( 2016 ) found levels of lead gunshot ingestion to be about five times higher in 660 ring-necked pheasant ( Phasianus colchicus ) harvested on shooting preserves in South Dakota USA then in 1301 birds from non-preserve areas where lead gunshot availability was presumably lower (3.9%, 95% CI 2.7–5.7% vs 0.8%, 95% CI 0.4–1.4% respectively).
Mateo et al. ( 2013 ) reviewed lead poisoning studies from Spain and reported that high densities of lead gunshot in various internationally important wetlands for waterfowl resulted in proportions of birds with ingested gunshot close to 70% in some species, such as pintail ( Anas acuta ). This study found that lead poisoning is a major cause of mortality of the white-headed duck ( Oxyura leucocephala ), listed as Endangered in the IUCN Red List. High proportions of birds with ingested gunshot (9.3% of 461 birds) were reported in chukars ( Alectoris chukar ) harvested in north-western Utah, USA, and 8.3% of 121 birds analysed had elevated liver lead (Bingham et al. 2015 ). Few studies have previously been published from Argentina. Ferreyra et al. ( 2014 ) investigated gunshot ingestion and blood lead concentrations in 415 hunter-killed ducks and 96 live-trapped ducks of 5 species in Argentina. Overall 10.4% of ducks contained ingested gunshot. Blood lead levels were elevated in 28% of ducks and exceeded 100 µg/dl, a threshold for clinical toxicity, in 8.6% of birds. Lead poisoning has been reported in the globally Vulnerable nene ( Branta sandvicensis ) in Hawaii (Work et al. 2015 ). Recent studies from Iranian wetlands found elevated levels of lead in the livers and/or kidneys of some sampled common pochard ( Aythya ferina ), mallard ( Anas platyrhynchos ), teal ( Anas crecca ) and gadwall ( Anas strepera ), with ingested gunshot suggested as a possible source (Sinkakarimi et al. 2015 , 2018 ).
Using lead isotope analyses of blood lead levels, Binkowski et al. ( 2016 ) concluded that lead gunshot currently available in Poland was unlikely to be the source of blood lead (mean of 0.241 ppm—24 µg/dl) in mute swans ( Cygnus olor ) wintering in northern Poland, although these authors did not examine how the relationship between blood and ammunition isotope ratios changes with blood lead concentration, and 204 Pb, upon which this conclusion relies, is not readily analysed using Q-ICP-MS (Ellam 2010 ).
Ingestion of lead from ammunition by raptors and scavengers
Lead from ammunition is available to predators and scavengers in the flesh of their prey either as whole gunshot/bullets or ammunition fragments. Exposure occurs when shot animals are killed but not retrieved, when parts of the carcass (e.g. offal) are discarded, or when animals are wounded but survive (and may be more vulnerable to early death later or to predation). Large numbers of some quarry species can survive carrying lead gunshot, commonly 20–30% in some wildfowl populations (Table I, Pain et al. 2015 ). Recent studies investigating the presence of lead fragments and/or elevated tissue lead concentrations add to evidence of the contamination of both small and large game species with lead ammunition (Warner et al. 2014 ; Cruz-Martinez et al. 2015 ; Andreotti et al. 2016 ; Ertl et al. 2016 ; Herring et al. 2016 ). Using wild and captive birds (ravens ( Corvus corax ), white-tailed eagles ( Haliaeetus albicilla ) and common buzzards ( Buteo buteo) ) Nadjafzadeh et al. ( 2015 ) found that birds more frequently ingested smaller metal fragments. However, they only avoided those > 8.8 mm, which is considerably larger than most gunshot or bullet fragments. Analyses of ‘trash’ items from the nest area of California condors ( Gymnogyps californianus ) (Finkelstein et al. 2015 ) and a literature review (Golden et al. 2016 ) confirmed the view that, while different sources of lead are available in the landscape, most lead poisoning of scavenging birds appears to result from lead-based ammunition ingested in their food. Low tissue lead levels (liver lead < 2.1 ppm dw; N = 11) have been reported in the lesser-spotted eagle ( Clanga pomarina brehm ), a species that breeds in Europe but migrates to Africa, thus avoiding the European hunting season (Kitowski et al. 2017c ). In contrast, a greater-spotted eagle ( Clanga clanga ), a related species that is associated with wetlands and susceptible to lead poisoning as it feeds on unretrieved quarry (BirdLife International 2018 ), was found wintering in the Ebro Delta, Spain, with an elevated blood lead level of 33.6 µg/dl (Mateo et al. 2001 ).
Previously, most studies documenting this exposure pathway were of condors and eagles (particularly California condor, bald eagle ( Haliaeetus leucocephalus ) and golden eagle ( Aquila chrysaetos ) in North America, white-tailed eagle in Europe and Japan and Steller’s sea eagle ( Haliaeetus pelagicus ) in Japan). However, many other species have been reported as exposed to and/or poisoned by lead ammunition. While fewer studies had been conducted on other species, recent research has documented exposure via this pathway in new species, taxa and locations, considerably strengthening the evidence-base and highlighting the significance of this exposure pathway. These studies are summarised in Table 2 , to which previous studies, reviewed in Pain et al. ( 2009 ) add at least another 14 species.
Table 2
Recent studies on exposure to and poisoning from lead from ammunition in predatory and scavenging birds
a We have no knowledge of previous reports in the published literature of lead poisoning in the wild in this species; for Andean condor, elevated feather lead with isotopic signals compatible with ammunition sources had been reported previously from Patagonia in Argentina (Lambertucci et al. 2011 )
b Ingestion of or poisoning by lead from ammunition has been reported in captive birds—see review of Pain et al. ( 2009 )
c Excluding species already listed above in the table
d Concentrations are given in the units presented in the references: ppm = µg/g = mg/kg
New studies highlight the potential for exposure of mammalian scavengers and predators to ammunition lead. Legagneux et al. ( 2014 ) used camera traps to identify species scavenging on moose ( Alces alces ) viscera left by hunters in eastern Quebec, Canada and these included black bears ( Ursus americanus ). In a study of brain metal concentrations in 9 mammal species from north-western Poland, Kalisinska et al. ( 2016 ) found that raccoon dogs ( Nyctereutes procyonoides ) from an area where hunting is prohibited had a lower brain lead compared to those from hunting grounds, and speculated that the elevated levels could have resulted from ingesting lead from animals shot by hunters. Two captive cheetahs that had routinely been fed on hunted antelope or game birds were suspected to have died from lead poisoning; they had ingested bullets in their stomachs, elevated tissue lead levels and associated clinical sign of poisoning (North et al. 2015 ). Hivert et al. ( 2018 ) found significantly higher blood lead concentrations in captive than wild Tasmanian devils ( Sarcophilus harrisii ). Captive Tasmanian devils were fed the meat of wild animals shot with lead ammunition. Subsequent removal from the Tasmanian devil’s diet of the lead-containing heads and wounds from shot animals resulted in a significant decrease in blood lead concentrations in animals at one of the captive study sites. These studies suggest that mammalian predators and scavengers that eat game species may also be at risk.
Movement of lead from embedded ammunition into body tissues
It is well established that lead from ammunition that has been shot into and become embedded in human body tissues can be mobilised and give rise to health effects (Weiss et al. 2017 ). Until recently, little was known about this possible pathway of exposure in wildlife. One study found that 2 white-tailed deer ( Odocoileus virginianus) with retained lead ammunition from previous gunshot wounds had muscle tissue lead concentrations similar to controls although one had elevated bone lead levels (Zimmer and Osier 2018 ). Other recent work on birds suggests that some embedded lead may be mobilised. Berny et al. ( 2017 ) found that birds of prey in French wildlife centres that had embedded lead projectiles had significantly higher blood lead concentrations than those without (22.4 vs 14.3 µg/dl), suggesting that embedded lead projectiles may release lead and have long-term health effects. In Peru, 6 of 9 Andean condors ( Vultur grypphus ) at a rehabilitation centre had detectable blood lead levels (3.7 µg/dl to 17.4 µg/dl), with a mean of 9.95 µg/dl. The highest value was from a condor admitted due to a gunshot wound and found on radiographic examination to be carrying 45 lead pellets embedded in body tissues as confirmed by X-ray examination (L. Schaefer pers. com. cited in Wiemeyer et al. 2017 ). In a study of wild California condors, Finkelstein et al. ( 2014 ) found one bird that had been shot and retained embedded birdshot (small sized gunshot) in its tissues. The blood lead level was 16.6 µg/dl and the isotope ratios of birdshot and blood lead were indistinguishable. The following year the same bird was captured with clinical lead poisoning (blood lead levels 556 µg/dl) and radiography showed that it had ingested a buckshot (large sized gunshot), and still retained embedded birdshot from the previous shooting incident. The blood and buckshot lead isotope ratios were indistinguishable at this time, but the buckshot isotope ratio was measurably different from that of the birdshot. This illustrates that ingested shot presents a far greater risk to this species than embedded shot, but nonetheless that some transference of lead from embedded shot appears to occur. Similarly LaDouceur et al. ( 2015 ) measured tissue lead concentrations in 14 individual wildlife cases with embedded lead projectiles that were unrelated to the cause of death. Clinically significant liver lead concentrations were only found in two cases suggesting that embedded lead carries a relatively low risk for lead poisoning.
While the number of studies on this pathway remains small, collectively, they suggest that some of the lead from embedded gunshot may be mobilised, resulting in increased blood lead concentrations, with potential long-term effects. It currently appears that absorption of lead from embedded ammunition is likely to be modest compared with that following the ingestion of lead from ammunition, and that effects may be sub-lethal. Several authors have previously reported associations between embedded gunshot and reduced body condition or survival in birds (Madsen and Noer 1996 ; Tavecchia et al. 2001 ; Merkel et al. 2006 ), but it is unclear whether these effects were related to the injuries caused by shooting, sub-lethal effects of lead absorbed from embedded gunshot, a combination of these or some other explanation.
While effects from this pathway appear likely to be small in comparison to those from ingested lead, large numbers of birds could be affected because a substantial proportion of some populations of game birds survive being shot, carrying gunshot in their flesh (e.g. commonly 20–30% in some wildfowl populations—Pain et al. 2015 ). Should this pathway be further verified, then numbers of birds suffering welfare effects from sub-lethal poisoning would be far greater than previously supposed.
Impacts of lead poisoning on wildlife
Sub-lethal and welfare effects.
Most birds that ingest lead ammunition suffer some effects as a result of absorbing above background levels of lead. These may be sub-clinical or clinical and will affect the birds’ welfare to varying degrees. In recent years considerable effort has been put into investigating the sub-lethal effects of lead from ammunition on birds, both under experimental conditions and in the wild.
Using transmission electron microscopy, Pineau et al. ( 2017 ) found marked subcellular toxicity in the liver associated with the ingestion of a single lead shot (0.177 ± 0.03 g) in experimentally dosed mallards ( n = 21) compared with controls ( n = 10). Experimental studies involving the dosing of pheasants (Gasparik et al. 2012 ; Runia and Solem 2017 ) and northern bobwhites ( Colinus virginianus ) (Tannenbaum 2012 ) with lead gunshot suggest that these species appear less susceptible to the acute effects of lead poisoning than others, such as mourning doves ( Zenaida macroura ), chukars or waterfowl. While tissue (blood and/or liver) lead concentrations of pheasants and northern bobwhites increased, and some of the biological parameters measured were negatively affected (two studies), this was to a lesser degree than in other species. Other experimental work has shown the sensitivity of certain terrestrial species to sub-lethal effects of lead, sometimes at low exposure levels. Maternal consumption of one 95-mg lead pellet affected egg size and hatchling organ development in domesticated roller pigeons ( Columba livia ) (Williams et al. 2017 ). Vallverdú-Coll et al. ( 2015a ) reported impacts on immune response and other variables in red-legged partridges ( Alectoris rufa ) dosed with 1–3 lead gunshot.
Vallverdú-Coll et al. ( 2016a ) found that female red-legged partridges dosed with three lead pellets (330 mg) had reduced egg hatching rate and males had decreased acrosome integrity and sperm motility. In contrast, when exposed to the lower dose of 1 pellet (110 mg), females produced heavier eggs and chicks and males presented increased sperm vigour. Then authors suggested that at the low exposure levels lead-induced endocrine disruption could explain the production of heavier and larger eggs by exposed females, although this effect has rarely been studied in female birds. Espín et al. ( 2014 ) investigated blood lead concentrations that cause effects on oxidative stress biomarkers using blood taken from 66 griffon vultures ( Gyps fulvus ) in Spain, and found that levels > 15 µg/dl can result in oxidative stress, risking damage to cell components. These and previous experimental studies suggest inter-specific variation in susceptibility to lead poisoning. Inter- and intraspecific variation in lead toxicity relates to factors that influence absorption, retention, detoxification and elimination of lead. Among these are diet (considered a key variable influencing lead absorption), age, sex, physiological condition and environmental factors such as temperature (Pattee and Pain 2003 ). Disentangling intrinsic variation in susceptibility from the effects of experimental conditions is complex.
Several recent studies have reported sub-lethal effects of lead in wild birds, supplementing earlier research. A histopathological study of the eyes of a bald eagle provided the first evidence of ocular lesions associated with sub-lethal but extremely elevated blood lead levels (c. 610 µg/dl—Eid et al. 2016 ). The prognosis for this rehabilitated bird’s vision was too poor for it to be released back to the wild. Along with other effects, body condition was negatively associated with liver lead of hunter-killed wild ducks in Argentina (Ferreyra et al. 2015 ) and with blood lead levels of female common eider ( Somateria mollissima ) in Canada (Provencher et al. 2016 ). Vallverdú-Coll et al. ( 2016b ) found that in mallards from the Ebro delta (north-eastern Spain), lead exposure was associated with increased oxidative stress, affected colour expression, and impaired constitutive immunity in ways that differed between the sexes. Through analysing mineral chemistry and crystallinity, Álvarez-Lloret et al. ( 2014 ) found that lead contamination altered bone remodelling of red-legged partridges from a farmland area in Albacete, Spain, in a concentration-dependent way and that this occurred at low bone lead concentrations (< 4 ppm dw bone lead).
Several papers have reported sub-lethal effects at lower blood lead levels (PbB) than previously suggested (Table 1 ). This mirrors research into the effects of chronic low-level exposure in humans, with reference values for elevated PbB considered significant by the Centers for Disease Control and Prevention decreasing markedly over time (CDC https://www.cdc.gov/ ). Vallverdú-Coll et al. ( 2015b ) found that lead gunshot ingestion in mallards can result in maternal transfer of lead to offspring, affect their developing immune system and reduce early life stage survival. In mallard eggs from the Ebro delta (Spain) eggshell lead and duckling blood lead levels were positively correlated, and ducklings with blood levels > 18 µg/dl had reduced body mass and died during the first week post hatching. Newth et al. ( 2016 ) found elevated (> 20 µg/dl lead in blood) levels in 41.7% (125/300) of whooper swans ( Cygnus cygnus ). Blood lead content was significantly negatively associated with winter body condition when levels were ≥ 44 µg/dl (27/260 = 10.4%), indicating that sub-lethal impacts of lead on body condition occur at the lower end of previously recommended clinical thresholds and that a relatively high proportion of individuals in this population may be affected. Analysis of tracking data from 16 adult golden eagles trapped in northern Sweden indicated that sub-lethal blood lead concentrations reduced mean flight height and movement rate (Ecke et al. 2017 ). Blood lead levels of c.2.5 µg/dl appeared to reduce flight height by 10%, levels of 4.3 µg/dl by 20%, and in birds with the highest blood levels by 50%. These lead blood concentrations fall far below previously suggested thresholds (Table 1 ), but because of the small sample the authors suggest that further data should be collected. González et al. ( 2017 ) studied blood parameters and lead concentrations in griffon vultures submitted to Wildlife Rehabilitation Centres in Spain. 26% of birds had blood lead > 20 µg/dl. Blood lead was negatively correlated with haematocrit and digestive signs such as stasis and weight loss, though not with other clinical signs. The authors suggested that this species may be more sensitive to the toxic effects of lead than previously thought.
Deaths from lead poisoning
Few recent studies have added to the substantial body of information on deaths from lead poisoning in wildfowl with most new research on mortality covering the previously less well-studied groups of raptors and scavengers. Despite this disparity, a number of species of raptor and scavenger had previously been reported as dying of lead poisoning in Europe and North America (reviewed in Mateo 2009 ; Pain and Green 2015 ; Golden et al. 2016 ). As awareness of this poisoning in raptors has increased, so too has the number of research studies. Table 2 includes many recent examples of mortality from ingesting lead from ammunition (or likely from ammunition) in raptors and scavengers including Andean condor, cape vulture ( Gyps coprotheres ), griffon vulture, golden eagle, red kite ( Milvus milvus ), white-tailed eagle, bald eagle and Steller’s sea eagle and ranging geographically from Europe, the Middle East and Japan to Africa, North and South America. Many other species had already been reported as being poisoned in this way (e.g. see Pain et al. 2009 ).
Recent studies illustrate that some partial bans on the use of lead ammunition do little to reduce lead poisoning mortality in raptors and scavengers. In North America, lead poisoning continues to be a significant cause of mortality in bald and golden eagles, despite the ban in autumn 1991 on the use of lead ammunition for shooting waterfowl (Russell and Franson 2014 ; Warner et al. 2014 ; Yaw et al. 2017 ). This is largely because eagles consume lead bullet fragments in offal and carcasses left behind during big game hunting. In Japan, lead poisoning remains a problem for Steller’s sea eagles and white-tailed eagles after partial bans on the use of lead ammunition (first for shooting sika deer ( Cervus nippon ) and then all large game in Hokkaido in 2000, 2001 and 2004—Ishii et al. 2017 ). Bans on only certain types of lead ammunition (either gunshot or bullets) or only for the shooting of certain animals frequently have limited effect, and can be difficult to police (e.g. see Cromie et al. 2015 for UK example), although at one site in Spain good enforcement improved compliance (Mateo et al. 2014 ). In California, lead poisoning has been a major factor in both causing the extinction in the wild and then limiting the recovery of the reintroduced California condor population. After successive types of limited ban, a total ban—on the use of all lead ammunition for all hunting—will come into force in California from 2019 (Rendon Act 2013 ).
Numbers of birds affected
Sufficient data are available for approximate estimates to be made of annual numbers of deaths caused by lead poisoning in wildfowl. These are necessarily imprecise as many external factors affect lead-poisoning mortality from year to year, notably food availability and weather (Pattee and Pain 2003 ). Estimates follow the method of Bellrose ( 1959 ) and are based on proportions of birds found to have ingested different numbers of gunshot, turnover rates of gunshot in the intestine and mortality from experimental studies. Andreotti et al. ( 2018a ) estimated that about 700 000 individuals of 16 waterbird species die annually in the European Union (EU) (6.1% of the wintering population) and one million across Europe (7.0%) as a direct effect of lead poisoning, with three times more birds suffering sub-lethal effects. This is similar to the number previously estimated by Mateo ( 2009 ). Pain et al. ( 2015 ) estimated that in the UK in the order of 50 000–100 000 wildfowl (c. 1.5–3.0% of the wintering population) die each winter (i.e. during the shooting season) as a direct result of lead poisoning (Pain et al. 2015 ), with several hundred thousand birds suffering sub-lethal poisoning and welfare effects. Wildfowl that die from delayed effects outside of the shooting season will be additional, as will those that ingest gunshot outside of the shooting season or die of causes exacerbated by lead poisoning (e.g. infectious diseases, collisions).
Pain et al. ( 2015 ) considered estimates of mortality for terrestrial game birds in the UK to be less accurate and precise than those for wildfowl. However, it is suggested that some hundreds of thousands of terrestrial game birds may die from lead poisoning annually. These estimated numbers are mainly due to the large numbers of pheasants and other game birds bred and released to be killed for sport in the UK every year (about 50 million—DEFRA 2013 ; Larkman et al. 2015 ). Even if the proportion of birds with ingested shot at a given time is only a few percent, several million birds are likely to ingest gunshot in the course of a year in the UK. It is possible that some birds (e.g. pheasants) may be less sensitive to the effects of lead than other terrestrial birds or waterbirds (as described in previous sections), and this may affect estimates of the numbers dying because of lead poisoning.
Most birds that ingest lead from ammunition probably suffer some effect on their welfare. These effects are likely to be severe in birds that subsequently die of lead poisoning. As described earlier, some of the lead that is embedded in the flesh of birds that have been shot but survive may also be absorbed into the blood and result in elevated PbB, albeit to a far lesser degree than ingested lead gunshot. Should this be the case, numbers of birds suffering welfare effects may be higher than current estimates. In the EU an estimated 6 million waterfowl are shot annually (AMEC 2012 ). While crippling ratios (the number of birds injured to those killed) are variable (e.g. Clausen et al. 2017 ), numbers crippled and carrying embedded gunshot may be large and may number over a million waterfowl, along with many more terrestrial birds. However, there is currently insufficient evidence to evaluate either the severity of impacts or numbers of birds potentially affected by embedded gunshot with any certainty.
Insufficient information exists to estimate numbers of raptors impacted in Europe and elsewhere although many scavenging and predatory species are affected (Table 2 ; Mateo 2009 ; Golden et al. 2016 ), probably including some currently unstudied species. This is consistent with what would be predicted from the source of lead and pathway of exposure.
Effects on populations
In some countries, there has been considerable debate about effects of lead from ammunition on bird population size and trend. Absence of robust information on this topic is sometimes cited as a reason for political inaction (Truss 2016 ). Any additional mortality of wildlife has the potential to affect population size and trends to some extent, and is certain to do so in the absence of complete, or perfect, density dependence. If density dependence exists but is not complete, population size will be lower because of lead poisoning, but it will stabilise at this lower level if the strength of density dependence is sufficient. If density dependence is absent or weak, any additional mortality, including that caused by lead poisoning, will cause a previously stable population to decline markedly or go extinct. Only if there is perfect density dependence which operates on demographic rates within the annual cycle and after the time when lead poisoning occurs, would deaths caused by lead poisoning be completely compensated for by density-dependent enhancement of survival or breeding success. Only then would lead poisoning be expected to have no effect on population size. It is difficult to measure the strength and form of density dependence in wild populations, so it is rarely known with any precision how large the effect of additional mortality on population size will be. However, a given proportion of birds poisoned is likely to have the biggest impact on species that naturally have the lowest annual mortality and reproductive rates, such as eagles and vultures.
Perfect or complete density dependence has rarely been demonstrated and is thought to be rare in the bird species affected by lead poisoning. For example, exhaustive studies of the effects of hunting on populations of mallards in North America provide no indication that the additional mortality it causes are compensated for by density-dependent processes (Nichols et al. 2015 ). Therefore, it is reasonable and precautionary to take evidence of additional mortality as evidence of an effect on population size and trend. Cases of populations exposed to ammunition-derived lead in which density dependence is clearly too weak to compensate for deaths caused by lead poisoning include those of the California condor in California and in the Grand Canyon. Both of these populations would decline to extinction because of poisoning from ammunition-derived dietary lead were it not for the capture, diagnosis and veterinary treatment of affected animals (Green et al. 2008 ).
Another approach to the assessment of population-level effects of lead poisoning is to correlate variation in population growth rates observed among geographical regions or species with levels of exposure to lead. Green and Pain ( 2016 ) found that for the eight duck species that winter in freshwater habitats in the UK, inter-specific variation in mean annual population growth rate during the period 1990/1991 to 2013/2014 was significantly negatively correlated with two independent measures of the prevalence of ingested lead gunshot in the UK and Europe. This relationship was also found for annual growth rates in the period 1966/1967 to 2013/2014, derived from smoothed population trajectories and was insensitive to the choice of period over which the effect was investigated. Duck species with high prevalence of ingested lead were more likely to have undergone a long-term population decline. These findings support the hypothesis that ingested lead gunshot affects population trend. The authors expressed particular concern about the possible impact of ingested lead gunshot on the common pochard, a species now listed as globally threatened (Vulnerable) because of population declines (BirdLife International 2015 ). Previously, the prevalence of lead shot ingestion was found to be negatively correlated with population trends in 15 waterbird species across Europe, in the last decades of the twentieth century (Fig. 3 ).

Correlation between the prevalence of lead shot ingestion and the trend of the wintering population in Europe of 15 species of waterfowl. Modified from Mateo ( 2009 )
Conducting a modelling study of spectacled eider ( Somateria fischeri ), Flint et al. ( 2016 ) suggested that populations would respond most dramatically to changes in adult female survival and that reductions in adult female survival related to lead poisoning were locally important. As most mortality from lead exposure occurs over winter, the related reduction in adult survival may be impeding recovery of local populations.
Meyer et al. ( 2016 ) used population models to create example scenarios demonstrating how mortality from lead poisoning and other poisons might affect the populations of three susceptible species: grey partridge ( Perdix perdix ) in continental Europe, common buzzard in Germany and red kite in Wales. Lead gunshot ingestion and poisoning at modelled levels (4–16% for lead poisoning depending on species) affected populations by reducing population size and slowing population growth. Lead gunshot alone reduced the population size of grey partridges by 10%, and reduced annual growth rate of the red kite population from 6.5% to 4%, slowing recovery. Decrease in the common buzzard mean population size by lead gunshot and poisons combined was much smaller (≤ 1%). The effects are somewhat higher if ingestion of these substances additionally causes sub-lethal reproductive impairment (which we know that lead can do—as described above). While these results are subject to uncertainty, they suggest that declining or recovering populations are most sensitive to poisoning by lead from ammunition or other poisons. These example scenarios may not be replicable to other places where exposure levels differ but they do illustrate how poisoning can hypothetically affect population levels and growth rates.
Population-level effects of lead poisoning are also supported by previous research on lead fishing weights that were responsible for widespread lead poisoning in mute swans. Following a ban on the sale and use of most lead fishing weights in England and Wales in 1987 there was a sharp reduction in most areas in the numbers of mute swans dying or sick from lead poisoning. This has been considered crucial to the subsequent increase in the species’ population (Sears and Hunt 1991 ; Kirby et al. 1994 ; Perrins et al. 2003 ). In a long-term study (1982–2012) lead fishing weights have recently been shown to have a population-level effect on common loons ( Gavia immer ) in New Hampshire, USA where their ingestion was responsible for 48.6% of adult loon deaths. The authors modelled the loon population retrospectively and estimated that mortality caused by ingestion of lead tackle reduced the population growth rate by 1.4% and the state-wide population by 43% during the years of the study (Grade et al. 2018 ).
The known effects of lead upon physiology, behaviour and reproduction, the widespread mortality in birds known to occur as a result of exposure to lead from ammunition, and the recent studies described here provide compelling evidence that lead from ammunition can, and sometimes does, negatively affect population levels and trends, and not only in quarry species. For this reason, it is reasonable and precautionary to take evidence of additional mortality as a result of lead poisoning as evidence of population-level effects.
Conclusions
Recent research supports and supplements more than a century of work on lead poisoning from ammunition sources in birds. The results of this work show that lead poisoning of birds is likely to occur wherever lead ammunition is used and a pathway of exposure exists. Cases of lead poisoning in new species and countries simply reflect that these had not previously been studied. Taxa beyond birds are also affected; recent research highlights risks to mammalian scavengers and predators although this has not been extensively investigated. Trends in lead poisoning research in birds reflect those in human medicine, with effects detected at ever lower levels of lead exposure and absorption. Large numbers of wild birds suffer welfare effects and are killed by spent lead ammunition annually, and it affects populations. Alternative non-toxic ammunition exists and some has been in widespread use for decades. Removing this avoidable source of environmental contamination and suffering and mortality of wildlife is a matter of political will (e.g. Arnemo et al. 2016 ; Kanstrup et al. 2018 ).
Acknowledgements
The authors would like to thank Esther Descalzo for her help in a literature review that provided useful information for this paper.
Biographies
is an Honorary Research Fellow in the Department of Zoology, University of Cambridge and an Ambassador for the Wildfowl & Wetlands Trust. Her research interests include diagnosing the causes of declines in threatened bird species and developing and testing practical and policy solutions to reverse them. She has an interest in ecotoxicology.
is an Associate Professor at the University of Castilla-La Mancha (UCLM), and Researcher at the Institute for Game and Wildlife Research (IREC). His research interests include wildlife ecotoxicology and ecophysiology.
is an Honorary Professor of Conservation Science in the Department of Zoology at the University of Cambridge. His research interests include the effects of human activities on population size and demographic rates of wild species. He uses statistical and simulation models fitted to data on these effects to devise practical interventions that land managers can use to reduce negative effects on wild species so as to improve their conservation status.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deborah J. Pain, Phone: +44(0)7826 550522, Email: [email protected] .
Rafael Mateo, Email: [email protected] .
Rhys E. Green, Email: ku.ca.mac@92ger .
- Aloupi M, Kazantzidis S, Akriotis T, Bantikou E, Hatzidaki VO. Lesser white-fronted ( Anser erythropus ) and greater white-fronted ( A. albifrons ) geese wintering in Greek wetlands are not threatened by Pb through shot ingestion. Science of the Total Environment. 2015; 527 :279–286. doi: 10.1016/j.scitotenv.2015.04.083. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Álvarez-Lloret P, Rodríguez-Navarro AB, Romanek CS, Ferrandis P, Martínez-Haro M, Mateo R. Effects of lead shot ingestion on bone mineralization in a population of red-legged partridge ( Alectoris rufa ) Science of the Total Environment. 2014; 466 :34–39. doi: 10.1016/j.scitotenv.2013.06.103. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- AMEC Environment and Infrastructure UK Limited. 2012. European Chemicals Agency. Abatement Costs of Certain Hazardous Chemicals. Lead in shot: Final Report December 2012. Report for European Chemicals Agency (ECHA). Contract No: ECHA 2011/140, Annankatu 18, 00121 Helsinki, Finland.
- Andreotti A, Borghesi F, Aradis A. Lead ammunition residues in the meat of hunted woodcock: A potential health risk to consumers. Italian Journal of Animal Science. 2016; 15 :22–29. doi: 10.1080/1828051X.2016.1142360. [ CrossRef ] [ Google Scholar ]
- Andreotti A, Fabbri I, Menotta S, Borghesi F, Menotta S. Lead gunshot ingestion by a Peregrine Falcon. Ardeola-International Journal of Ornithology. 2018; 65 :53–58. [ Google Scholar ]
- Andreotti A, Guberti V, Nardelli R, Pirrello S, Serra L, Volponi S, Green RE. Economic assessment of wild bird mortality induced by the use of lead gunshot in European wetlands. Science of the Total Environment. 2018; 610 :1505–1513. doi: 10.1016/j.scitotenv.2017.06.085. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arnemo JM, Andersen O, Stokke S, Thomas VG, Krone O, Pain DJ, Mateo R. Health and environmental risks from lead-based ammunition: Science versus socio-politics. EcoHealth. 2016; 13 :618–622. doi: 10.1007/s10393-016-1177-x. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- ATSDR. 2007. Toxicological profile for lead. Agency for Toxic Substances and Disease Registry, Atlanta Georgia USA. https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf . [ PubMed ]
- Behmke S, Fallon J, Duerr AE, Lehner A, Buchweitz J, Katzner T. Chronic lead exposure is epidemic in obligate scavenger populations in eastern North America. Environment International. 2015; 79 :51–55. doi: 10.1016/j.envint.2015.03.010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bellrose FC. Lead poisoning as a mortality factor in waterfowl populations. Illinois Natural History Survey Bulletin. 1959; 27 :235–288. [ Google Scholar ]
- Berny PJ, Mas E, Vey D. Embedded lead shots in birds of prey: The hidden threat. European Journal of Wildlife Research. 2017; 63 :101. doi: 10.1007/s10344-017-1160-z. [ CrossRef ] [ Google Scholar ]
- Berny P, Vilagines L, Cugnasse JM, Mastain O, Chollet JY, Joncour G, Razin M. Vigilance poison: Illegal poisoning and lead intoxication are the main factors affecting avian scavenger survival in the Pyrenees (France) Ecotoxicology and Environmental Safety. 2015; 118 :71–82. doi: 10.1016/j.ecoenv.2015.04.003. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bingham RJ, Larsen RT, Bissonette JA, Hall JO. Widespread ingestion of lead pellets by wild chukars in northwestern Utah. Wildlife Society Bulletin. 2015; 39 :94–102. doi: 10.1002/wsb.527. [ CrossRef ] [ Google Scholar ]
- Binkowski ŁJ, Meissner W, Trzeciak M, Izevbekhai K, Barker J. Lead isotope ratio measurements as indicators for the source of lead poisoning in mute swans ( Cygnus olor ) wintering in Puck Bay (northern Poland) Chemosphere. 2016; 164 :436–442. doi: 10.1016/j.chemosphere.2016.08.120. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- BirdLife International. 2015. Aythya ferina . The IUCN Red List of Threatened Species 2015: e.T22680358A59966548. Retrieved 04 October, 2018, from https://www.iucnredlist.org/species/22680358/59966548 .
- BirdLife International. 2018. Species factsheet: Clanga clanga . Retrieved 09 October, 2018, from http://datazone.birdlife.org/index.php/species/factsheet/greater-spotted-eagle-clanga-clanga/text .
- Calvert HS. Pheasants poisoned by swallowing shot. The Field. 1876; 47 :189. [ Google Scholar ]
- Carneiro MA, Oliveira PA, Brandão R, Francisco ON, Velarde R, Lavín S, Colaço B. Lead poisoning due to lead-pellet ingestion in griffon vultures ( Gyps fulvus ) from the Iberian Peninsula. Journal of Avian Medicine and Surgery. 2016; 30 :274–279. doi: 10.1647/2014-051. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carneiro M, Colaço B, Brandão R, Azorín B, Nicolas O, Colaço J, Pires MJ, Agustí S, et al. Assessment of the exposure to heavy metals in Griffon vultures ( Gyps fulvus ) from the Iberian Peninsula. Ecotoxicology and Environmental Safety. 2015; 113 :295–301. doi: 10.1016/j.ecoenv.2014.12.016. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carneiro M, Colaço B, Brandão R, Ferreira C, Santos N, Soeiro V, Colaço A, Pires MJ, et al. Biomonitoring of heavy metals (Cd, Hg, and Pb) and metalloid (As) with the Portuguese common buzzard ( Buteo buteo ) Environmental Monitoring and Assessment. 2014; 186 :7011–7021. doi: 10.1007/s10661-014-3906-3. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Clausen KK, Holm TE, Haugaard L, Madsen J. Crippling ratio: A novel approach to assess hunting-induced wounding of wild animals. Ecological Indicators. 2017; 80 :242–246. doi: 10.1016/j.ecolind.2017.05.044. [ CrossRef ] [ Google Scholar ]
- Cromie, R., J. Newth, J. Reeves, M. O’Brien, K. Beckmann, and M. Brown. 2015. The sociological and political aspects of reducing lead poisoning from ammunition in the UK: why the apparently simple solution is so difficult. In Proceedings of the Oxford Lead Symposium. Lead ammunition: understanding and minimising the risks to human and environmental health , eds. R.J. Delahay, and C.J. Spray, pp. 104–124. Edward Grey Institute, University of Oxford.
- Cruz-Martinez L, Grund MD, Redig PT. Quantitative assessment of bullet fragments in viscera of sheep carcasses as surrogates for white-tailed deer. Human-Wildlife Interactions. 2015; 9 :211–218. [ Google Scholar ]
- Delahay R.J., and C.J. Spray (Eds.) 2015. Proceedings of the Oxford Lead Symposium: Lead Ammunition: Understanding and Minimizing the Risks to Human and Environmental Health , 152 pp. Oxford University, Edward Grey Institute.
- DEFRA. 2013. Great Britain poultry register statistics: 2013. Department for Environment, Food and Rural Affairs. Retrieved March, 2018, from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/314715/pub-avian-gbpr13.pdf (Accessed March 2018).
- Descalzo, E., and R. Mateo. 2018. La contaminación por munición de plomo en Europa: el plumbismo aviary las implicaciones en la seguridad de la carne de caza. Instituto de Investigación en Recursos Cinegéticos (IREC), Ciudad Real, España. 82 pp. http://www.irec.es/wp-content/uploads/2018/12/Descalzo-y-Mateo-2018-Revision-Plomo-Europa.pdf .
- ECHA. 2017. European Chemicals Agency Annex XV restriction report—lead in shot, 96 pp. https://echa.europa.eu/documents/10162/6ef877d5-94b7-a8f8-1c49-8c07c894fff7 .
- Ecke F, Singh NJ, Arnemo JM, Bignert A, Helander B, Berglund ÅMM, Borg H, Bröjer C, et al. Sublethal lead exposure alters movement behavior in free-ranging golden eagles. Environmental Science and Technology. 2017; 51 :5729–5736. doi: 10.1021/acs.est.6b06024. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- EFSA. 2010. Scientific opinion on lead in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA Journal . 8(4): 1570. 10.2903/j.efsa.2010.1570. Retrieved March, 2018, from: http://www.efsa.europa.eu/en/efsajournal/pub/1570 .
- Eid R, Guzman DSM, Keller KA, Wiggans KT, Murphy CJ, LaDouceur EEB, Keel MK, Reilly CM. Choroidal vasculopathy and retinal detachment in a bald eagle ( Haliaeetus leucocephalus ) with lead toxicosis. Journal of Avian Medicine and Surgery. 2016; 30 :357–363. doi: 10.1647/2015122. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eisler, R. 1988. Lead hazards to fish, wildlife, and invertebrates: a synoptic review. Contaminant Hazard Reviews. U.S. Fish and Wildlife Service Biological Report, 85 (1.14).
- Ellam RM. The graphical presentation of lead isotope data for environmental source apportionment. Science of the Total Environment. 2010; 408 :3490–3492. doi: 10.1016/j.scitotenv.2010.03.037. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ertl K, Kitzer R, Goessler W. Elemental composition of game meat from Austria. Food Additives and Contaminants: Part B. 2016; 9 :120–126. doi: 10.1080/19393210.2016.1151464. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Espín S, Martínez-López E, Jiménez P, María-Mojica P, García-Fernández AJ. Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture ( Gyps fulvus ) Environmental Research. 2014; 129 :59–68. doi: 10.1016/j.envres.2013.11.008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ferreyra H, Beldomenico PM, Marchese K, Romano M, Caselli A, Correa AI, Uhart M. Lead exposure affects health indices in free-ranging ducks in Argentina. Ecotoxicology. 2015; 24 :735–745. doi: 10.1007/s10646-015-1419-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ferreyra H, Romano M, Beldomenico P, Caselli A, Correa A, Uhart M. Lead gunshot pellet ingestion and tissue lead levels in wild ducks from Argentine hunting hotspots. Ecotoxicology and Environmental Safety. 2014; 103 :74–81. doi: 10.1016/j.ecoenv.2013.10.015. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Finkelstein ME, Brandt J, Sandhaus E, Grantham J, Mee A, Schuppert PJ, Smith DR. Lead exposure risk from trash ingestion by the endangered California condor ( Gymnogyps californianus ) Journal of Wildlife Diseases. 2015; 51 :901–906. doi: 10.7589/2014-10-253. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Finkelstein ME, Kuspa ZE, Welch A, Eng C, Clark M, Burnett J, Smith DR. Linking cases of illegal shootings of the endangered California condor using stable lead isotope analysis. Environmental Research. 2014; 134 :270–279. doi: 10.1016/j.envres.2014.07.022. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Flint PL, Grand JB, Petersen MR, Rockwell RF. Effects of lead exposure, environmental conditions, and metapopulation processes on population dynamics of spectacled eiders. North American Fauna. 2016; 81 :1–41. doi: 10.3996/nafa.81.0001. [ CrossRef ] [ Google Scholar ]
- Franson JC, Pain DJ. Lead in birds. In: Beyer WN, Meador JP, editors. Environmental contaminants in biota: Interpreting tissue concentrations. Boca Raton: Taylor and Francis Group; 2011. pp. 563–593. [ Google Scholar ]
- Friend, M., and C.J. Franson. (Eds). 1999. Field Manual of Wildlife Diseases. U.S. Geological Survey, Biological Resources Division, ITR 1999-001 National Wildlife Health Center, 6006 Schroeder Rd., Madison, WI 53711 https://www.nwhc.usgs.gov/publications/field_manual/field_manual_of_wildlife_diseases.pdf .
- Ganz K, Jenni L, Madry MM, Kraemer T, Jenny H, Jenny D. Acute and chronic lead exposure in four avian scavenger species in Switzerland. Archives of Environmental Contamination and Toxicology. 2018; 75 :566–575. doi: 10.1007/s00244-018-0561-7. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garbett, R., G. Maude, P. Hancock, D. Kenny, D. Reading, and A. Amar. 2018. Association between hunting and elevated blood lead levels in the critically endangered African white-backed vulture Gyps africanus. Science of the Total Environment 630: 1654–1665. https://www.sciencedirect.com/science/article/pii/S0048969718306193?via%3Dihub . [ PubMed ]
- Gasparik J, Venglarcik J, Slamecka J, Kropil R, Smehyl P, Kopecky J. Distribution of lead in selected organs and its effect on reproduction parameters of pheasants ( Phasianus colchicus ) after an experimental per oral administration. Journal of Environmental Science and Health: Part A. 2012; 47 :1267–1271. doi: 10.1080/10934529.2012.672127. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gil-Sánchez JM, Molleda S, Sánchez-Zapata JA, Bautista J, Navas I, Godinho R, García-Fernández AJ, Moleón M. From sport hunting to breeding success: Patterns of lead ammunition ingestion and its effects on an endangered raptor. Science of the Total Environment. 2018; 613 :483–491. doi: 10.1016/j.scitotenv.2017.09.069. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Golden, N.H., S.E. Warner, and M.J. Coffey. 2016. A review and assessment of spent lead ammunition and its exposure and effects to scavenging birds in the United States. In Reviews of Environmental Contamination and Toxicology , eds. W. de Voogt, 237: 123–191. 10.1007/978-3-319-23573-8_6. [ PubMed ]
- González F, López I, Suarez L, Moraleda V, Rodríguez C. Levels of blood lead in griffon vultures from a Wildlife Rehabilitation Center in Spain. Ecotoxicology and Environmental Safety. 2017; 143 :143–150. doi: 10.1016/j.ecoenv.2017.05.010. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grade TJ, Pokras MA, Laflamme EM, Vogel HS. Population-level effects of lead fishing tackle on common loons. Journal of Wildlife Management. 2018; 82 :155–164. doi: 10.1002/jwmg.21348. [ CrossRef ] [ Google Scholar ]
- Green RE, Hunt WG, Parish CN, Newton I. Effectiveness of action to reduce exposure of free-ranging California condors in Arizona and Utah to lead from spent ammunition. PLoS ONE. 2008; 3 :e4022. doi: 10.1371/journal.pone.0004022. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Green RE, Pain DJ. Possible effects of ingested lead gunshot on populations of ducks wintering in the UK. Ibis. 2016; 158 :699–710. doi: 10.1111/ibi.12400. [ CrossRef ] [ Google Scholar ]
- Group of Scientists. 2013. Health risks from lead-based ammunition in the environment: A consensus statement of scientists. Retrieved March, 2018, from: https://escholarship.org/uc/item/6dq3h64x .
- Group of Scientists. 2014. Wildlife and human health risks from lead-based ammunition in Europe: a consensus statement by scientists. Retrieved March, 2018, from: http://www.zoo.cam.ac.uk/leadammuntionstatement/ (Accessed March 2018).
- Herring G, Eagles-Smith CA, Wagner MT. Ground squirrel shooting and potential lead exposure in breeding avian scavengers. PLoS ONE. 2016; 11 :e0167926. doi: 10.1371/journal.pone.0167926. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hivert LG, Clarke JR, Peck SJ, Lawrence C, Brown WE, Huxtable S, Schaap D, Pemberton D, et al. High blood lead concentrations in captive Tasmanian devils ( Sarcophilus harrisii ): A threat to the conservation of the species? Australian Veterinary Journal. 2018; 96 :442–449. doi: 10.1111/avj.12753. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Horowitz IH, Yanco E, Nadler RV, Anglister N, Landau S, Elias R, Lublin A, Perl S, et al. Acute lead poisoning in a griffon vulture ( Gyps fulvus ) in Israel. Israel Journal of Veterinary Medicine. 2014; 69 :163–168. [ Google Scholar ]
- Ishii C, Nakayama SMM, Ikenaka Y, Nakata H, Saito K, Watanabe Y, Mizukawa H, Tanabe S, et al. Lead exposure in raptors from Japan and source identification using Pb stable isotope ratios. Chemosphere. 2017; 186 :367–373. doi: 10.1016/j.chemosphere.2017.07.143. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Isomursu M, Koivusaari J, Stjernberg T, Hirvelä-Koski V, Venäläinen ER. Lead poisoning and other human-related factors cause significant mortality in white-tailed eagles. Ambio. 2018; 47 :858–868. doi: 10.1007/s13280-018-1052-9. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jenni L, Madry MM, Kraemer T, Kupper J, Naegeli H, Jenny H, Jenny D. The frequency distribution of lead concentration in feathers, blood, bone, kidney and liver of golden eagles Aquila chrysaetos : Insights into the modes of uptake. Journal of Ornithology. 2015; 156 :1095–1103. doi: 10.1007/s10336-015-1220-7. [ CrossRef ] [ Google Scholar ]
- Kalisinska E, Lanocha-Arendarczyk N, Kosik-Bogacka D, Budis H, Podlasinska J, Popiolek M, Pirog A, Jedrzejewska E. Brains of native and alien mesocarnivores in biomonitoring of toxic metals in Europe. PLoS ONE. 2016; 11 :e0159935. doi: 10.1371/journal.pone.0159935. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kanstrup N, Swift J, Stroud DA, Lewis M. Hunting with lead ammunition is not sustainable: European perspectives. Ambio. 2018; 47 :846–857. doi: 10.1007/s13280-018-1042-y. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kelly A, Kelly S. Are mute swans with elevated blood lead levels more likely to collide with overhead power lines? Waterbirds. 2005; 28 :331–334. doi: 10.1675/1524-4695(2005)028[0331:AMSWEB]2.0.CO;2. [ CrossRef ] [ Google Scholar ]
- Kirby J, Delany S, Quinn J. Mute swans in Great Britain: A review, current status and long-term trends. Hydrobiologia. 1994; 279 :467–482. doi: 10.1007/BF00027878. [ CrossRef ] [ Google Scholar ]
- Kitowski I, Sujak A, Wiącek D, Strobel W, Komosa A, Stobiński M. Heavy metals in livers of raptors from Eastern Poland: The importance of diet composition. Belgian Journal of Zoology. 2016; 146 :3–13. [ Google Scholar ]
- Kitowski I, Jakubas D, Wiącek D, Sujak A. Concentrations of lead and other elements in the liver of the white-tailed eagle ( Haliaeetus albicilla ), a European flagship species, wintering in Eastern Poland. Ambio. 2017; 46 :825–841. doi: 10.1007/s13280-017-0929-3. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kitowski I, Wiacek D, Sujak A, Komosa A, Świetlicki M. Factors affecting trace element accumulation in livers of avian species from East Poland. Turkish Journal of Zoology. 2017; 41 :901–913. doi: 10.3906/zoo-1606-43. [ CrossRef ] [ Google Scholar ]
- Kitowski I, Sujak A, Wiącek D, Komosa A. Ecological factors helping to avoid the toxic element accumulation in livers of the lesser spotted eagle ( Clanga pomarina Brehm) from Eastern Poland. Journal of Elementology. 2017; 22 :305–314. [ Google Scholar ]
- Koeppel KN, Kemp LV. Lead toxicosis in a southern ground hornbill Bucorvus leadbeateri in South Africa. Journal of Avian Medicine and Surgery. 2015; 29 :340–344. doi: 10.1647/2014-037. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- LaDouceur EE, Kagan R, Scanlan M, Viner T. Chronically embedded lead projectiles in wildlife: A case series investigating the potential for lead toxicosis. Journal of Zoo and Wildlife Medicine. 2015; 46 :438–442. doi: 10.1638/2015-0026R.1. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- LAG. 2015. Lead Ammunition, Wildlife and Human Health. A report prepared for the Department for Environment, Food and Rural Affairs and the Food Standards Agency in the United Kingdom. Available at: http://www.leadammunitiongroup.org.uk/reports/ and Appendices http://www.leadammunitiongroup.org.uk/wp-content/uploads/2015/06/LAG-Report-June-2015-Appendices-without-Appendix-6.pdf .
- Lambertucci SA, Donázar JA, Huertas AD, Jiménez B, Sáez M, Sanchez-Zapata JA, Hiraldo F. Widening the problem of lead poisoning to a South-American top scavenger: Lead concentrations in feathers of wild Andean condors. Biological Conservation. 2011; 144 :1464–1471. doi: 10.1016/j.biocon.2011.01.015. [ CrossRef ] [ Google Scholar ]
- Langner HW, Domenech R, Slabe VA, Sullivan SP. Lead and mercury in fall migrant golden eagles from western North America. Archives of Environmental Contamination and Toxicology. 2015; 69 :54–61. doi: 10.1007/s00244-015-0139-6. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Larkman, A., I. Newton, R.E. Feber, and D. Macdonald. 2015. Small farmland bird declines, and changes in seed sources. In Wildlife Conservation on Farmland , eds. D.W. Macdonald, and R.E. Feber, pp. 181–202. Oxford University Press.
- Legagneux P, Suffice P, Messier JS, Lelievre F, Tremblay JA, Maisonneuve C, Saint-Louis R, Bety J. High risk of lead contamination for scavengers in an area with high moose hunting success. PLoS ONE. 2014; 9 :e111546. doi: 10.1371/journal.pone.0111546. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lindblom RA, Reichart LM, Mandernack BA, Solensky M, Schoenebeck CW, Redig PT. Influence of snowfall on blood lead levels of free-flying bald eagles ( Haliaeetus leucocephalus ) in the Upper Mississippi River Valley. Journal of Wildlife Diseases. 2017; 53 :816–823. doi: 10.7589/2017-02-027. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Madry MM, Kraemer T, Kupper J, Naegeli H, Jenny H, Jenni L, Jenny D. Excessive lead burden among golden eagles in the Swiss Alps. Environmental Research Letters. 2015; 10 :034003. doi: 10.1088/1748-9326/10/3/034003. [ CrossRef ] [ Google Scholar ]
- Madsen J, Noer H. Decreased survival of pink-footed geese Anser brachyrhynchus carrying shotgun pellets. Wildlife Biology. 1996; 2 :75–82. doi: 10.2981/wlb.1996.035. [ CrossRef ] [ Google Scholar ]
- Mariussen E, Heier LS, Teienb HC, Pettersen MN, Holth TF, Salbub B, Rosselande BO. Accumulation of lead (Pb) in brown trout ( Salmo trutta ) from a lake downstream a former shooting range. Ecotoxicology and Environmental Safety. 2017; 135 :327–336. doi: 10.1016/j.ecoenv.2016.10.008. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Martin PA, Hughes KD, Campbell GD, Shutt JL. Metals and organohalogen contaminants in Bald Eagles ( Haliaeetus leucocephalus ) from Ontario, 1991–2008. Archives of Environmental Contamination and Toxicology. 2018; 74 :305–317. doi: 10.1007/s00244-017-0479-5. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mateo R. Lead poisoning in wild birds in Europe and the regulations adopted by different countries. In: Watson RT, Fuller M, Pokras M, Hunt WG, editors. Ingestion of lead from spent ammunition: Implications for wildlife and humans. Boise: The Peregrine Fund; 2009. pp. 71–98. [ Google Scholar ]
- Mateo R, Cadenas R, Máñez M, Guitart R. Lead shot ingestion in two raptor species from Doñana, Spain. Ecotoxicology and Environmental Safety. 2001; 48 :6–10. doi: 10.1006/eesa.2000.1996. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mateo R, Vallverdú Coll N, Ortiz Santaliestra ME. Intoxicación por munición de plomo en aves silvestres en España y medidas para reducir el riesgo. Ecosistemas. 2013; 22 :61–67. doi: 10.7818/ECOS.2013.22-2.10. [ CrossRef ] [ Google Scholar ]
- Mateo R, Petkov N, Lopez-Antia A, Rodríguez-Estival J, Green AJ. Risk assessment of lead poisoning and pesticide exposure in the declining population of red-breasted goose ( Branta ruficollis ) wintering in Eastern Europe. Environmental Research. 2016; 151 :359–367. doi: 10.1016/j.envres.2016.07.017. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mateo R, Vallverdú-Coll N, López-Antia A, Taggart MA, Martínez-Haro M, Guitart R, Ortiz-Santaliestra ME. Reducing Pb poisoning in birds and Pb exposure in game meat consumers: The dual benefit of effective Pb shot regulation. Environment International. 2014; 63 :163–168. doi: 10.1016/j.envint.2013.11.006. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mateo-Tomás P, Olea PP, Jiménez-Moreno M, Camarero PR, Sánchez-Barbudo IS, Rodríguez Martin-Doimeadios RC, Mateo R. Mapping the spatio-temporal risk of lead exposure in apex species for more effective mitigation. Proceedings of the Royal Society B: Biological Sciences. 2016; 283 :20160662. doi: 10.1098/rspb.2016.0662. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Merkel FR, Falk K, Jamieson SE. Effect of embedded lead shot on body condition of common eiders. Journal of Wildlife Management. 2006; 70 :1644–1649. doi: 10.2193/0022-541X(2006)70[1644:EOELSO]2.0.CO;2. [ CrossRef ] [ Google Scholar ]
- Meyer CB, Meyer JS, Francisco AB, Holder J, Verdonck F. Can ingestion of lead shot and poisons change population trends of three European birds: Grey partridge, common buzzard, and red kite? PLoS ONE. 2016; 11 :e0147189. doi: 10.1371/journal.pone.0147189. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Molenaar FM, Jaffe JE, Carter I, Barnett EA, Shore RF, Rowcliffe JM, Sainsbury AW. Poisoning of reintroduced red kites ( Milvus milvus ) in England. European Journal of Wildlife Research. 2017; 63 :94. doi: 10.1007/s10344-017-1152-z. [ CrossRef ] [ Google Scholar ]
- Nadjafzadeh M, Hofer H, Krone O. Lead exposure and food processing in white-tailed eagles and other scavengers: An experimental approach to simulate lead uptake at shot mammalian carcasses. European Journal of Wildlife Research. 2015; 61 :763–774. doi: 10.1007/s10344-015-0953-1. [ CrossRef ] [ Google Scholar ]
- Naidoo V, Wolter K, Botha CJ. Lead ingestion as a potential contributing factor to the decline in vulture populations in southern Africa. Environmental Research. 2017; 152 :150–156. doi: 10.1016/j.envres.2016.10.013. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Newth JL, Rees EC, Cromie RL, McDonald RA, Bearhop S, Pain DJ, Norton GJ, Deacon C, et al. Widespread exposure to lead affects the body condition of free-living whooper swans Cygnus cygnus wintering in Britain. Environmental Pollution. 2016; 209 :60–67. doi: 10.1016/j.envpol.2015.11.007. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nichols JD, Johnson FA, Williams BK, Boomer GS. On formally integrating science and policy: Walking the walk. Journal of Applied Ecology. 2015; 52 :539–543. doi: 10.1111/1365-2664.12406. [ CrossRef ] [ Google Scholar ]
- North MA, Lane EP, Marnewick K, Caldwell P, Carlisle G, Hoffman LC. Suspected lead poisoning in two captive cheetahs ( Acinonyx jubatus jubatus ) in South Africa, in 2008 and 2013. Journal of the South African Veterinary Association. 2015; 86 :1286. doi: 10.4102/jsava.v86i1.1286. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- O’Donoghue, B. 2017. R.A.P.T.O.R. Recording and Addressing Persecution and Threats to Our Raptors. National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht. https://www.npws.ie/sites/default/files/publications/pdf/2017-raptor-report.pdf .
- O’Halloran J, Myers AA, Duggan PF. Lead poisoning in swans and sources of contamination in Ireland. Journal of Zoology. 1988; 216 :211–223. doi: 10.1111/j.1469-7998.1988.tb02426.x. [ CrossRef ] [ Google Scholar ]
- Pain, D. J. (ed.). 1992. Lead Poisoning in Waterfowl. Proc. IWRB Workshop, Brussels, Belgium 1991. IWRB Spec. Publ. 16, Slimbridge UK.
- Pain, D.J., J.J. Fisher, and V.G. Thomas. 2009. A global update of lead poisoning in terrestrial birds from ammunition sources. In Ingestion of Lead from Spent Ammunition: Implications for Wildlife and Humans , eds. R.T. Watson, M. Fuller, M. Pokras, W.G. Hunt, pp. 289–301. Boise: The Peregrine Fund
- Pain, D.J., and R.E. Green. 2015. An evaluation of the risks to wildlife in the UK from lead derived from ammunition. A report to the UK Government, 120 p. http://www.leadammunitiongroup.org.uk/wp-content/uploads/2015/06/LAG-Report-June2015-Appendices-without-Appendix-6.pdf
- Pain, D. J., R. Cromie, and R.E. Green. 2015. Poisoning of birds and other wildlife from ammunition-derived lead in the UK. In Proceedings of the Oxford Lead Symposium. Lead ammunition: understanding and minimising the risks to human and environmental health, eds., R.J. Delahay, and C.J. Spray, pp. 58–84. Edward Grey Institute, University of Oxford.
- Pattee, O., and D. Pain. 2003. Lead in the environment. In Handbook of ecotoxicology , eds. D.J. Hoffman, B.A. Rattner, G.A. Burton Jr., and J. Cairns Jr, Second ed., pp. 373–408. Boca Raton, Florida, USA: CRC Press.
- Perrins CM, Cousquer G, Waine J. A survey of blood lead levels in mute swans Cygnus olor . Avian Pathology. 2003; 32 :205–212. doi: 10.1080/0307946021000071597. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pineau A, Fauconneau B, Plouzeau E, Fernandez B, Quellard N, Levillain P, Guillard O. Ultrastructural study of liver and lead tissue concentrations in young mallard ducks ( Anas platyrhynchos ) after ingestion of single lead shot. Journal of Toxicology and Environmental Health-Part A-Current Issues. 2017; 80 :188–195. doi: 10.1080/15287394.2017.1279093. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Provencher JF, Forbes MR, Hennin HL, Love OP, Braune BM, Mallory ML, Gilchrist HG. Implications of mercury and lead concentrations on breeding physiology and phenology in an Arctic bird. Environmental Pollution. 2016; 218 :1014–1022. doi: 10.1016/j.envpol.2016.08.052. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rendon Act. 2013. Assembly Bill No. 711. CHAPTER 742 An act to amend Section 3004.5 of the Fish and Game Code, relating to hunting. https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201320140AB711 .
- Rodríguez-Seijo A, Cachada A, Gavina A, Duarte AC, Vega FA, Andrade ML, Pereira R. Lead and PAHs contamination of an old shooting range: A case study with a holistic approach. Science of the Total Environment. 2017; 575 :367–377. doi: 10.1016/j.scitotenv.2016.10.018. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Runia TJ, Solem AJ. Spent lead shot availability and ingestion by ring-necked pheasants in South Dakota. Wildlife Society Bulletin. 2016; 40 :477–486. doi: 10.1002/wsb.681. [ CrossRef ] [ Google Scholar ]
- Runia TJ, Solem AJ. Pheasant response to lead ingestion. The Prairie Naturalist. 2017; 49 :13–18. [ Google Scholar ]
- Russell RE, Franson JC. Causes of mortality in eagles submitted to the National Wildlife Health Center 1975–2013. Wildlife Society Bulletin. 2014; 38 :697–704. doi: 10.1002/wsb.469. [ CrossRef ] [ Google Scholar ]
- Sinkakarimi MH, Pourkhabbaz AR, Hassanpour M, Levengood JM. Study on metal concentrations in tissues of Mallard and Pochard from two major wintering sites in Southeastern Caspian Sea, Iran. Bulletin of Environmental Contamination and Toxicology. 2015; 95 :292–297. doi: 10.1007/s00128-015-1591-8. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sinkakarimi MH, Binkowski LJ, Hassanpour M, Ghasem R, Ahmadpour M, Levengood J. Metal concentrations in tissues of Gadwall and common teal from Miankaleh and Gomishan international wetlands, Iran. Biological Trace Element Research. 2018; 185 :177–184. doi: 10.1007/s12011-017-1237-2. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sears J, Hunt A. Lead poisoning in mute swans, Cygnus olor , in England. Wildfowl Supplement. 1991; 1 :383–388. [ Google Scholar ]
- Stroud, D.A. 2015. Regulation of some sources of lead poisoning: a brief review. pp 8-26. In Proceedings of the Oxford Lead Symposium: Lead Ammunition: Understanding and Minimizing the Risks to Human and Environmental Health, eds., R.J. Delahay, and C.J. Spray, 152 pp. Oxford University: Edward Grey Institute.
- Tannenbaum L. Evidence of high tolerance to ecologically relevant lead shot pellet exposures by an upland bird. Human and Ecological Risk Assessment. 2012; 20 :479–496. doi: 10.1080/10807039.2012.746143. [ CrossRef ] [ Google Scholar ]
- Tavecchia G, Pradel R, Lebreton JD, Johnson AR, Mondain-Monval JY. The effect of lead exposure on survival of adult mallards in the Camargue, southern France. Journal of Applied Ecology. 2001; 38 :1197–1207. doi: 10.1046/j.0021-8901.2001.00684.x. [ CrossRef ] [ Google Scholar ]
- Truss, E. 2016. Letter from Elizabeth Truss to John Swift. Retrieved 12 January, 2019, from: http://www.leadammunitiongroup.org.uk/wp-content/uploads/2016/07/Letter-from-Elizabeth-Truss-to-John-Swift-13-July-2016.pdf .
- Vallverdú-Coll N, Ortiz-Santaliestra ME, Mougeot F, Vidal D, Mateo R. Sublethal Pb exposure produces season-dependent effects on immune response, oxidative balance and investment in carotenoid-based coloration in red-legged partridges. Environmental Science and Technology. 2015; 49 :3839–3850. doi: 10.1021/es505148d. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vallverdú-Coll N, López-Antia A, Martinez-Haro M, Ortiz-Santaliestra ME, Mateo R. Altered immune response in mallard ducklings exposed to lead through maternal transfer in the wild. Environmental Pollution. 2015; 205 :350–356. doi: 10.1016/j.envpol.2015.06.014. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vallverdú-Coll N, Mougeot F, Ortiz-Santaliestra ME, Castaño C, Santiago-Moreno J, Mateo R. Effects of lead exposure on sperm quality and reproductive success in an avian model. Environmental Science and Technology. 2016; 50 :12484–12492. doi: 10.1021/acs.est.6b04231. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vallverdú-Coll N, Mougeot F, Ortiz-Santaliestra ME, Rodriguez-Estival J, López-Antia A, Mateo R. Lead exposure reduces carotenoid-based coloration and constitutive immunity in wild mallards. Environmental Toxicology and Chemistry. 2016; 35 :1516–1525. doi: 10.1002/etc.3301. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Warner SE, Britton EE, Becker DN, Coffey MJ. Bald eagle lead exposure in the Upper Midwest. Journal of Fish and Wildlife Management. 2014; 5 :208–216. doi: 10.3996/032013-JFWM-029. [ CrossRef ] [ Google Scholar ]
- Watson, R.T., M. Fuller, M. Pokras, and W. Hunt. (eds). 2009. Proceedings of the conference ingestion of lead from spent ammunition: implications for wildlife and humans. The Peregrine Fund, Boise, ID, USA.
- Weiss, D., C.D. Tomasallo, G. Meiman, W. Alarcon, N.M. Grabe, K.M. Bisgard, and H.A. Anderson. 2017. Elevated blood lead levels associated with retained bullet fragments: United States, 2003–2012 MMWR - Morbidity and Mortality Weekly Report 66: 130–133. [ PMC free article ] [ PubMed ]
- West CJ, Wolfe JD, Wiegardt A, Williams-Claussen T. Feasibility of California condor recovery in northern California, USA: Contaminants in surrogate turkey vultures and common ravens. Condor. 2017; 119 :720–731. doi: 10.1650/CONDOR-17-48.1. [ CrossRef ] [ Google Scholar ]
- Wiemeyer GM, Perez MA, Bianchini LT, Sampietro L, Bravo GF, Jacome NL, Astore V, Lambertucci SA. Repeated conservation threats across the Americas: High levels of blood and bone lead in the Andean condor widen the problem to a continental scale. Environmental Pollution. 2017; 220 :672–679. doi: 10.1016/j.envpol.2016.10.025. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Williams RJ, Tannenbaum LV, Williams SM, Holladay SD, Tuckfield RC, Sharma A, Humphrey DJ, Gogal RM. Ingestion of a single 2.3 mm lead pellet by laying roller pigeon hens reduces egg size and adversely affects F1 generation hatchlings. Archives of Environmental Contamination and Toxicology. 2017; 73 :513–521. doi: 10.1007/s00244-017-0406-9. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wobester GA. Diseases of the wild waterfowl. 2. New York: Plenum Press; 1997. [ Google Scholar ]
- Work TM, Dagenais J, Rameyer R, Breeden R. Mortality patterns in endangered Hawaiian geese (Nene; Branta sandvicensis ) Journal of Wildlife Diseases. 2015; 51 :688–696. doi: 10.7589/2014-11-256. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yaw T, Neumann K, Bernard L, Cancilla J, Evans T, Martin-Schwarze A, Zaffarano B. Lead poisoning in bald eagles admitted to wildlife rehabilitation facilities in Iowa, 2004–2014. Journal of Fish and Wildlife Management. 2017; 8 :465–473. doi: 10.3996/122015-JFWM-124. [ CrossRef ] [ Google Scholar ]
- Zimmer MC, Osier TL. Lead concentrations in white-tailed deer tissue due to retained bullets. Human-Wildlife Interactions. 2018; 12 :444–448. [ Google Scholar ]
Lead Poisoning in Northwestern Nigeria (The Village of Gold) Essay
Introduction.
An outbreak has erupted in Zamfara province in the northwestern section of Nigeria. Doctors confirmed that three villages within Zamfara province are at the heart of the crisis (CDC, n.d). The deadly outbreak known as lead poisoning has claimed 118 children below the age of five. The number of sick children has grown to 1,400, with 1,150 victims hospitalized. A first stop outside the villages depicts a horrific scene of 100 small newly dug graveyards (CDC, n.d). The situation is alarming because the outbreak came from the mining activities done within the area. The challenge is that the natives cannot stop this business because it is their only source of income. The most exposed population are children because they play on the ground where the contaminants are more concentrated (Kurup et al., 2019). Their parents also return from work soaked in dust that is confirmed to be toxic which increases poisoning. This paper explores some of the epidemiological processes applied in the identification process of lead poisoning (Kurup et al., 2019).
Solve the Outbreak Challenge
“The Village of Gold” is a Center for Disease Control and Prevention game that requires an individual to answer specific entries to define an outbreak (CDC, n.d). After answering the prompts in the game, I discovered the outbreak affecting the people of Zamfara Province (CDC, n.d). Lead poisoning was an outbreak that emerged from the mining activities performed in the area, and this contributed to the poisoning and death of children in the region. The causes, effects, and impact of mineral extractions are revealed through the use of epidemiological processes.
The Epidemiological Processes Applied
Epidemiology is an unbiased process of collection, evaluation, and interpretation of data within a specific population. The principles of epidemiology seek to define the frequency and the pattern of a health situation in a specified area (CDC, n.d). In this scenario of lead poisoning in the Zamfara province, I applied some of the epidemiological checklists approved by the Field Epidemiology Training Program (FETP), which helps extract data on the overall characteristics of an outbreak report and the steps applied (Kurup et al., 2019). The FETP approach used to identify and assess the outbreak included the following steps.
- Confirmation of the outbreak.
- Verification of the diagnosis.
- Case definition.
- Case finding.
- Descriptive epidemiology.
- Hypothesis.
- Analytical epidemiology.
Confirmation
The sudden surge in the mortality rate and the number of hospitalized children indicated an epidemic. I used the confirmation technique to analyze data gathered from Zamfara province and concluded that there was a rising curve in the number of affected (CDC, n.d.) Initially, the number of infections was at 150, which increased to a total of 1,400 affected children with more than 1000 hospitalized (CDC, n.d). The rising number of the affected automatically indicated an outbreak.
Verification of Diagnosis
Some of the children admitted to the hospital showed symptoms characterized by abdominal pain, headache, vomiting, and convulsions (CDC, n.d). Doctors had tried administering anti-malaria and antibiotic drugs, and none have worked since the first administration. A laboratory test had to be conducted to confirm the outbreak because it could not be physically examined. Laboratory results reveal that the patients (Majorly children) have a high level of lead in their blood, confirming that they have been exposed to lead poisoning (CDC, n.d).
Case Definition
After the test results, it was evident that the patients were suffering from Lead poisoning, something they acquired from the mining site where they spend most of their time (CDC, n.d). Some reported having extended their mining activities to their homes to make ends meet. The incubation period was unknown, and all the residents were at risk.
Descriptive Epidemiology
This technique helped me to analyze the information about the persons associated with the outbreak (Kurup et al., 2019).
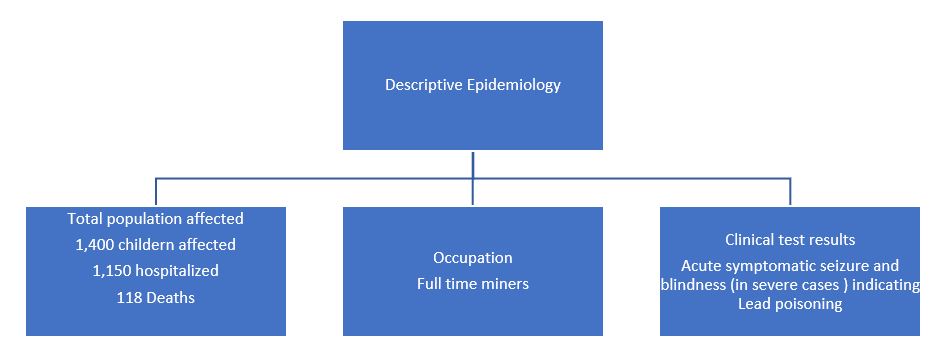
The primary cause of lead poisoning is exposure to lead-contaminated soil and air. During the extraction of minerals, exposure to lead-contaminated dust and soil is inevitable (Kurup et al., 2019). Children play with the soil, and this interaction increases their risk of being poisoned (CDC, n.d). Also, the natives do not use any protective clothing during mining, which means that they breathe in a lot of contaminated dust. If the natives take extra caution during mining by wearing protective gear and residing in far areas away from the site, the poisoning could decline.
Analytical epidemiology
At this stage, I applied the case-control study, which compares the exposure among cases and controls (Kurup et al., 2019). In this analysis, I realized that the poverty rate in Zamfara province was a contributing factor to poisoning. The people had no alternative income-generating activity, and this meant that they had to mine. Also, they could not afford to buy protective clothing that secured them from lead exposure.
The epidemiological processes of disease identification have played an essential role in explaining the outbreak investigation. For the Zamfara case scenario, the epidemiological techniques have enabled me to describe the outbreak report by focusing on specific characteristics such as the population affected, the prevalence of infection, zoned area, and the type of outbreak. The descriptive and analytic epidemiology have supported the explanation of the occurrence of the outbreak by providing a comprehensive framework that helped assess the exposures and risks associated with this lead poisoning.
Center for Disease Control and Prevention. (n.d). Solve the outbreak; the village of gold. Web.
Kurup, K. K., John, D., Ponnaiah, M., & George, T. (2019). Use of systematic epidemiological methods in outbreak investigations from India, 2008–2016: A systematic review . Clinical Epidemiology and Global Health, 7(4) , 648-653. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, July 20). Lead Poisoning in Northwestern Nigeria (The Village of Gold). https://ivypanda.com/essays/lead-poisoning-in-northwestern-nigeria-the-village-of-gold/
"Lead Poisoning in Northwestern Nigeria (The Village of Gold)." IvyPanda , 20 July 2022, ivypanda.com/essays/lead-poisoning-in-northwestern-nigeria-the-village-of-gold/.
IvyPanda . (2022) 'Lead Poisoning in Northwestern Nigeria (The Village of Gold)'. 20 July.
IvyPanda . 2022. "Lead Poisoning in Northwestern Nigeria (The Village of Gold)." July 20, 2022. https://ivypanda.com/essays/lead-poisoning-in-northwestern-nigeria-the-village-of-gold/.
1. IvyPanda . "Lead Poisoning in Northwestern Nigeria (The Village of Gold)." July 20, 2022. https://ivypanda.com/essays/lead-poisoning-in-northwestern-nigeria-the-village-of-gold/.
Bibliography
IvyPanda . "Lead Poisoning in Northwestern Nigeria (The Village of Gold)." July 20, 2022. https://ivypanda.com/essays/lead-poisoning-in-northwestern-nigeria-the-village-of-gold/.
- Abbott Northwestern Hospital: VBP
- Northwestern Memorial Hospital: The Customer Service Model
- Pumpkin- A Northwestern Favorite
- Abbott Northwestern Hospital's Leadership and Delivery
- Nurse Leader and Abbott Northwestern Hospital
- Northwestern and New York Business Schools Comparison
- Northwestern Paper Company's Transfer Pricing
- North-Western Medical Faculty Foundation's Human Resource Department
- Northwestern Memorial Hospital Managing Diversity
- Companies Web Search: PWC, Northwestern Mutual and Target
- The Public Health Prevention Model and the Institute of Medicine Framework
- Recovery Movement and the Recovery Movement’s Approach
- Minnesota Multiphasic Personality Inventory II (MMPI-II)
- Measles: Definition and Assessment
- Income Influence on Child Obesity
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Environmental Factor
Your online source for niehs news, grantee shines new light on cause of ciguatera seafood poisoning.
Keystone Lecturer Alison Robertson, Ph.D., shared how tracking toxic algae in the marine food web can help to prevent ciguatera outbreaks.
By Ben Richardson
Ciguatera poisoning is the most common non-bacterial seafood-borne illness in the world. More than 50,000 cases are reported annually, but scientists think that the true numbers may be much higher, because the symptoms — vomiting, diarrhea, tingling, muscle weakness and pain, and tactile sensitivity to cold — can be easily mistaken for other seafood illnesses.
During a Feb. 27 Keystone Science Lecture, Alison Robertson, Ph.D. , described her team’s efforts to track the accumulation and movement of ciguatoxins — the source of ciguatera poisoning — in reef food webs across the world. There is no diagnostic test for ciguatoxins, which is why tracking their movement is so important.

“On a weekly basis, our team is contacted about poisoning outbreaks that are happening among people who have eaten seafood and ended up sick,” said Robertson, an associate professor at the University of South Alabama and senior marine scientist at the Dauphin Island Sea Lab . “We’ve seen an increase in the number of these reports at our lab, because not a lot is known about ciguatera, and people are desperate to find answers.”

Her team is interested in understanding how, when, and why the microscopic marine algae produce these potent neurotoxins, and how fish metabolize and respond to them when exposed. By tracing this complicated food web, they aim to develop better methods of predicting and monitoring ciguatoxin buildup in the environment and ultimately improve seafood safety.
“Dr. Robertson demonstrated that understanding the delicate balance of the aquatic ecosystem and marine life is crucial to answering some long-standing questions about ciguatera poisoning in humans around the globe that are pertinent for the development of prevention strategies,” said Anika Dzierlenga, Ph.D. , a scientific program director in the Genes, Environment, and Health Branch and lecture host. “Her work is an excellent example of One Health research capturing the interrelatedness of environmental, animal, and human health.”
Uncovering the source
Robertson explained ciguatoxins first enter the food web when they are produced by some algae of the genera Gambierdiscus and Fukuyoa that live on degraded surfaces, as well as sea grasses and macroalgae in coral reefs. These microscopic algae are eaten by a wide variety of fish and marine invertebrates, which are, in turn, consumed by other reef predators, such as triggerfish, snappers, and grouper. If the fish have been feeding in an area where ciguatoxins are being produced locally, they also have the potential to bioaccumulate ciguatoxins and cause ciguatera if consumed by people.
In tracing ciguatoxin movement in the marine food web, Robertson and her collaborative team identified for the first time the specific type of algal ciguatoxin — a neurotoxin called Caribbean CTX-5 — that caused ciguatera in the Caribbean.
“We had identified Gambierdiscus silvae as the likely algal source species (along with a few others), but we had not yet worked out the toxin it was producing,” Robertson said. “Through strong partnerships and collaborations with the National Research Council and the Norwegian Veterinary Institute, we were finally able to uncover the toxins responsible, which makes tracing them through the environment a realistic goal, and something many in the field have been chasing for decades.”
Given the lack of pre-market fish testing, diagnostic testing, and medication available to treat ciguatera poisoning, Robertson said she hopes her research will lead to more robust environmental monitoring of CTX-5 and other fish metabolites of the ciguatoxins to reduce ciguatera outbreaks in vulnerable communities.

“If money was no object, we would have teams in the water in all heavily impacted areas trying to do spatial surveys of the algae,” Robertson said. “Fishermen know quite a lot about areas that are causing illness, and they’re avoiding them. I would like to see teams that can integrate environmental sampling and monitoring with geospatial analysis and fisheries science to get to some kind of predictive capacity.”
(Ben Richardson, Ph.D., is a Presidential Management Fellow in the NIEHS Office of Communications and Public Liaison.)
Related Articles
Human health linked to animals, environment, say experts

Society of Toxicologic Pathology looks to One Health

One Health draws international group to NIEHS

One Health conference draws experts to Thailand
- Share full article
Advertisement
Supported by
Maureen Dowd
Donald Trump’s Insatiable Bloodlust

By Maureen Dowd
Opinion Columnist, writing from Washington.
An earthquake. An eclipse. A bridge collapse. A freak blizzard. A biblical flood. Donald Trump leading in battleground states.
Apocalyptic vibes are stirred by Trump’s violent rhetoric and talk of blood baths.
If he’s not elected, he bellowed in Ohio, there will be a blood bath in the auto industry. At his Michigan rally on Tuesday, he said there would be a blood bath at the border, speaking from a podium with a banner reading, “Stop Biden’s border blood bath.” He has warned that, without him in the Oval, there will be an “Oppenheimer”-like doomsday; we will lose World War III and America will be devastated by “weapons, the likes of which nobody has ever seen before.”
“And the only thing standing between you and its obliteration is me,” Trump has said.
An unspoken Trump threat is that there will be a blood bath again in Washington, like Jan. 6, if he doesn’t win.
That is why he calls the criminals who stormed the Capitol “hostages” and “unbelievable patriots.” He starts some rallies with a dystopian remix of the national anthem, sung by the “J6 Prison Choir,” and his own reciting of the Pledge of Allegiance.
The bloody-minded Trump luxuriates in the language of tyrants.
In “Macbeth,” Shakespeare uses blood imagery to chart the creation of a tyrant. Those words echo in Washington as Ralph Fiennes stars in a thrilling Simon Godwin production of “MacBeth” for the Shakespeare Theater Company, opening Tuesday.
“The raw power grab that excites Lady Macbeth and incites her husband to regicide feels especially pertinent now, when the dangers of autocracy loom over political discussions,” Peter Marks wrote in The Washington Post about the production with Fiennes and Indira Varma (the lead sand snake in “Game of Thrones.”)
Trump’s raw power grab after his 2020 loss may have failed, but he’s inflaming his base with language straight out of Macbeth’s trip to hell.
“Blood will have blood,” as Macbeth says. One of the witches, the weird sisters, urges him, “Be bloody, bold and resolute.”
Another weird sister, Marjorie Taylor Greene, is predicting end times. “God is sending America strong signs to tell us to repent,” she tweeted on Friday. “Earthquakes and eclipses and many more things to come. I pray that our country listens.”
Like Macbeth, Trump crossed a line and won’t turn back. The Irish say, “You may as well be hanged for a sheep as a lamb.” Macbeth killed his king, then said: “I am in blood. Stepped in so far that, should I wade no more, Returning were as tedious as go o’er.”
The Washington Post’s Josh Dawsey reported that since Trump put his daughter-in-law in charge of the Republican National Committee, prospective employees are asked if they think the election was stolen. Republicans once burbled on about patriotism and defending America. Now denying democracy is a litmus test for employment in the Formerly Grand Old Party.
My Irish immigrant father lived through the cruel “No Irish Need Apply” era. I’m distraught that our mosaic may shatter.
But Trump embraces Hitleresque phrases to stir racial hatred. He has talked about immigrants “poisoning the blood of our country.” Last month, he called migrants “animals,” saying, “I don’t know if you call them ‘people,’ in some cases. They’re not people, in my opinion.”
Trump’s obsession with bloodlines was instilled by his father, the son of a German immigrant. He thinks there is good blood and bad blood, superior blood and inferior blood. Fred Trump taught his son that their family’s success was genetic, reminiscent of Hitler’s creepy faith in eugenics.
“The family subscribes to a racehorse theory of human development,” the Trump biographer Michael D’Antonio told PBS. “They believe that there are superior people and that if you put together the genes of a superior woman and a superior man, you get a superior offspring.”
Trump has been talking about this as far back as an “Oprah” show in 1988. The “gene believer” brought it up in a 2020 speech in Minnesota denouncing refugees.
“A lot of it is about the genes, isn’t it, don’t you believe?” he told the crowd about their pioneer lineage, adding: “The racehorse theory, you think we’re so different? You have good genes in Minnesota.”
As Stephen Greenblatt writes in “Tyrant: Shakespeare on Politics,” usurpers don’t ascend to the throne without complicity. Republican enablers do all they can to cozy up to their would-be dictator, even introducing a bill to rename Dulles airport for Trump. Democrats responded by introducing a bill to name a prison in Florida for Trump.
“Why, in some circumstances, does evidence of mendacity, crudeness or cruelty serve not as a fatal disadvantage but as an allure, attracting ardent followers?” Greenblatt asked. “Why do otherwise proud and self-respecting people submit to the sheer effrontery of the tyrant, his sense that he can get away with saying and doing anything he likes, his spectacular indecency?”
Like Macbeth’s castle, the Trump campaign has, as Lady Macbeth put it, “the smell of blood,” and “all the perfumes of Arabia will not sweeten” it.
The Times is committed to publishing a diversity of letters to the editor. We’d like to hear what you think about this or any of our articles. Here are some tips . And here’s our email: [email protected] .
Follow the New York Times Opinion section on Facebook , Instagram , TikTok , WhatsApp , X and Threads .
Maureen Dowd is an Opinion columnist for The Times. She won the 1999 Pulitzer Prize for distinguished commentary. @ MaureenDowd • Facebook

IMAGES
VIDEO
COMMENTS
cases of oral lead poisoning result from small amounts of lead -containing material such as contaminated dust or soil, flakes of lead paint, contaminated food and spices, lead -containing traditional medicines or from ingestion of lead as a foreign body. Inhalation of lead as fumes or particles is a major occupational route of exposure.
Lead poisoning causes a variety of symptoms, including abnormal behaviour which varies from person to person, while time of exposure plays an important role (Kosnett, 2005). There are also studies which show no symptoms of lead poisoning even with elevated levels of lead in the body (Mycyk et al., 2005). The question what makes such differences ...
Lead Poisoning Essay. Sort By: Page 1 of 50 - About 500 essays. Better Essays. Lead Poisoning In Children. 2024 Words; 9 Pages; Lead Poisoning In Children. is lead, it is a soft metal that is a silver color. It is one of the four metals that has the most damaging effects on human health. (Lenntech, 2016) Lead is toxic to everyone especially ...
Lead became a common occupational toxin with the birth of the Industrial Revolution, by the end of the 19-century childhood lead poisoning secondary to exposure to lead-based paints was beginning to be recognized. As the 20 century progressed, so did the appreciation for increasingly subtle and even subclinical manifestations of lead toxicity.
Lead poisoning is usually caused by eating or drinking (ingesting) lead, but touching or breathing in the toxic metal can also cause it. Lead poisoning is when any detectable amount of lead is found in your child's blood. Lead can affect many parts of your child's body, including their brain, nerves, blood, digestive organs and more.
Lead poisoning is a wholly preventable disease. This monograph is one in a series of self-instructional publications designed to increase the primary care provider's knowledge of hazardous substances in the environment and to aid in the evaluation of potentially exposed patients. See page 27 for more information about continuing medical ...
Lead Poisoning Essay. Considered the oldest metal out there, lead can be very poisonous when ingested or inhaled into the body. It has been used throughout many centuries for several of every day household products. Many people really don't know how poisonous lead can be to the system. Lead is used throughout the world and causes problems ...
Abstract. Childhood lead poisoning is a multi-faceted, complex condition, which affects not only the child's health and well-being, but also the family's housing security, economic status, job security, and stress level. This review updates the emergency department clinician on the management of childhood lead poisoning.
Lead poisoning symptoms in adults. Although children are primarily at risk, lead poisoning is also dangerous for adults. Signs and symptoms in adults might include: High blood pressure. Joint and muscle pain. Difficulties with memory or concentration. Headache. Abdominal pain. Mood disorders.
Lead has also led to occupational hazards where certain occupations predispose individuals to poisoning. One common impact of lead on the health of populations is its toxicity among children. Lead poisoning among children was first identified approximately 100 years ago when 10 children were reported dead.
Lead exposure is a type of poisoning that may be prevented and recognized. The most common cause of lead exposure is lead-based paint. ... This essay, "Lead Exposure: The Common Cause" is published exclusively on IvyPanda's free essay examples database. You can use it for research and reference purposes to write your own paper.
Lead poisoning in Asian nations, such as China and India, is a significant health issue. In the US, lead poisoning caused by retaining bullets is a rare, but severe source of Pb poisoning. Over 115,000 people are injured by firearms each year in the United States (Gotsch Ke et al., 2001). According to data from the Adult Blood Lead Epidemiology ...
the middle and late twentieth century, and although the thesis of chronic lead poisoning as causing the decline ofRome had first been presented by Robert. in 1909,2 this theory did not achieve a notoriety until it was restated and resummarized by Gilfillan in 1965.3 The operative notion is simple: lead.
Lead Paint Screening egulations in California Childhood Lead Poisoning Prevention (105275-105310) Agency esponsible The agency responsible for giving guidelines is the United States Center for Disease Control. The Department of Health is further responsible for enforcing this law. Incentives and Enforcement This regulation gives several incentives and enforcements that encourage compliance ...
Essay On Lead Poisoning. 610 Words3 Pages. The Romans used lead for just about everything they possibly could. It was used in cosmetics, jewelry, utensils, cooking pots, plumbing pipes (Powered By Osteons), seasoning for food, and as a wine preservative. It was even used for pigment in paint.
Lead poisoning has been a concern for many years. In fact, because of the affects of lead poisoning, there has been an extensive decline in its use. "Many people believe lead poisoning is no longer a threat, yet millions of homes contain lead based paint" (Heck, J., 2005, para. 1).
The points argued in this essay will give examples where increasing interest in lead poisoning will give to solve such issues like the Flint, Michigan water crisis 2016, which according to an article on MSNBC, 200 children under the age of 6 were reported to have high levels of lead in their blood, and 9000 others that were exposed to lead ...
Essay On Lead Poisoning. Lead is a naturally occurring element found in small amounts in the earth's crust. Although it has some beneficial uses, it can be toxic to humans and animals causing health effects. Lead can be found in many places, much because of human activity through burning fossil fuels, mining, and manufacturing.
18 of 43 dead birds collected after a ban on the use of lead bullets for hunting sika deer had elevated liver lead concentrations (> 2 ppm w.w.) associated with poisoning. Isotopic analysis was consistent with lead ammunition. One bird that died in 2013 had a lead bullet in the stomach and a liver lead of 36.3 ppm ww.
Lead Poisoning Essay. 754 Words4 Pages. Lead Poisoning The way lead poisoning had widely spread is due to the lack of care that a lot of companies have stopped watching for in their lead based paints and how it is affecting kids. The reason why this poses such an issue is because there are a lot of different toy products that have lead based paint.
Studies have shown that lead is able to mimic certain metals that are significant in biological processes, leading to a collection of developmental and physiological conditions commonly grouped as "lead poisoning.". In brief, lead acts on small biological systems that affect a major system, an example of which are zinc -finger proteins.
An outbreak has erupted in Zamfara province in the northwestern section of Nigeria. Doctors confirmed that three villages within Zamfara province are at the heart of the crisis (CDC, n.d). The deadly outbreak known as lead poisoning has claimed 118 children below the age of five. The number of sick children has grown to 1,400, with 1,150 ...
By Ben Richardson. Ciguatera poisoning is the most common non-bacterial seafood-borne illness in the world. More than 50,000 cases are reported annually, but scientists think that the true numbers may be much higher, because the symptoms — vomiting, diarrhea, tingling, muscle weakness and pain, and tactile sensitivity to cold — can be ...
He has talked about immigrants "poisoning the blood of our country." Last month, he called migrants "animals," saying, "I don't know if you call them 'people,' in some cases. They ...