- Search by keyword
- Search by citation
Page 1 of 26

The financial and social impacts of the COVID-19 pandemic on youth with eating disorders, their families, clinicians and the mental health system: a mixed methods cost analysis
The onset of the COVID-19 pandemic has had an adverse impact on children, youth, and families with eating disorders (EDs). The COVID-19 pandemic exacerbated pre-existing personal and financial costs to youth, ...
- View Full Text
Pediatric hospital utilization for patients with avoidant restrictive food intake disorder
Avoidant restrictive food intake disorder (ARFID) is a relatively new feeding and eating disorder added to the DSM-5 in 2013 and ICD-10 in 2018. Few studies have examined hospital utilization for patients with...
Fostering positive attitudes toward food in individuals with restrained eating: the impact of flexible food-related inhibition
Individuals exhibiting restrained eating behaviors demonstrate increased inhibitory control when exposed to food-related stimuli, indicating the presence of an automatic food-inhibition association. Existing l...
Meeting abstracts from the 2022 ANZAED conference
This article is part of a Supplement: Volume 12 Supplement 1
Temperament impact on eating disorder symptoms and habit formation: a novel model to inform treatment
Temperament has long been described as the biological dimension of personality. Due to advancing brain-imaging technology, our understanding of temperament has deepened and transformed over the last 25 years. ...
Reliability generalization meta-analysis of orthorexia nervosa using the ORTO-11/12/15/R scale in all populations and language versions
The ORTO scale was developed in 2004 as a self-report questionnaire to assess symptoms of orthorexia nervosa (ON). ON is an unhealthy preoccupation with eating healthy food. The scale aims to measure obsessive...
The relationship between internalised weight bias and biopsychosocial outcomes in children and youth: a systematic review
To synthesise the evidence on the relationships between internalised weight bias (IWB) and biopsychosocial health outcomes in individuals ≤ 25 years.
Transitions from child and adolescent to adult mental health services for eating disorders: an in-depth systematic review and development of a transition framework
Eating disorders (EDs) peak in mid-to-late adolescence and often persist into adulthood. Given their early onset and chronicity, many patients transition from child and adolescent mental health services (CAMHS...
“Maze Out”: a study protocol for a randomised controlled trial using a mix methods approach exploring the potential and examining the effectiveness of a serious game in the treatment of eating disorders
Eating Disorders (ED) are severe and costly mental health disorders. The effects of existing treatment approaches are limited and there is a need to develop novel interventions, including digital strategies th...
Further evidence of the association between social media use, eating disorder pathology and appearance ideals and pressure: a cross-sectional study in Norwegian adolescents
Few studies have investigated how the plethora of contemporary social media (SM) platforms relate to, and influence eating disorder (ED) pathology, appearance ideals and pressure to conform to these ideals in ...
Longitudinal associations between response-style strategies and abnormal eating behaviors/attitudes in adolescents: a cross-lagged panel model
Previous studies have suggested that response-style strategies (rumination, problem-solving, and distraction) can be risk or protective factors for the development of abnormal eating behaviors/attitudes (AEB) ...
Views of German mental health professionals on the use of digital mental health interventions for eating disorders: a qualitative interview study
Digital mental health interventions (DMHIs) are getting increasingly important for mental health care. In the case of eating disorders (EDs), DMHIs are still in early stages. Few studies so far investigated th...
The various facets of orthorexic eating behavior: five case reports of individuals with supposed orthorexia nervosa
Orthorexia nervosa, defined as a fixation on eating healthy according to subjective criteria, is recently being discussed as another variant of disordered eating behavior. Further characteristics are rigid ad...
Psychometric properties of the nine-item avoidant/restrictive food intake disorder screen (NIAS) in Turkish children
The nine item avoidant/restrictive food intake disorder screen (NIAS) is a short and practical assessment tool specific to ARFID with three ARFID phenotypes such as “Picky eating,” “Fear,” and “Appetite”. This...
Association between body composition standards and eating disorder medical claims among active-duty service women
Eating disorders are a worldwide public health concern with the United States having a particularly high prevalence. Eating disorders are of particular concern to the Department of Defense and Military Health ...
Correction: Food addiction and binge eating disorder are linked to shared and unique deficits in emotion regulation among female seeking bariatric surgery
The original article was published in Journal of Eating Disorders 2023 11 :97
Early weight gain as a predictor of weight restoration in avoidant/restrictive food intake disorder
Previous research has demonstrated that early weight gain in family-based treatment (FBT) is predictive of remission for adolescents with anorexia nervosa (AN). However, no published data has addressed if earl...
New understandings meet old treatments: putting a contemporary face on established protocols
In the twenty years since the publication of the most widely used treatment manuals describing evidence-based therapies for eating disorders, there have been some substantial advances in the field. New methods...
Hedonic hunger, food addiction, and night eating syndrome triangle in adolescents and ıts relationship with body mass ındex
The relationship between adolescent obesity and eating disorders is an issue that needs urgent attention. Screening for eating disorders is as important as dietary interventions to treat obesity. This study ai...
A systematic review, meta-analysis, and meta-regression of the prevalence of self-reported disordered eating and associated factors among athletes worldwide
The purpose of this meta-analysis was to provide a pooled prevalence estimate of self-reported disordered eating (SRDE) in athletes based on the available literature, and to identify risk factors for their occ...
“It’s beautiful and it’s messy and it’s tragic”: exploring the role of compassion in the eating disorder recovery processes of 2S/LGBTQ + Canadians
This research explores experiences of compassion among 2S/LGBTQ + Canadians living with eating disorders in the context of eating disorder treatment and community support. There is a growing body of scholarshi...
Adolescent utilization of eating disorder higher level of care: roles of family-based treatment adherence and demographic factors
Outpatient family-based treatment (FBT) is effective in treating restrictive eating disorders among adolescents. However, little is known about whether FBT reduces higher level of care (HLOC) utilization or if...
Carotid wave analysis in young adults with a history of adolescent anorexia nervosa: a case control study
Anorexia nervosa (AN) is associated with abnormalities that may increase the risk of future cardiovascular disease. This study assessed the cardiovascular health of individuals who recovered from AN during ado...
Key-in-session identity negotiations in a first line treatment for adult anorexia nervosa
Exploration of client identity negotiations during treatment for Anorexia Nervosa (AN) is a relatively new area of research. Research suggests that difficulties with identity negotiations may present as a barr...
Avoidant/restrictive food intake disorder differs from anorexia nervosa in delay discounting
Avoidant/restrictive food intake disorder (ARFID) and anorexia nervosa (AN) are the two primary restrictive eating disorders; however, they are driven by differing motives for inadequate dietary intake. Despit...
How evaluative pairings improve body dissatisfaction in adult women: evidence from a randomized-controlled online study
Many young women are dissatisfied with their bodies. This study investigated the effect on current body dissatisfaction levels of a newly developed evaluative conditioning procedure that paired self-similar an...
Preliminary identification of clinical cut-off of the vegetarian vegan eating disorder screener (V-EDS) in a community and self-reported clinical sample of vegetarians and vegans
The vegetarian vegan eating disorder screener (V-EDS) is an 18-item self-report screening tool designed to assess the unique elements of eating disorder symptomology in vegetarians and vegans. Previous results...
Identifying overcontrol and undercontrol personality types among young people using the five factor model, and the relationship with disordered eating behaviour, anxiety and depression
Overcontrol and undercontrol personality types have been associated with an increase in eating pathology, depression and anxiety. The aim of the research was to explore whether latent overcontrol and undercont...
Clinical characteristics, treatment course and outcome of adults treated for avoidant/restrictive food intake disorder (ARFID) at a tertiary care eating disorders program
Avoidant/restrictive food intake disorder (ARFID) is now recognized as a feeding/eating disorder that affects individuals across the lifespan, but research on ARFID in general and particularly in adults remain...
Confirmatory factor analysis and gender invariance of Persian version of the modified Yale food addiction scale (mPYFAS) 2.0: insight from a large scale Iranian sample
The Modified Yale Food Addiction Scale 2.0 (mYFAS 2.0) was developed with the primary objective of evaluating food addiction (FA). The present study aimed to undertake the translation, pilot testing, and evalu...
Expanding considerations for treating avoidant/restrictive food intake disorder at a higher level of care
Existing descriptions of the treatment of avoidant/restrictive food intake disorder (ARFID) at higher levels of care (HLOC) for eating disorders are limited, despite HLOC settings frequently serving patients w...
Anorexia nervosa through the lens of a severe and enduring experience: ‘ lost in a big world’
Severe and enduring anorexia nervosa (SE-AN), is a serious and persistent illness, despite ‘state of the art’ treatment. Criteria have been theoretically proposed, but not tested, and may not adequately captur...
How young people perceive change to occur in family therapy for anorexia nervosa: a qualitative study
Family therapy for anorexia nervosa (FT-AN) is the first line recommended treatment for child and adolescent anorexia nervosa. Despite evidence of its efficacy, little is understood about the treatment mechani...
Relationship of self-reported pica and avoidant restrictive food intake disorder symptomology with dimensions of impulsivity, perceived stress among Pakistani University students
Pica and avoidant/restrictive food intake disorder are two of the three new eating and feeding disorders introduced in the DSM-5, this inclusion has drawn attention to the immediate need for research into thei...
Early evaluation of a DBT-informed online intervention for people with eating disorders
Eating disorders (EDs) have a worldwide prevalence of 7.8%, with towering mortality rates and high healthcare costs. The current recommended treatment for EDs principally works by directly targeting ED thought...
The buffet challenge: a behavioral assessment of eating behavior in adolescents with an eating disorder
Eating disorders are characterized by disturbances in nutritional intake and abnormal mealtime behaviors. Laboratory eating paradigms offer a unique opportunity to accurately measure dietary intake and eating ...
Tuning in to recovery: influence of music on emotional well-being during mealtime in inpatient facilities for eating disorders
In rehabilitating eating disorders (ED), mealtimes are critical but often induce stress, both for restrictive and binge-purge disorders. Although preliminary data indicate a positive effect of music during mea...
Longitudinal associations between community violence exposure, posttraumatic stress symptoms, and eating disorder symptoms
Eating disorder (ED) symptoms have been associated with different types of traumatic events, such as exposure to sexual and physical violence, and emotional abuse. However, the relation between ED symptoms and...
Do risk factors differentiate DSM-5 and drive for thinness severity groups for anorexia nervosa?
The current study examined whether risk factors for anorexia nervosa (AN) were related to different levels of severity based on (a) the DSM-5/body mass index (BMI) and (b) drive for thinness (DT) severity rati...
Development and preliminary validation of a novel eating disorder screening tool for vegetarians and vegans: the V-EDS
Eating disorders have one of the highest mortality of all mental illnesses but are associated with low rates of screening and early intervention. In addition, there remains considerable uncertainty regarding t...
Eating disorder hospitalizations among children and youth in Canada from 2010 to 2022: a population-based surveillance study using administrative data
Eating disorders (EDs) are severe mental illnesses associated with significant morbidity and mortality. EDs are more prevalent among females and adolescents. Limited research has investigated Canadian trends o...
Associations between physical activity, mental health concerns, eating disorder symptoms, and emotional intelligence in adolescent athletes transitioning from COVID-19
It is well known that COVID-19 significantly disrupted the routines of school sports for adolescent athletes. In transitioning from this “change event,” athletes may need support with resuming their pre-pandem...
Improving motivation and treatment uptake behaviors of patients with eating disorders using patient narrative videos: study protocol of a pilot randomized controlled trial
Patients with eating disorders (ED) typically report delays between the onset of symptoms and engagement with treatment services. Personal barriers including stigma, shame, and guilt, as well as the availabili...
Psychometric properties of self-report measures of eating disorder cognitions: a systematic review
Although eating disorder (ED) models display some differences in theory and treatment approach, cognitive-behavioural, schema-focused, and disorder-specific models all highlight the fundamental nature of cogni...
Cultural adaptation of an integrated eating disorders prevention and healthy weight management program
Both eating disorder (ED) prevention and weight management interventions often focus on the thin ideal. Yet, many Black and Latina women do not view thinness as their body ideal. This study used focus groups t...
Motives for using social networking sites: a uses & gratifications perspective amongst people with eating disorder symptoms
Studies investigating motives for social networking sites (SNS) use amongst people with eating disorder (ED) symptoms are scarce. The uses and gratifications theory states that people actively select media con...
How does COVID-19-related social media usage influence disordered eating? A daily diary study among Chinese adults during lockdown
Despite previous studies highlighting the benefits of social media use during the COVID-19 pandemic, particularly under lockdown, limited research has identified the potential detrimental consequences of socia...
Correction: Childhood maltreatment, shame, psychological distress, and binge eating: testing a serial mediational model
The original article was published in Journal of Eating Disorders 2023 11 :96
Glial cell changes in the corpus callosum in chronically-starved mice
Anorexia nervosa (AN) is characterized by emaciation, hyperactivity, and amenorrhea. Imaging studies in AN patients have revealed reductions in grey and white matter volume, which correlate with the severity o...
Pharmacotherapy for attention deficit/hyperactivity disorder in youth with avoidant restrictive food intake disorder: a case series of patients prescribed stimulant medication in a partial hospitalization program for eating disorders
Appetite suppression and weight loss are established potential side effects of most medications for attention deficit/hyperactivity disorder (ADHD). These side effects may be especially problematic when using ...
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 4.1 - 2-year Impact Factor 4.2 - 5-year Impact Factor 1.209 - SNIP (Source Normalized Impact per Paper) 0.833 - SJR (SCImago Journal Rank)
2023 Speed 11 days submission to first editorial decision for all manuscripts (Median) 119 days submission to accept (Median)
2023 Usage 1,680,918 downloads 2,607 Altmetric mentions
- More about our metrics
Journal of Eating Disorders
ISSN: 2050-2974
- General enquiries: [email protected]
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
APA Releases Updated Guideline for Treating Eating Disorders
- Nick Zagorski
Search for more papers by this author
The new practice guideline focuses on evidence-based pharmacological, psychotherapeutic, and other nonpharmacological treatments for eating disorders in adolescents and adults.
APA has released an updated practice guideline on how to treat patients with eating disorders— the first full update since 2006.
There have been many clinical advances and diagnostic changes related to eating disorders in the past 17 years, noted Laura Fochtmann, M.D., M.B.I., a professor of psychiatry, pharmacological sciences and biomedical informatics at Stony Brook University School of Medicine and the medical editor for APA’s practice guidelines. Fochtmann was also the vice-chair of the writing group for this newest practice guideline.
Notably, the publication of DSM-5 in 2013 and DSM-5-TR in 2022 resulted in broader diagnostic criteria for both anorexia nervosa and bulimia nervosa as well as the additions of binge-eating disorder and avoidant/restrictive food intake disorder (ARFID) categories. Binge-eating and avoidant/restrictive food intake disorders are both referenced in the updated guideline: There is a comprehensive section on assessing and treating binge-eating disorder, and there is also information on assessing patients with ARFID, Fochtmann noted. (Due to limited clinical data, there are no specific recommendations for how to treat patients with ARFID.)
The fourth edition of APA’s practice guideline for eating disorders includes 16 clinical recommendations or suggestions, depending on the level of scientific evidence. Here are some of the recommendations:
Screening for the presence of an eating disorder as part of an initial psychiatric evaluation.
Conducting comprehensive patient evaluations, including laboratory tests such as electrocardiograms.
Ensuring the treatment plan is patient-centered and culturally sensitive.
Setting individualized weight goals for patients with anorexia.
Treating patients with anorexia, bulimia, and binge-eating disorders with eating disorder–focused psychotherapy.
Including family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
In addition to the guideline, APA has developed supplemental materials to help both health professionals and patients and families, such as a clinician pocket guide and educational slides. The centerpiece of these resources is an online tool that offers a step-by-step guide on how to assess symptoms and screen patients for a potential eating disorder. This clinical decision tool will be customized for different professionals (for example, psychiatrists, psychologists, and pediatricians) to match their skillsets and the symptoms they might expect when encountering someone with an eating disorder.
The development of the online toolkit and other supporting resources was made possible through a grant from the Council of Medical Specialty Societies.
The release of this new practice guideline comes at an important juncture for people with eating disorders (“ Special Report: Youth With Eating Disorders—Time Is of the Essence in Achieving Remission ”). Studies have reported upticks in individuals developing symptoms of and requiring hospitalization for disordered eating since the start of the COVID-19 pandemic in 2020. As noted in the guideline, maintaining a structured plan for meals and snacks is an important component of treatment, and the pandemic disrupted normal routines and increased stress and anxiety.
“Early identification and treatment of an eating disorder is critical for achieving positive long-term outcomes,” said Joel Yager, M.D., a professor emeritus of psychiatry at the University of Colorado School of Medicine and the chair of the writing group for all three previous editions of the eating disorder practice guideline. “We hope that this tool will help anyone make an informed diagnosis regardless of their previous experience with eating disorders.” ■
“ The American Psychiatric Association Practice Guideline for the Treatment of Patients With Eating Disorders, Fourth Edition ”
Practice Guideline for Treatment of Patients with Eating Disorders .

- Patient Care & Health Information
- Diseases & Conditions
- Anorexia nervosa
If your doctor suspects that you have anorexia nervosa, he or she will typically do several tests and exams to help pinpoint a diagnosis, rule out medical causes for the weight loss, and check for any related complications.
These exams and tests generally include:
- Physical exam. This may include measuring your height and weight; checking your vital signs, such as heart rate, blood pressure and temperature; checking your skin and nails for problems; listening to your heart and lungs; and examining your abdomen.
- Lab tests. These may include a complete blood count (CBC) and more-specialized blood tests to check electrolytes and protein as well as functioning of your liver, kidney and thyroid. A urinalysis also may be done.
- Psychological evaluation. A doctor or mental health professional will likely ask about your thoughts, feelings and eating habits. You may also be asked to complete psychological self-assessment questionnaires.
- Other studies. X-rays may be taken to check your bone density, check for stress fractures or broken bones, or check for pneumonia or heart problems. Electrocardiograms may be done to look for heart irregularities.
Your mental health professional also may use the diagnostic criteria for anorexia in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), published by the American Psychiatric Association.
More Information
- Bone density test
- Complete blood count (CBC)
- Electrocardiogram (ECG or EKG)
- Liver function tests
Treatment for anorexia is generally done using a team approach, which includes doctors, mental health professionals and dietitians, all with experience in eating disorders. Ongoing therapy and nutrition education are highly important to continued recovery.
Here's a look at what's commonly involved in treating people with anorexia.
Hospitalization and other programs
If your life is in immediate danger, you may need treatment in a hospital emergency room for such issues as a heart rhythm disturbance, dehydration, electrolyte imbalances or a psychiatric emergency. Hospitalization may be required for medical complications, severe psychiatric problems, severe malnutrition or continued refusal to eat.
Some clinics specialize in treating people with eating disorders. They may offer day programs or residential programs rather than full hospitalization. Specialized eating disorder programs may offer more-intensive treatment over longer periods of time.
Medical care
Because of the host of complications anorexia causes, you may need frequent monitoring of vital signs, hydration level and electrolytes, as well as related physical conditions. In severe cases, people with anorexia may initially require feeding through a tube that's placed in their nose and goes to the stomach (nasogastric tube).
Care is usually coordinated by a primary care doctor or a mental health professional, with other professionals involved.
Restoring a healthy weight
The first goal of treatment is getting back to a healthy weight. You can't recover from anorexia without returning to a healthy weight and learning proper nutrition. Those involved in this process may include:
- Your primary care doctor, who can provide medical care and supervise your calorie needs and weight gain
- A psychologist or other mental health professional, who can work with you to develop behavioral strategies to help you return to a healthy weight
- A dietitian, who can offer guidance getting back to regular patterns of eating, including providing specific meal plans and calorie requirements that help you meet your weight goals
- Your family, who will likely be involved in helping you maintain normal eating habits
- Psychotherapy
These types of therapy may be beneficial for anorexia:
- Family-based therapy. This is the only evidence-based treatment for teenagers with anorexia. Because the teenager with anorexia is unable to make good choices about eating and health while in the grips of this serious condition, this therapy mobilizes parents to help their child with re-feeding and weight restoration until the child can make good choices about health.
- Individual therapy. For adults, cognitive behavioral therapy — specifically enhanced cognitive behavioral therapy — has been shown to help. The main goal is to normalize eating patterns and behaviors to support weight gain. The second goal is to help change distorted beliefs and thoughts that maintain restrictive eating.
Medications
No medications are approved to treat anorexia because none has been found to work very well. However, antidepressants or other psychiatric medications can help treat other mental health disorders you may also have, such as depression or anxiety.
Treatment challenges in anorexia
One of the biggest challenges in treating anorexia is that people may not want treatment. Barriers to treatment may include:
- Thinking you don't need treatment
- Fearing weight gain
- Not seeing anorexia as an illness but rather a lifestyle choice
People with anorexia can recover. However, they're at increased risk of relapse during periods of high stress or during triggering situations. Ongoing therapy or periodic appointments during times of stress may help you stay healthy.
- Acupuncture
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
When you have anorexia, it can be difficult to take care of yourself properly. In addition to professional treatment, follow these steps:
- Stick to your treatment plan. Don't skip therapy sessions and try not to stray from meal plans, even if they make you uncomfortable.
- Talk to your doctor about appropriate vitamin and mineral supplements. If you're not eating well, chances are your body isn't getting all of the nutrients it needs, such as Vitamin D or iron. However, getting most of your vitamins and minerals from food is typically recommended.
- Don't isolate yourself from caring family members and friends who want to see you get healthy. Understand that they have your best interests at heart.
- Resist urges to weigh yourself or check yourself in the mirror frequently. These may do nothing but fuel your drive to maintain unhealthy habits.
Alternative medicine
Dietary supplements and herbal products designed to suppress the appetite or aid in weight loss may be abused by people with anorexia. Weight-loss supplements or herbs can have serious side effects and dangerously interact with other medications. These products do not go through a rigorous review process and may have ingredients that are not posted on the bottle.
Keep in mind that natural doesn't always mean safe. If you use dietary supplements or herbs, discuss the potential risks with your doctor.
Anxiety-reducing approaches that complement anorexia treatment may increase the sense of well-being and promote relaxation. Examples of these approaches include massage, yoga and meditation.
Coping and support
You may find it difficult to cope with anorexia when you're hit with mixed messages by the media, culture, and perhaps your own family or friends. You may even have heard people joke that they wish they could have anorexia for a while so that they could lose weight.
Whether you have anorexia or your loved one has anorexia, ask your doctor or mental health professional for advice on coping strategies and emotional support. Learning effective coping strategies and getting the support you need from family and friends are vital to successful treatment.
Preparing for your appointment
Here's some information to help you get ready for your appointment and know what to expect from your doctor or mental health professional.
You may want to ask a family member or friend to go with you. Someone who accompanies you may remember something that you missed or forgot. A family member may also be able to give your doctor a fuller picture of your home life.
What you can do
Before your appointment, make a list of:
- Any symptoms you're experiencing, including any that may seem unrelated to the reason for the appointment. Try to recall when your symptoms began.
- Key personal information, including any major stresses or recent life changes.
- All medications, vitamins, herbal products, over-the-counter medications and other supplements that you're taking, and their dosages.
- Questions to ask your doctor so that you'll remember to cover everything you wanted to.
Some questions you might want to ask your doctor or mental health professional include:
- What kinds of tests do I need? Do these tests require any special preparation?
- Is this condition temporary or long lasting?
- What treatments are available, and which do you recommend?
- Is there a generic alternative to the medicine you're prescribing?
- Are there any brochures or other printed material that I can have? What websites do you recommend?
Don't hesitate to ask other questions during your appointment.
What to expect from your doctor
Your doctor or mental health professional is likely to ask you a number of questions, including:
- How long have you been worried about your weight?
- Do you exercise? How often?
- What ways have you used to lose weight?
- Are you having any physical symptoms?
- Have you ever vomited because you were uncomfortably full?
- Have others expressed concern that you're too thin?
- Do you think about food often?
- Do you ever eat in secret?
- Have any of your family members ever had symptoms of an eating disorder or been diagnosed with an eating disorder?
Be ready to answer these questions to reserve time to go over any points you want to focus on.
- Sim LA (expert opinion). Mayo Clinic, Rochester, Minn. Jan. 31, 2018.
- Anorexia nervosa. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th ed. Arlington, Va.: American Psychiatric Association; 2013. http://dsm.psychiatryonline.org. Accessed Nov. 13, 2017.
- Hales RE, et al. Anorexia nervosa. In: The American Psychiatric Publishing Textbook of Psychiatry. 6th ed. Washington, D.C.: American Psychiatric Publishing; 2014. http://psychiatryonline.org. Accessed Nov. 13, 2017.
- Klein D, et al. Anorexia nervosa in adults: Clinical features, course of illness, assessment, and diagnosis. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Mehler P. Anorexia nervosa in adults and adolescents: Medical complications and their management. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Mehler P. Anorexia nervosa in adults: Evaluation for medical complications and criteria for hospitalization to manage these complications. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Pike K. Anorexia nervosa in adults: Cognitive behavioral therapy (CBT). https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Walsh BT. Anorexia nervosa in adults: Pharmacotherapy. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Anorexia nervosa. Merck Manual Professional Version. http://www.merckmanuals.com/professional/psychiatric-disorders/eating-disorders/anorexia-nervosa. Accessed Nov. 13, 2017.
- Harrington BC, et al. Initial evaluation, diagnosis, and treatment of anorexia nervosa and bulimia nervosa. American Family Physician. 2015;91:46.
- Brockmeyer T, et al. Advances in the treatment of anorexia nervosa: A review of established and emerging interventions. Psychological Medicine. In press. Accessed Nov. 13, 2017.
- Davis H, et al. Pharmacotherapy of eating disorders. Current Opinion in Psychiatry. 2017;30:452.
- Herpertz-Dahlmann B. Treatment of eating disorders in child and adolescent psychiatry. Current Opinion in Psychiatry. 2017;30:438.
- Fogarty S, et al. The role of complementary and alternative medicine in the treatment of eating disorders: A systematic review. Eating Behaviors. 2016;21:179.
- Eating disorders. National Alliance on Mental Illness. https://www.nami.org/Learn-More/Mental-Health-Conditions/Eating-Disorders/Overview. Accessed Nov. 13, 2017.
- Lebow J, et al. Is there clinical consensus in defining weight restoration for adolescents with anorexia nervosa? Eating Disorders. In press. Accessed Dec. 4, 2017.
- Lebow J, et al. The effect of atypical antipsychotic medications in individuals with anorexia nervosa: A systematic review and meta-analysis. International Journal of Eating Disorders. 2013;46:332.
- Five things to know about safety of dietary supplements for children and teens. National Center for Complementary and Integrative Health. https://nccih.nih.gov/health/tips/child-supplements. Accessed Feb. 9, 2018.
Associated Procedures
Products & services.
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Mayo Clinic in Rochester, Minnesota, has been recognized as one of the top Psychiatry hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Eating Disorder Treatment and Recovery
- Bulimia Nervosa: Signs, Symptoms, and Treatment
Helping Someone with an Eating Disorder
- Orthorexia Nervosa: Signs, Symptoms, and Treatment
Binge Eating Disorder
- Body Shaming: The Effects and How to Overcome it
- Cognitive Behavioral Therapy (CBT): What it is, How it Helps
Body Dysmorphic Disorder (BDD)
- Online Therapy: Is it Right for You?
- Mental Health
- Health & Wellness
- Children & Family
- Relationships
Are you or someone you know in crisis?
- Bipolar Disorder
- Eating Disorders
- Grief & Loss
- Personality Disorders
- PTSD & Trauma
- Schizophrenia
- Therapy & Medication
- Exercise & Fitness
- Healthy Eating
- Well-being & Happiness
- Weight Loss
- Work & Career
- Illness & Disability
- Heart Health
- Childhood Issues
- Learning Disabilities
- Family Caregiving
- Teen Issues
- Communication
- Emotional Intelligence
- Love & Friendship
- Domestic Abuse
- Healthy Aging
- Aging Issues
- Alzheimer’s Disease & Dementia
- Senior Housing
- End of Life
- Meet Our Team
What is anorexia nervosa?
Types of anorexia, am i anorexic, signs and symptoms of anorexia, anorexia causes and risk factors, effects of anorexia, getting help, anorexia treatment, tip 1: understand this is not really about weight or food, tip 2: learn to tolerate your feelings, tip 3: challenge damaging mindsets, tip 4: develop a healthier relationship with food, helping someone with anorexia, anorexia nervosa: symptoms, causes, and treatment.
Are you or a loved one struggling with anorexia? Explore the warning signs, symptoms, and causes of this serious eating disorder—as well as how to get the help you need.

Anorexia nervosa is a serious eating disorder characterized by a refusal to maintain a healthy body weight, an intense fear of gaining weight, and a distorted body image. Anorexia can result in unhealthy, often dangerous weight loss. In fact, the desire to lose weight may become more important than anything else. You may even lose the ability to see yourself as you truly are.
While it is most common among adolescent women, anorexia can affect women and men of all ages. You may try to lose weight by starving yourself, exercising excessively, or using laxatives, vomiting, or other methods to purge yourself after eating. Thoughts about dieting, food, and your body may take up most of your day—leaving little time for friends, family, and other activities you used to enjoy. Life becomes a relentless pursuit of thinness and intense weight loss. But no matter how skinny you become, it’s never enough.
The intense dread of gaining weight or disgust with how your body looks, can make eating and mealtimes very stressful. And yet, food and what you can and can’t eat is practically all you can think about.
But no matter how ingrained this self-destructive pattern seems, there is hope. With treatment, self-help, and support, you can break the self-destructive hold anorexia has over you, develop a more realistic body image, and regain your health and self-confidence.
There are three types of anorexia:
- Restricting type of anorexia is where your weight loss is achieved by restricting calories (following drastic diets, fasting, exercising to excess).
- Purging type of anorexia is where your weight loss is achieved by vomiting or using laxatives and diuretics.
- Atypical anorexia is where you have all the symptoms and dangerous obsessions of anorexia, except you’re not underweight (often due to your genetic makeup). Even though you may still be in a healthy weight range, your dieting or exercise habits put severe stress on your body.
Ask yourself the following questions:
- Do you feel fat even though people tell you you’re not?
- Are you terrified of gaining weight?
- Do you lie about how much you eat or hide your eating habits from others?
- Are your friends or family concerned about your weight loss, eating habits, or appearance?
- Do you diet, compulsively exercise, or purge when you’re feeling overwhelmed or bad about yourself?
- Do you feel powerful or in control when you go without food, over-exercise, or purge?
- Do you base your self-worth on your weight or body size?
Speak to a Licensed Therapist
BetterHelp is an online therapy service that matches you to licensed, accredited therapists who can help with depression, anxiety, relationships, and more. Take the assessment and get matched with a therapist in as little as 48 hours.
While people with anorexia often exhibit different habits, one constant is that living with anorexia means you’re constantly hiding those habits. This can make it hard at first for friends and family to spot the warning signs. When confronted, you might try to explain away your disordered eating and wave away concerns. But as anorexia progresses, people close to you won’t be able to deny their instincts that something is wrong—and neither should you. If eating and weight control your life, you don’t have to wait until your symptoms have progressed or your health is dangerously poor before seeking help.
Food behavior symptoms
Dieting despite being thin. Following a severely restricted diet. Eating only certain low-calorie foods. Banning “bad” foods such as carbohydrates and fats.
Obsession with calories, fat grams, and nutrition. Reading food labels, measuring and weighing portions, keeping a food diary, reading diet books.
Pretending to eat or lying about eating. Hiding, playing with, or throwing away food to avoid eating. Making excuses to get out of meals (“I had a huge lunch” or “My stomach isn’t feeling good”).
Preoccupation with food. Constantly thinking about food. Cooking for others, collecting recipes, reading food magazines, or making meal plans while eating very little.
Strange or secretive food rituals. Refusing to eat around others or in public places. Eating in rigid, ritualistic ways (e.g. cutting food “just so,” chewing food and spitting it out, using a specific plate).
Appearance and body image symptoms
Dramatic weight loss. Rapid, drastic weight loss with no medical cause.
Feeling fat, despite being underweight. You may feel overweight in general or just “too fat” in certain places, such as the stomach, hips, or thighs.
Fixation on body image. Obsessed with weight, body shape, or clothing size. Frequent weigh-ins and concern over tiny fluctuations in weight.
Harshly critical of appearance. Spending a lot of time in front of the mirror checking for flaws. There’s always something to criticize. You’re never thin enough.
[Read: Body Shaming: Causes, Effects, and Improving Your Body Image]
Denial that you’re too thin. You may deny that your low body weight is a problem, while trying to conceal it (drinking a lot of water before being weighed, wearing baggy or oversized clothes).
Purging symptoms
Using diet pills, laxatives, or diuretics. Abusing water pills, herbal appetite suppressants, prescription stimulants, ipecac syrup, and other drugs for weight loss.
Throwing up after eating. Frequently disappearing after meals or going to the bathroom. May run the water to disguise sounds of vomiting or reappear smelling like mouthwash or mints.
Compulsive exercising. Following a punishing exercise regimen aimed at burning calories. Exercising through injuries, illness, and bad weather. Working out extra hard after bingeing or eating something “bad.”
There are no simple answers to the causes of anorexia. Anorexia is a complex condition that arises from a combination of many social, emotional, and biological factors. Although our culture’s idealization of thinness plays a powerful role, there are many other contributing factors, including your family environment, emotional difficulties, low self-esteem, and traumatic experiences you may have gone through in the past.
Psychological causes . People with anorexia are often perfectionists and overachievers. They tend to be the “good” daughters and sons who do what they’re told, excel in everything they do, and focus on pleasing others. But while they may appear to have it all together, inside they feel helpless, inadequate, and worthless. Through their harshly critical lens, if they’re not perfect, they’re a total failure.
Family and social pressures . In addition to the cultural pressure to be thin, there are other family and social pressures that can contribute to anorexia. These include participation in an activity that demands slenderness, such as ballet, gymnastics, or modeling. It can also include having parents who are overly controlling, put a lot of emphasis on looks, diet themselves, or criticize their children’s bodies and appearance. Stressful life events—such as the onset of puberty, a breakup, or going away to school—can also trigger anorexia.
Biological causes . Research suggests that a genetic predisposition to anorexia may run in families. If a girl has a sibling with anorexia, she is 10 to 20 times more likely than the general population to develop anorexia herself. Brain chemistry also plays a significant role. People with anorexia tend to have high levels of cortisol, the brain hormone most related to stress, and decreased levels of serotonin and norepinephrine, which are associated with feelings of well-being.
Risk factors for anorexia
- Body dissatisfaction
- Strict dieting
- Low self-esteem
- Emotional difficulties
- Perfectionism
- Troubled family relationships
- History of physical or sexual abuse
- Other traumatic experiences
- Family history of eating disorders
While the causes of anorexia are uncertain, the physical effects are clear. When your body doesn’t get the fuel it needs to function normally, it goes into starvation mode and slows down to conserve energy. Essentially, your body begins to consume itself. If self-starvation continues and more body fat is lost, medical complications pile up and your body and mind pay the price.
Source: National Women’s Health Information Center
Deciding to get help for anorexia is not an easy choice to make. It’s not uncommon to feel like anorexia is part of your identity—or even your “friend.” You may think that anorexia has such a powerful hold over you that you’ll never be able to overcome it. But while change is hard, it is possible.
Admit you have a problem. Up until now, you’ve been invested in the idea that life will improve—that you’ll finally feel good—if you lose more weight. The first step in anorexia recovery is admitting that your relentless pursuit of thinness is out of your control and acknowledging the physical and emotional damage that you’ve suffered because of it.
Talk to someone. It can be hard to talk about what you’re going through, especially if you’ve kept your anorexia a secret for a long time. You may be ashamed, ambivalent, or afraid. But it’s important to understand that you’re not alone. Find a good listener—someone who will support you as you try to heal.
Stay away from people, places, and activities that trigger your obsession with being thin. You may need to avoid looking at fashion or fitness magazines, spend less time with friends who constantly diet and talk about losing weight, and stay away from weight loss websites and “pro-ana” sites that promote anorexia.
Seek treatment from trained eating disorder professionals.
Treating anorexia involves three steps:
- Getting back to a healthy weight.
- Starting to eat more food.
- Changing how you think about yourself and food.
[Read: Eating Disorder Treatment and Recovery]
Medical treatment . The first priority in anorexia treatment is addressing and stabilizing any serious health issues. Hospitalization may be necessary if you are dangerously malnourished or so distressed that you no longer want to live. You may also need to be hospitalized until you reach a less critical weight. Outpatient treatment is an option when you’re not in immediate medical danger.
Nutritional treatment . A second component of anorexia treatment is nutritional counseling. A nutritionist or dietician will teach you about healthy eating and proper nutrition. The nutritionist will also help you develop and follow meal plans that include enough calories to reach or maintain a normal, healthy weight.
Therapy . Therapy is crucial to anorexia treatment. Its goal is to identify the negative thoughts and feelings that fuel your eating disorder and replace them with healthier, less distorted beliefs. Therapy can also help you deal with difficult emotions, relationship problems, and stress in a productive, rather than a self-destructive, way.
Along with professional treatment, the following tips can guide you on the road to recovery:
The food and weight-related issues are in fact symptoms of a deeper issue: depression, anxiety, loneliness, insecurity, pressure to be perfect, or feeling out of control. Problems that no amount of dieting or weight loss can cure.
The difference between dieting and anorexia
In order to overcome anorexia, you first need to understand that it meets a need in your life. For example, maybe you feel powerless in many parts of your life, but you can control what you eat. Saying “no” to food, getting the best of hunger, and controlling the number on the scale may make you feel strong and successful—at least for a short while. You may even come to enjoy your hunger pangs as reminders of a “special talent” that most people don’t possess.
Anorexia may also be a way of distracting yourself from difficult emotions. When you spend most of your time thinking about food, dieting, and weight loss, you don’t have to face other problems in your life or deal with complicated emotions. Restricting food may provide an emotional numbness, anesthetizing you from feelings of anxiety, sadness, or anger, perhaps even replacing those emotions with a sense of calm or safety.
Unfortunately, any boost you get from starving yourself or shedding pounds is extremely short-lived—and at some point, it will stop working for you at all. Dieting and weight loss can’t repair the negative self-image at the heart of anorexia. The only way to do that is to identify the emotional need that self-starvation fulfills and find other ways to meet it.
“I feel fat”
While your weight usually remains quite constant over the course of, say, a week, feelings of fatness can fluctuate wildly. Often, feeling fat is a mislabeling of other emotions, such as shame, boredom, frustration, or sadness. In other words, “I feel fat” really means “I feel anxious,” or “I feel lonely.” And those feelings are unlikely to ever be changed by a diet.
Identifying the underlying issues that drive your eating disorder is the first step toward recovery, but insight alone is not enough. Let’s say, for example, that following restrictive food rules makes you feel safe and powerful. When you take that coping mechanism away, you will be confronted with the feelings of fear and helplessness your anorexia helped you avoid.
Reconnecting with your feelings can be extremely uncomfortable. It’s why you may feel worse at the beginning of your recovery. But the answer isn’t to return to the destructive eating habits you previously used to distract yourself; it’s to learn how to accept and tolerate all of your feelings—even the negative ones.
Using mindfulness to cope with difficult emotions
When you start to feel overwhelmed by negativity, discomfort, or the urge to restrict food, take a moment to stop whatever you’re doing and investigate what’s going on inside.
Identify the emotion you’re feeling. Is it guilt? Shame? Helplessness? Loneliness? Anxiety? Disappointment? Fear? Insecurity?
Accept the experience you’re having. Avoidance and resistance only make negative emotions stronger. Instead, try to accept what you’re feeling without judging yourself.
Dig deeper. Where do you feel the emotion in your body? What kinds of thoughts are going through your head?
Distance yourself. Realize that you are NOT your feelings. Emotions are passing events, like clouds moving across the sky. They don’t define who you are.
Once you learn how to accept and tolerate your feelings, they’ll no longer seem so scary. You’ll realize that you’re still in control and that negative emotions are only temporary. Once you stop fighting them, they’ll quickly pass.
For a step-by-step guide to learning how to manage stress and uncomfortable emotions, check out HelpGuide’s free Emotional Intelligence Toolkit .
New ways to find emotional fulfillment
Once you understand the link between your emotions and your disordered eating patterns—and can identify your triggers—you still need to find alternatives to dieting that you can turn to for emotional fulfillment. For example:
If you’re depressed or lonely, call someone who always makes you feel better, schedule time with family or friends, watch a comedy show, or play with a dog or cat.
If you’re anxious, expend your nervous energy by dancing to your favorite music, squeezing a stress ball, or taking a brisk walk or bike ride.
If you’re exhausted, treat yourself with a hot cup of tea, go for a walk, take a bath, or light some scented candles.
If you’re bored, read a good book, explore the outdoors, visit a museum, or turn to a hobby you enjoy (playing the guitar, knitting, shooting hoops, scrapbooking, etc.).
People with anorexia are often perfectionists and overachievers. They’re the “good” daughters and sons who do what they’re told, try to excel in everything they do, and focus on pleasing others. But while they may appear to have it all together, inside they feel helpless, inadequate, and worthless.
If that sounds familiar to you, here’s the good news: these feelings don’t reflect reality. They’re fueled by irrational, self-sabotaging ways of thinking that you can learn to overcome.
Damaging mindsets that fuel anorexia
All-or-nothing thinking. Through this harshly critical lens, if you’re not perfect, you’re a total failure. You have a hard time seeing shades of gray, at least when it comes to yourself.
Emotional reasoning. You believe if you feel a certain way, it must be true. “I feel fat” means “I am fat.” “I feel hopeless” means you’ll never get better.
Musts, must-nots, and have-tos . You hold yourself to a rigid set of rules ( “I must not eat more than x number of calories , “ “I have to get straight A’s,” “ I must always be in control.” etc.) and beat yourself up if you break them.
Labeling. You call yourself names based on mistakes and perceived shortcomings. “I’m unhappy with how I look” becomes “I’m disgusting.” Slipping up becomes “I’m a “failure.”
Catastrophizing. You jump to the worst-case scenario. If you backslide in recovery, for example, you assume that there’s no hope you’ll ever get better.
Put your thoughts on the witness stand
Once you identify the destructive thoughts patterns that you default to, you can start to challenge them with questions such as:
- “What’s the evidence that this thought is true? Not true?”
- “What would I tell a friend who had this thought?”
- “Is there another way of looking at the situation or an alternate explanation?”
- “How might I look at this situation if I didn’t have anorexia?”
As you cross-examine your negative thoughts, you may be surprised at how quickly they crumble. In the process, you’ll develop a more balanced perspective.
Even though anorexia isn’t fundamentally about food, over time you’ve developed harmful food habits that can be tough to break. Developing a healthier relationship with food entails:
- Getting back to a healthy weight
- Starting to eat more food
- Changing how you think about yourself and food
Let go of rigid food rules. While following rigid rules may help you feel in control, it’s a temporary illusion. The truth is that these rules are controlling you, not the other way around. In order to get better, you’ll need to let go. This is a big change that will feel scary at first, but day by day, it will get easier.
Get back in touch with your body. If you have anorexia, you’ve learned to ignore your body’s hunger and fullness signals. You may not even recognize them anymore. The goal is to get back in touch with these internal cues, so you can eat based on your physiological needs.
Allow yourself to eat all foods. Instead of putting certain food off limits, eat whatever you want, but pay attention to how you feel physically after eating different foods. Ideally, what you eat should leave you feeling satisfied and energized.
Get rid of your scale. Instead of focusing on weight as a measurement of self-worth, focus on how you feel. Make health and vitality your goal, not a number on the scale.
Develop a healthy meal plan. If you need to gain weight, a nutritionist or dietician can help you develop a healthy meal plan that includes enough calories to get you back to a normal weight. While you can do this on your own, you’re probably out of touch with what a normal meal or serving size looks like.
Getting past your fear of gaining weight
Getting back to a normal weight is no easy task. The thought of gaining weight is probably extremely frightening, and you may be tempted to resist.
But this fear is a symptom of your anorexia. Reading about anorexia or talking to other people who have lived with it can help. It also helps to be honest about your feelings and fears. The better your family and treatment team understand what you’re going through, the better support you’ll receive.
Having anorexia can distort the way your loved one thinks—about their body, the world around them, even your motivations for trying to help. Add to that the defensiveness and denial involved in anorexia and you’ll need to tread lightly.
Waving around articles about the dire effects of anorexia or declaring, “you’ll die if you don’t eat!” probably won’t work. A better approach is to gently express your concerns and let the person know that you’re available to listen. If your loved one is willing to talk, listen without judgment, no matter how out of touch the person sounds.
[Read: Helping Someone with an Eating Disorder]
Think of yourself as an “outsider.” As someone not suffering from anorexia, there isn’t a lot you can do to “solve” your loved one’s condition. It is ultimately their choice to decide when they are ready.
Encourage your loved one to get help. The longer an eating disorder remains undiagnosed and untreated, the harder it is on the body and the more difficult it is to overcome.
Seek advice from a health professional, even if your friend or family member won’t. And you can bring others—from peers to parents—into the circle of support.
Be a role model for healthy eating, exercising, and body image. Don’t make negative comments about your own body or anyone else’s.
Don’t act like the food police. A person with anorexia needs compassion and support, not an authority figure standing over the table with a calorie counter.
Avoid threats, scare tactics, angry outbursts, and put-downs. Anorexia is often a symptom of extreme emotional distress and develops out of an attempt to manage emotional pain, stress, and/or self-hate. Negative communication will only make it worse.
Hotlines and support
National Eating Disorders Association or call 1-800-931-2237 (National Eating Disorders Association)
Beat Eating Disorders or call 0345 643 1414 (Helpfinder)
Butterfly Foundation for Eating Disorders or call 1800 33 4673 (National Eating Disorders Collaboration)
Service Provider Directory or call 1-866-633-4220 (NEDIC)
More Information
- Almost Anorexic – Is My (or My Loved One’s) Relationship with Food a Problem? - (Harvard Health Books)
- Treatment - Tips on eating disorder treatment. (National Eating Disorders Association)
- Anorexia nervosa - FAQs on anorexia and its treatment. (Office on Women’s Health)
- Anorexia Nervosa - Includes risk factors such as body image, self esteem, and perfectionism. (Eating Disorders Victoria)
- Feeding and Eating Disorders. (2013). In Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association. Link
- Anorexia nervosa | Office on Women’s Health. (n.d.). Retrieved July 27, 2022, from Link
- Anorexia Nervosa—Psychiatric Disorders—Merck Manuals Professional Edition. (n.d.). Retrieved July 27, 2022, from Link
- Anorexia Nervosa—StatPearls—NCBI Bookshelf. (n.d.). Retrieved July 27, 2022, from Link
- Lloyd, S., Yiend, J., Schmidt, U., & Tchanturia, K. (2014). Perfectionism in Anorexia Nervosa: Novel Performance Based Evidence. PLoS ONE, 9 (10), e111697. Link
- Moskowitz, Lindsay, and Eric Weiselberg. “Anorexia Nervosa/Atypical Anorexia Nervosa.” Current Problems in Pediatric and Adolescent Health Care 47, no. 4 (April 1, 2017): 70–84. Link
- Harrington, Brian C., Michelle Jimerson, Christina Haxton, and David C. Jimerson. “Initial Evaluation, Diagnosis, and Treatment of Anorexia Nervosa and Bulimia Nervosa.” American Family Physician 91, no. 1 (January 1, 2015): 46–52. Link
- National Eating Disorders Association. “Anorexia Nervosa,” March 31, 2023. Link
- Tozzi, Federica, Patrick F. Sullivan, Jennifer L. Fear, Jan McKenzie, and Cynthia M. Bulik. “Causes and Recovery in Anorexia Nervosa: The Patient’s Perspective.” International Journal of Eating Disorders 33, no. 2 (2003): 143–54. Link
- Woerwag-Mehta, Sabine, and Janet Treasure. “Causes of Anorexia Nervosa.” Psychiatry , Eating disorders, 7, no. 4 (April 1, 2008): 147–51. Link
- Fairburn, C. G., Z. Cooper, H. A. Doll, and S. L. Welch. “Risk Factors for Anorexia Nervosa: Three Integrated Case-Control Comparisons.” Archives of General Psychiatry 56, no. 5 (May 1999): 468–76. Link
- Zipfel, Stephan, Katrin E Giel, Cynthia M Bulik, Phillipa Hay, and Ulrike Schmidt. “Anorexia Nervosa: Aetiology, Assessment, and Treatment.” The Lancet Psychiatry 2, no. 12 (December 1, 2015): 1099–1111. Link
More in Eating Disorders
How to overcome your eating disorder and gain true self-confidence

Bulimia Nervosa
Signs, symptoms, treatment and self-help tips

Advice for parents, family members and friends offering support

Orthorexia Nervosa
How to recognize if your healthy eating has gone too far

Symptoms, treatment and help for compulsive overeating

Body Shaming
Improving your body image and achieving body acceptance

Cognitive Behavioral Therapy (CBT)
How it can help with anxiety, depression, PTSD, substance abuse, and more

Always focusing on your physical flaws? You may have BDD.
Professional therapy, done online
BetterHelp makes starting therapy easy. Take the assessment and get matched with a professional, licensed therapist.
Help us help others
Millions of readers rely on HelpGuide.org for free, evidence-based resources to understand and navigate mental health challenges. Please donate today to help us save, support, and change lives.
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- April 01, 2024 | VOL. 181, NO. 4 CURRENT ISSUE pp.255-346
- March 01, 2024 | VOL. 181, NO. 3 pp.171-254
- February 01, 2024 | VOL. 181, NO. 2 pp.83-170
- January 01, 2024 | VOL. 181, NO. 1 pp.1-82
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Anorexia Nervosa
- Evelyn Attia M.D.
- B. Timothy Walsh M.D.
Search for more papers by this author
At the suggestion of her pediatrician, “Rachel,” a 19-year-old college freshman at a competitive liberal arts college, was brought by her parents for psychiatric evaluation during spring break. According to her parents, Rachel had lost 16 lb since her precollege physical the previous August, falling to a weight of 104 lb at a height of 5 feet, 5 inches. Rachel’s chief complaint was that “everyone thinks I have an eating disorder.” She explained that she had been a successful student and field hockey player in high school. Having decided not to play field hockey in college, she began running several mornings each week during the summer and “cut out junk food” to protect herself from gaining “that freshman 10.” Rachel lost a few pounds that summer and received compliments from friends and family for looking so “fit.” She reported feeling more confident and ready for college than she had expected as the summer drew to a close. Once she began school, Rachel increased her running to daily, often skipped breakfast in order to get to class on time, and selected from the salad bar for her lunch and dinner. She worked hard in school, made the dean’s list the first semester, and announced to her family that she had decided to pursue a premed program. When Rachel returned home for Christmas vacation, her family noticed that she looked thin and tired. Despite encouragement to catch up on rest, she awoke early each morning to maintain her running schedule. She displayed a newfound interest in cooking and spent much of the day planning, shopping, and preparing dinner for her family. Rachel returned to school in January and thought she might be developing depression. Courses seemed less interesting, and she wondered whether the college she attended was right for her after all. She was sleeping less well and felt cold much of the day. Rachel’s parents asked her to step on the bathroom scale the night she returned home for spring break. Rachel was surprised to learn that her weight had fallen to 104 lb, and she agreed to a visit to her pediatrician, who found no evidence of a general medical illness and recommended a psychiatric consultation. Does Rachel have anorexia nervosa? If so, how should she be treated?
Anorexia nervosa is a serious mental illness characterized by the maintenance of an inappropriately low body weight, a relentless pursuit of thinness, and distorted cognitions about body shape and weight. Anorexia nervosa commonly begins during middle to late adolescence, although onsets in both prepubertal children and older adults have been described. Anorexia nervosa has a mortality rate as high as that seen in any psychiatric illness (1) and is associated with physiological alterations in virtually every organ system, although routine laboratory test results are often normal and physical examination may reveal only marked thinness.
Current Definition
DSM-IV (2) lists four criteria for the diagnosis of anorexia nervosa:
1. Refusal to maintain body weight at or above a minimally normal weight for age and height
2. Intense fear of gaining weight or becoming fat, even though underweight
3. Disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or denial of the seriousness of the current low body weight
4. In postmenarchal females, amenorrhea (i.e., the absence of at least three consecutive menstrual cycles)
DSM-IV describes two subtypes of anorexia nervosa—the restricting subtype, consisting of those individuals whose eating behavior is characterized by restriction of type and quantity of food without binge eating or purging behaviors, and the binge-purge subtype, consisting of those who also exhibit binge eating and/or purging behaviors, such as vomiting or misuse of laxatives.
Diagnostic Challenges
The DSM-IV criteria are most easily applied when patients are both sufficiently ill to fulfill all four diagnostic criteria and able to describe their ideation and behavior accurately. However, because ambivalence and denial frequently lead those with anorexia nervosa to minimize their symptoms, the clinician must make inferences about mental state and behavior.
An additional problem in diagnosis is that many individuals meet some but not all of the formal diagnostic criteria. For example, some women who meet all other criteria for anorexia nervosa continue to report some spontaneous menstrual activity. In a community-based sample of 84 female patients with full- or partial-syndrome anorexia nervosa, those with amenorrhea were not statistically different from those without across a number of clinical variables (3) , which raises questions about the utility of this diagnostic criterion (4 , 5) .
Differential Diagnosis
Proper diagnosis of any condition that includes low weight and restrictive eating must include consideration of other psychiatric and medical conditions that include these problems. Psychotic disorders, including schizophrenia and schizoaffective and delusional disorders, as well as anxiety disorders, such as obsessive-compulsive disorder, can include symptoms of food avoidance and distorted beliefs about one’s body. Medical conditions, including endocrine disturbances (such as thyroid disease and diabetes mellitus), gastrointestinal disturbances (such as inflammatory bowel and celiac disease), infections (such as hepatitis), and neoplastic processes may present with weight loss and should be considered when evaluating a patient for a possible eating disorder.
Anorexia nervosa has been recognized for centuries. Sir William Gull coined the term anorexia nervosa in 1873, but Richard Morton likely offered the first medical description of the condition in 1689 (6 , 7) . Despite its long-standing recognition, remarkably little is known about the etiology of, and effective treatment for, anorexia nervosa. A 2002 review in the American Journal of Psychiatry concluded that little progress was made during the second half of the 20th century in understanding the etiology, prognosis, or treatment of the disorder (8) .
Epidemiology
Prevalence rates for anorexia nervosa are generally described as ranging from 0.5% to 1.0% among females (9 , 10) , with males being affected about one-tenth as frequently (10 , 11) . A recent study describing a large population-based cohort of Swedish twins born between 1935 and 1958 found the overall prevalence of anorexia nervosa among the 31,406 study participants to be 1.20% and 0.29% for females and males, respectively; the prevalence of anorexia nervosa in both sexes was greater among those born after 1945 (12) .

Risk Factors
The identification of risk factors for anorexia nervosa is challenging because the low incidence of the disorder makes the conduct of prospective studies of sufficient size very difficult. A variety of possible risk factors have been identified, including early feeding difficulties, symptoms of anxiety, perfectionistic traits, and parenting style, but none can be considered to have been conclusively demonstrated (13 , 14) . Similarly, cultural factors undoubtedly play some role in the development of anorexia nervosa, although the disorder’s long history and its presence in regions around the globe (15 – 18) suggest that factors other than culture provide central contributions to the development of the disorder. In fact, one review that considers historical reports of eating disorders, data regarding changing incidence rates of eating disorders over time, and the prevalence of eating disorders in non-Western cultures concludes that anorexia nervosa is not a culture-bound syndrome (19) . Genetic factors are increasingly accepted as important contributors to the risk of anorexia nervosa. Twin studies of eating disorders have consistently found that a significant fraction of the variability in the occurrence of anorexia nervosa can be attributed to genetic factors, with heritability estimates ranging from 33% to 84% (20) .
Course of Illness
The course of anorexia nervosa is highly variable, with individual outcomes ranging from full recovery to a chronic and severe psychosocial disability accompanied by physical complications and death. Intervention early in the course of illness and full weight restoration appear to be associated with the best outcomes. Adolescent patients have a better prognosis than do adults. One-year relapse rates after initial weight restoration approach 50% (21) . Intermediate and long-term follow-up studies examining clinical samples find that while a significant fraction of patients achieve full psychological and physical recovery, at least 20% continue to meet full criteria for anorexia nervosa on follow-up assessment, with many others reporting significant residual eating disorder symptoms, even if they do not meet full criteria for anorexia nervosa (22) .
Physiological Disturbances
A multitude of biological disturbances may occur in underweight patients, but most appear to be normal physiological responses to starvation. Clinically significant abnormalities may develop in the cardiovascular, gastrointestinal, reproductive, and fluid and electrolyte systems (23) . These abnormalities usually do not require specific treatment beyond refeeding, and they return to normal on weight restoration. A worrisome possible exception is reduced bone density; since peak bone density is normally achieved during young adulthood, a prolonged episode of anorexia nervosa during this development stage may have a long-term impact on the risk of osteoporosis.
Neurobiological Hypotheses
The striking physical and behavioral characteristics of anorexia nervosa have prompted the development of a variety of neurobiological hypotheses over the years. Recently, results of several investigations have suggested that abnormalities in CNS serotonin function may play a role in the development and persistence of the disorder (24 , 25) . Notably, studies of long-term weight-recovered patients have described indications of increased serotonin activity, such as elevated levels of the serotonin metabolite 5-hydroxyindoleacetic acid in the CSF (26) and reduced binding potential of 5-HT 2A receptors, suggestive of higher levels of circulating CNS serotonin, in several brain regions (27) .
Kaye and colleagues (28) hypothesize that individuals with anorexia nervosa may have a trait disturbance characterized by high levels of CNS serotoninergic activity leading to symptoms of anxiety that are relieved by dieting, which leads to a reduction in serotonin production. However, this provocative hypothesis is based on assessments conducted after the onset of illness, which therefore cannot distinguish a predisposing trait from a long-lasting consequence of anorexia nervosa.
Another recent line of inquiry into the biological underpinnings of anorexia nervosa focuses on the perfectionistic and rigid behavioral style, including repetitive and stereotyped behaviors, characteristic of the syndrome. Investigators have hypothesized that these behaviors may result from a propensity to extreme fear conditioning and resistance to fear extinction (29) , suggesting that abnormalities may be present in limbic structures known to be involved in the acquisition of conditioned fear behavior. Other investigators have proposed that difficulties of individuals with anorexia nervosa in changing maladaptive behavior may relate to problems with set shifting, a function mediated by corticostriatothalamocortical neural circuits (30 , 31) .
Engaging a patient with anorexia nervosa to participate fully in the psychiatric evaluation may present a greater challenge than would be the case for patients with other disorders, including other eating disorders such as bulimia nervosa or binge eating disorder. Patients with anorexia nervosa often present for evaluation not because of their own interest in symptom relief but because of the concerns of family, friends, or health care providers. It may be necessary to obtain additional information from family members or others who know the patient well.
In addition, during the evaluation, it may be helpful to identify symptoms of the illness that are most likely to be ego-dystonic for the particular patient. Patients commonly minimize their concerns about low weight, but they may be more concerned, and therefore more likely to participate in the evaluation, if they recognize poor concentration, increased irritability, low bone density, hair loss, or feeling cold as developments associated with their restrictive eating pattern.
Medical issues should be reviewed, including weight and menstrual history. A complete review of systems is indicated, as anorexia nervosa can manifest a multitude of disturbances, including cardiovascular symptoms (e.g., bradycardia and other arrhythmias, including QTc prolongation, and hypotension), gastrointestinal symptoms (e.g., slow motility, esophageal inflammation associated with purging), endocrinologic symptoms (low estrogen in females, low testosterone in males, osteopenia, and osteoporosis), and dermatologic changes, such as the development of a layer of fine hair (lanugo) on the face and extremities.
The evaluation should include specific questions about eating behaviors, including the number and content of all meals and snacks on a recent day. The clinician should inquire about 1) restricting behaviors, including limiting permissible foods, as well as decreasing caloric amounts; 2) binge eating; 3) purging behaviors, including vomiting and misuse of laxatives and diuretics; and 4) exercise and hyperactive behaviors, including preferential walking and standing.
Given patients’ reluctance to endorse all of the diagnostic symptoms of anorexia nervosa on first meeting, the clinician may do well to identify the problem as “low weight” and explain that the treatment needs to include weight restoration, whether or not the patient meets full criteria for anorexia nervosa. Patients and their families are generally very interested in data from the World War II Minnesota study of semistarvation that documented the association between starvation and the development of psychological symptoms frequently identified with anorexia nervosa, such as depression, anxiety, obsessionality about food, and rigidity about eating behaviors (32) . The clinician may have better results engaging the patient with the identification of symptoms that are commonly associated with the state of starvation and that the patient has likely found troubling (such as thinking constantly about food) and therefore worth resolving.
Treatment Guidelines
All current treatment guidelines for anorexia nervosa emphasize weight restoration. There is no clearly defined algorithm for how to accomplish this goal, although common practice includes the selection of the least restrictive treatment setting that is likely to be effective. The APA practice guideline on treatment of eating disorders suggests that highly structured treatments are often needed to achieve weight gain for patients at weights <85% ideal body weight (33) . Hospital-based treatments may be used when weight is significantly low (e.g., <75% of ideal body weight) or when there has been rapid weight loss or medical signs of malnutrition, including significant bradycardia, hypotension, hypothermia, and so on.
Generally, outpatient treatments rely on a team of professionals. Medical monitoring, including weight and laboratory assessment, may be provided by an internist or pediatrician; psychological support is offered by a psychiatrist or other therapist; and nutritional counseling from a dietitian or nutritionist is often included. The team is generally led by the medical or psychiatric clinician—typically the one with the greatest expertise in the management of eating disorders.
Effective treatments generally assess outcome by weight and behavioral change. Nonspecific support needs to be paired with expectation of progress in measurable medical, behavioral, and psychological symptoms. Weight restoration is generally associated with improvement in a variety of psychological areas, including mood and anxiety symptoms (34 , 35) . In contrast, psychological improvement without accompanying changes in weight and eating behavior is of limited value. Patients and families should be informed about the physiology of weight gain, including the substantial number of calories required daily.
A family-based outpatient treatment for anorexia nervosa, also called the “Maudsley method,” may be helpful for younger patients (36) . This approach empowers the parents of a patient with anorexia nervosa to refeed their child, renegotiate the relationship between child and parents to involve issues other than food, and help their child resume normal adolescent development without an eating disorder. Several preliminary studies have shown promising results for family therapy with adolescent patients (37 , 38) .
For patients with anorexia nervosa who do not respond to outpatient treatments or those who do not have specialized outpatient treatments available in their vicinity, more structured treatments such as inpatient or partial hospital (day treatment) programs may be necessary. Structured treatments generally include observation during and after meals together with a consistently applied behavioral program that reinforces weight gain and normal eating behaviors. In recent years, the length of hospital stay for anorexia nervosa has decreased substantially because of economic limitations imposed by third-party payers; nonetheless, hospital programs can achieve a rate of weight gain of 2–4 pounds per week during active treatment (39) .
Controlled Treatment Trials
While structured settings have been used successfully for weight restoration treatments, there is little empirical support for a specific level of care or a particular psychosocial treatment for anorexia nervosa. As mentioned, a family-based approach appears promising for children and adolescents with anorexia nervosa; family therapy has been reported to be superior to individual therapy in two randomized controlled trials for adolescents with anorexia nervosa (40 , 41) . For adults with anorexia nervosa, a small study by Pike and colleagues (42) found cognitive behavior therapy superior to nutritional counseling in preventing relapse after hospital-based weight restoration. A recent study by McIntosh et al. (43) provocatively suggested that a patient-centered nonspecific supportive therapy may have been more helpful than cognitive behavior therapy or interpersonal therapy, as measured by a global rating of anorexia nervosa symptoms, in a sample of 56 underweight women with anorexia nervosa receiving treatment over a minimum of 20 weeks; unfortunately, the amount of weight gain was modest and not significantly different among the three study treatments.
Randomized controlled trials of medications for patients with anorexia nervosa have consistently reported disappointing results. Several psychopharmacologic agents have been studied, without identification of clear benefit, although studies have been limited by small sample sizes and the fact that most of the trials have been conducted in hospital settings where other treatment interventions are offered in addition to study medication (44) . While it has been suggested that psychotropic medications are rendered ineffective in underweight patients by the biological impact of starvation, a recent study comparing fluoxetine and placebo in weight-restored patients notably found no significant benefit to medication during the year following nutritional rehabilitation (45) .
Summary and Recommendations
Although recognized for centuries, anorexia nervosa remains enigmatic, often difficult to treat, and potentially lethal. The current approach to treatment includes careful medical assessment, ongoing medical and weight monitoring, and behaviorally oriented treatment aimed at normalizing weight and eating behaviors. Family-based treatment appears promising for younger patients.
With Rachel, the patient in the vignette, her typical presentation, her low weight (corresponding to a body mass index of 17.3), and her reluctance to restore her weight to its previously healthy level led the evaluating psychiatrist to conclude that Rachel indeed had anorexia nervosa. The psychiatrist recommended that Rachel attempt outpatient treatment but explained to her and her family that many patients require more structured settings for successful weight restoration. The psychiatrist recommended that Rachel see an eating disorder specialist knowledgeable about the characteristics of anorexia nervosa and experienced in dealing with the challenges of its treatment. The outpatient treatment plan included weekly psychotherapy sessions, along with regular visits with her pediatrician and a nutritionist. Although Rachel had complained of “depression,” the psychiatrist elected not to prescribe antidepressant medication, as there is no evidence of its utility in anorexia nervosa, and weight gain in this disorder is known to lead to improvement in mood. In the meetings with Rachel, the psychiatrist used cognitive behavior therapy techniques to help her in reevaluating her assumptions that low weight was somehow essential to her sense of self-worth. Treatment outcome was assessed by changes in weight and eating behavior. Rachel’s family participated by helping to supervise meals at the start of treatment and offering her more autonomy around eating as she made progress. Rachel was asked to gain weight at a rate of >1 lb per week and knew that failure to meet this goal would lead to transfer of treatment to a more structured setting. Rachel reached and maintained her premorbid weight and was able to return to school 6 months after initial presentation.
Received July 19, 2007; accepted Aug. 6, 2007 (doi: 10.1176/appi.ajp.2007.07071151). From the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York; and the Eating Disorders Research Unit, New York State Psychiatric Institute, New York. Address correspondence and reprint requests to Dr. Attia, New York State Psychiatric Institute, 1051 Riverside Dr., Unit 98, New York, NY 10032; [email protected] (e-mail).
CME Disclosure: Dr. Attia has received research support from Pfizer and Eli Lilly. Dr. Walsh has received research support from Abbott Pharmaceuticals.
APA policy requires disclosure by CME authors of unapproved or investigational use of products discussed in CME programs. Off-label use of medications by individual physicians is permitted and common. Decisions about off-label use can be guided by scientific literature and clinical experience.
1. Sullivan PF: Mortality in anorexia nervosa. Am J Psychiatry 1995; 152:1073–1074 Google Scholar
2. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994 Google Scholar
3. Garfinkel PE, Lin E, Goering P, Spegg C, Goldbloom D, Kennedy S, Kaplan AS, Woodside DB: Should amenorrhea be necessary for the diagnosis of anorexia nervosa? evidence from a Canadian community sample. Br J Psychiatry 1996; 168:500–506 Google Scholar
4. Cachelin F, Maher B: Is amenorrhea a critical criterion for anorexia nervosa? J Psychosom Res 1998;44:435–440 Google Scholar
5. Watson T, Andersen A: A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175–182 Google Scholar
6. Pearce JM: Richard Morton: origins of anorexia nervosa. Eur Neurol 2004; 52:191–192 Google Scholar
7. Silverman JA: Sir William Gull (1819–1890): limner of anorexia nervosa and myxoedema: an historical essay and encomium. Eat Weight Disord 1997; 2:111–116 Google Scholar
8. Steinhausen H-C: The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 2002; 159:1284–1293 Google Scholar
9. Hoek HW, van Hoeken D: Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003; 34:383–396 Google Scholar
10. Hudson JI, Hiripi E, Pope HG Jr, Kessler RC: The prevalence and correlates of eating disorders in the National Comorbidity Survey replication. Biol Psychiatry 2007; 61:348–358 Google Scholar
11. Lucas AR, Beard CM, O’Fallon WM, Kurland LT: 50-year trends in the incidence of anorexia nervosa in Rochester, Minn: a population-based study. Am J Psychiatry 1991; 148:917–922 Google Scholar
12. Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL: Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry 2006; 63:305–312 Google Scholar
13. Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WC: Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy: Psychol Bull 2004; 130:19–65 Google Scholar
14. Striegel-Moore RH, Bulik CM: Risk factors for eating disorders. Am Psychologist 2007; 62:181–198 Google Scholar
15. Lee HY, Lee EL, Pathy P, Chan YH: Anorexia nervosa in Singapore: an eight-year retrospective study. Singapore Med J 2005; 46:275–281 Google Scholar
16. Njenga FG, Kangethe RN: Anorexia nervosa in Kenya. East Afr Med J 2004; 81:188–193 Google Scholar
17. Bennett D, Sharpe M, Freeman C, Carson A: Anorexia nervosa among female secondary school students in Ghana. Br J Psychiatry 2004; 185:312–317 Google Scholar
18. Hoek HW, van Harten PN, Hermans KME, Katzman MA, Matroos GE, Susser ES: The incidence of anorexia nervosa on Curaçao. Am J Psychiatry 2005; 162:748–752 Google Scholar
19. Keel PK, Klump KL: Are eating disorders culture-bound syndromes? implications for conceptualizing their etiology. Psychol Bull 2003; 129:747–769 Google Scholar
20. Bulik CM: Exploring the gene-environment nexus in eating disorders. J Psychiatry Neurosci 2005; 30:335–339 Google Scholar
21. Pike KM: Long-term course of anorexia nervosa: response, relapse, remission, and recovery. Clin Psychol Rev 1998; 18:447–475 Google Scholar
22. Fisher M: The course and outcome of eating disorders in adults and in adolescents: a review. Adolesc Med 2003; 14:149–158 Google Scholar
23. Walsh BT: Eating disorders, in Harrison’s Principles of Internal Medicine, 17th ed. Edited by Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. New York, McGraw-Hill (in press) Google Scholar
24. Kaye WH, Frank GK, Bailer UF, Henry SE: Neurobiology of anorexia nervosa: clinical implications of alterations of the function of serotonin and other neuronal systems. Int J Eat Disord 2005; 37(suppl):S15–S19 Google Scholar
25. Attia E, Wolk S, Cooper T, Glasofer D, Walsh BT: Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biol Psychiatry 2005; 57:674–678 Google Scholar
26. Kaye WH, Gwirtsman HE, George DT, Ebert MH: Altered serotonin activity in anorexia nervosa after long-term weight restoration: does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch Gen Psychiatry 1991; 48:556–562 Google Scholar
27. Frank GK, Kaye WH, Meltzer CC, Price JC, Greer P, McConaha C, Skovira K: Reduced 5-HT 2A receptor binding after recovery from anorexia nervosa. Biol Psychiatry 2002; 52:896–906 Google Scholar
28. Kaye WH, Barbarich NC, Putnam K, Gendall KA, Fernstrom J, Fernstrom M, McConaha CW, Kishore A: Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord 2003; 33:257–267 Google Scholar
29. Strober M: Pathologic fear conditioning and anorexia nervosa: on the search for novel paradigms. Int J Eat Disord 2004; 35:504–508 Google Scholar
30. Holliday J, Tchanturia K, Landau S, Collier D, Treasure J: Is impaired set-shifting an endophenotype of anorexia nervosa? Am J Psychiatry 2005; 162:2269–2275 Google Scholar
31. Steinglass J, Walsh BT, Stern Y: Set shifting deficit in anorexia nervosa. J Int Neuropsychol Soc 2006; 12:431–435 Google Scholar
32. Schiele BC, Brozek J: “Experimental neurosis” resulting from semistarvation in man. Psychosom Med 1948; 10:31–50 Google Scholar
33. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Eating Disorders, 3rd ed. Am J Psychiatry 2006; 163(Jul suppl) Google Scholar
34. Meehan KG, Loeb KL, Roberto CA, Attia E: Mood change during weight restoration in patients with anorexia nervosa. Int J Eat Disord 2006; 39:587–589 Google Scholar
35. Attia E, Haiman C, Walsh BT, Flater SR: Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry 1998; 155:548–551 Google Scholar
36. Lock J, le Grange D, Agras WS, Dare C: Treatment Manual for Anorexia Nervosa: A Family Based Approach. New York, Guilford, 2001 Google Scholar
37. Lock J, le Grange D, Forsberg S, Hewell K: Is family therapy useful for treating children with anorexia nervosa? results of a case series. J Am Acad Child Adolesc Psychiatry 2006; 45:1323–1328 Google Scholar
38. Lock J, Agras WS, Bryson S, Kraemer HC: A comparison of short- and long-term family therapy for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry 2005; 44:632–639 Google Scholar
39. Guarda AS, Heinberg LJ: Inpatient and partial hospital approaches to the treatment of eating disorders, in Handbook of Eating Disorders and Obesity. Edited by Thompson JK. Hoboken, NJ, John Wiley & Sons, 2004, pp 297–322 Google Scholar
40. Russell GF, Szmukler GI, Dare C, Eisler I: An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry 1987; 44:1047–1056 Google Scholar
41. Robin AL, Siegel PT, Moye AW, Gilroy M, Dennis AB, Sikand A: A controlled comparison of family versus individual therapy for adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry 1999; 38:1482–1489 Google Scholar
42. Pike KM, Walsh BT, Vitousek K, Wilson GT, Bauer J: Cognitive behavior therapy in the posthospitalization treatment of anorexia nervosa. Am J Psychiatry 2003; 160:2046–2049 Google Scholar
43. McIntosh VVW, Jordan J, Carter FA, Luty SE, McKenzie JM, Bulik CM, Frampton CMA, Joyce PR: Three psychotherapies for anorexia nervosa: a randomized, controlled trial. Am J Psychiatry 2005; 162:741–747 Google Scholar
44. Attia E, Schroeder L: Pharmacologic treatment of anorexia nervosa: where do we go from here? Int J Eat Disord 2005; 37(suppl):S60–S63 Google Scholar
45. Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, Pike KM, Devlin MJ, Woodside B, Roberto CA, Rockert W: Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA 2006; 295:2605–2612 Google Scholar
- Musicothérapie réceptive et anorexie mentale. Évaluation du dispositif DéPi-AM de détente psychomusicale dans l’accompagnement d’adolescentes hospitalisées : étude pilote auprès de 8 patientes Neuropsychiatrie de l'Enfance et de l'Adolescence, Vol. 72, No. 2
- Anorexie mentale et musicothérapie Soins, Vol. 68, No. 881
- L’anorexie mentale, une pathologie familière et complexe Soins, Vol. 68, No. 881
- Cognitive flexibility and the risk of anorexia nervosa: An investigation using self-report and neurocognitive assessments Journal of Psychiatric Research, Vol. 151
- Eating Disorders 18 June 2021
- Severe and Enduring Anorexia Nervosa: Enduring Wrong Assumptions? 15 February 2021 | Frontiers in Psychiatry, Vol. 11
- Characterization of Anorexia Nervosa on Social Media: Textual, Visual, Relational, Behavioral, and Demographical Analysis 20 July 2021 | Journal of Medical Internet Research, Vol. 23, No. 7
- Cognitive flexibility in acute anorexia nervosa and after recovery: A systematic review Clinical Psychology Review, Vol. 81
- Exploring the Eating Disorder Examination Questionnaire, Clinical Impairment Assessment, and Autism Quotient to Identify Eating Disorder Vulnerability: A Cluster Analysis 2 September 2020 | Machine Learning and Knowledge Extraction, Vol. 2, No. 3
- A systematic review of blood-based serotonergic biomarkers in Bulimia Nervosa Psychiatry Research, Vol. 279
- Eating Disorders 12 March 2019
- Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, Vol. 21, No. 2
- Anorexia Nervosa, Bulimia Nervosa, and Other Eating Disorders
- Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity NeuroImage, Vol. 109
- Anorexia Nervosa 23 January 2015
- Boletín Médico del Hospital Infantil de México, Vol. 72, No. 4
- The association between the development of weighing technology, possession and use of weighing scales, and self-reported severity of disordered eating 9 January 2014 | Irish Journal of Medical Science (1971 -), Vol. 183, No. 3
- Eating Disorders among Adolescents with a History of Obesity Pakistan Journal of Nutrition, Vol. 13, No. 8
- Risk Factors and Antecedent Life Events in the Development of Anorexia Nervosa: A Portuguese Case-Control Study 27 February 2014 | European Eating Disorders Review, Vol. 22, No. 4
- Anorexia nervosa in adolescents: challenges remain The Lancet, Vol. 383, No. 9924
- Family and Family Therapy, Vol. 22, No. 2
- Recognising anorexia nervosa: approaches to treatment British Journal of Mental Health Nursing, Vol. 2, No. 1
- Journal of Clinical Densitometry, Vol. 16, No. 4
- Open Journal of Preventive Medicine, Vol. 03, No. 02
- Food Restriction and Reward in Rats 24 August 2012
- Back to life: How to use positive psychology to beat anorexia? 25 January 2012 | International Journal of Research Studies in Psychology, Vol. 1, No. 2
- Osteopathic Family Physician, Vol. 4, No. 6
- Anorexie mentale par procuration : une présentation inhabituelle La Presse Médicale, Vol. 40, No. 5
- Recognising anorexia nervosa British Journal of Wellbeing, Vol. 1, No. 9
- Identification and Management of Eating Disorders in Children and Adolescents 1 December 2010 | Pediatrics, Vol. 126, No. 6
- Medical futility and psychiatry: Palliative care and hospice care as a last resort in the treatment of refractory anorexia nervosa 14 May 2009 | International Journal of Eating Disorders, Vol. 43, No. 4
- Neural processing of negative word stimuli concerning body image in patients with eating disorders: An fMRI study NeuroImage, Vol. 50, No. 3
- Anorexie mentale à l'adolescence EMC - Pédiatrie - Maladies infectieuses, Vol. 5, No. 1
- Journal de Pédiatrie et de Puériculture, Vol. 23, No. 1
- Scandinavian Journal of Caring Sciences, Vol. 24, No. 3
- Pro‐anorexia websites: What a clinician should know 29 November 2008 | International Journal of Eating Disorders, Vol. 42, No. 4
- Expert Opinion on Investigational Drugs, Vol. 18, No. 5

Eating disorders among teens more severe than ever

Teen eating disorders have never been this rampant — or this severe.
Hospitalizations for eating disorders spiked during the pandemic, doubling among adolescent girls, according to the Centers for Disease Control and Prevention . While most teens have returned to a normal life of in-person school, sports and social activities, eating disorders, especially anorexia, remain at an all-time high, experts warn.
“The kids are not OK,” said Melissa Freizinger, the associate director of the eating disorder program at Boston Children’s Hospital. “As the pandemic started and then progressed, we kept thinking, ‘Oh, it’s going to get better in 2022. Oh, it’s going to get better in 2023. But it hasn’t.”
Eating disorder-related health visits — which include hospital stays, pediatrician visits, telehealth talk therapy, and everything in between — more than doubled among people younger than 17 in the past five years, according to a recent report from the data company Trilliant Health . From 2018 through mid-2022, visits among this age group jumped 107.4% across all eating disorders, from around 50,000 visits at the beginning of 2018 to more than 100,000 in 2022. Visits related to anorexia nervosa, which has the highest death rate of any mental illness, jumped 129.26%.
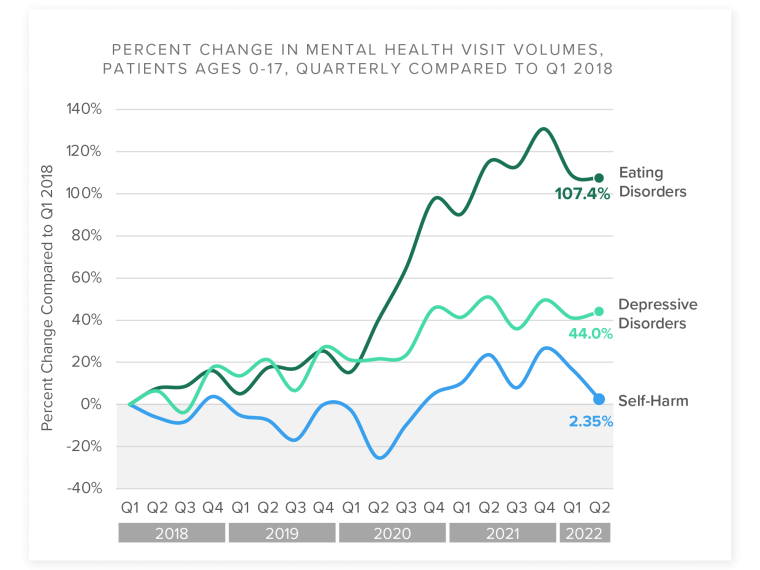
The pandemic worsened the incidence of anxiety and depression — both are risk factors for triggering or worsening eating disorders.
While eating disorder-related visits dipped slightly after a peak in 2021, they’re nowhere near pre-pandemic levels as adolescents and younger teens cope with the after-effects of Covid, such as grieving for family members who have died, falling behind in school or losing touch with friends.
And the patients coming in with eating disorders are in more serious condition now, with both mental and physical symptoms appearing more urgent, experts say.
“They’re sicker than before, and they’re more complicated than they were before,” said Boston Children’s Freizinger, noting that even after Covid, teens are being hospitalized at an alarming rate. Many require medical stabilization for malnourishment, and their psychiatric symptoms are more severe.
“We all have collective trauma from the pandemic, but many of these kids have PTSD," said Freizinger. "They’re also younger.”
Waitlists for eating disorders treatment
Seventeen-year-old Lana Elisha Garrido, who was first treated for anorexia at age 13 and then relapsed in December 2021, said she noticed younger patients at the Los Angeles facility where she received intensive treatment five months last year.
“When I was 13, everyone around me in treatment was an adult,” she said. “Now there’s like 20 people my age.” Garrido, who’s connected with other teens through volunteering with the National Eating Disorder Association (NEDA), said she’s been hearing about monthslong waitlists to begin treatment.
Despite the pervasive eating disorder stereotype — white, female and underweight — teens from racial and ethnic minority groups, as well as males and teens with larger bodies, develop certain eating disorders at even higher rates, according to research.

“I feel like a lot of marginalized people out there don’t know what to do or where to go or how to approach treatment,” said Garrido, a first-generation Filipino American whose parents are immigrants.
Over the years, Garrido said she’s noticed that almost all of the doctors in her various treatment facilities have been white, despite living in a city as racially and ethnically diverse as Los Angeles.
Eating disorders are less likely to be recognized among these underrepresented groups. In one 2006 study, doctors accurately diagnosed 17% of Black women, 41% of Latina women and 44% of white women with identical eating disorder symptoms.
Freizinger added that many eating disorder specialists don’t accept Medicaid or don’t accept insurance at all, which can make treatment access all the more difficult for underrepresented minority groups, especially Black and Hispanic populations who are more likely to have Medicaid or lack health insurance altogether than white Americans.
These missed diagnoses and treatment access barriers have meant very few research studies into eating disorder disparities, but several slightly older studies suggest certain eating disorders may be more prevalent among minorities. A 2011 JAMA Psychiatry study, for instance, found bulimia was more common among Hispanic teens than white teens, and binge-eating disorder was more common among both Black and Hispanic teens than white teens. In 2013, a survey of high schoolers identified eating disorder behaviors occurred nearly three times as often among transgender students.
‘The environment pulls the trigger’
Before Covid, Freizinger would typically start seeing patients around a parent’s divorce, a lost loved one or the transition from middle school to high school or high school to college.
“It’s a complicated process with biological, psychological, genetic and social-cultural factors,” she said.
Under typical circumstances, strong social connections can act as protective factors for teens with heightened risks, helping them avoid developing eating disorders.
That changed in 2020. During pandemic lockdowns, young people suddenly lost access to these connections.

Megan Bazzini, now 22, has struggled with anorexia since her early teens. She has since recovered, but said her symptoms worsened during the pandemic.
“Before Covid, if your thing was going out for dumplings with your friends, and you said you didn’t want to do that because of your eating disorder, you’d stop getting invited to hang out,” said Bazzini, who lives in New York City.
During Covid restrictions and lockdowns, those experiences vanished.
“I wasn’t in social situations where I felt like I needed to eat to make other people happy,” she said. “Eating disorders thrive in secret.”
Thinness ideals and social media
However, it's impossible to separate the teen eating disorder crisis from social media, experts insist.
Eighty-four percent of teens reported using social media, and the most popular apps were YouTube, Snapchat and TikTok, according to a survey from the nonprofit Common Sense Media. Experts say these platforms’ algorithms encourage eating disorder behaviors and reinforce negative body image.
“We’re seeing these algorithms target teens and make the content they see more extreme,” said Dr. Jessica Lin is an adolescent medicine physician who specializes in eating disorders at Cincinnati Children’s. She offered the example of a teen who started watching home exercise videos during the pandemic.
“Suddenly the algorithm says they’re interested in exercise and diet content, and it just keeps showing up and worsening,” she said. “It can just spiral from there.”
After several years in recovery, Garrido says her TikTok feed started recommending what the eating disorder community calls “pro-ana” content, meaning photos and videos glamorizing the eating disorder and encouraging followers to consume fewer calories. Garrido said these videos played a role in her recent relapse.
“I was like, ‘Why am I trying to recover from something someone else wants so desperately?’ Might as well just do it again.’”
Bazzini has stopped using most social media for this reason. “It’s just awful,” she said.
Social media companies, including TikTok, Meta — which owns Instagram — and Google — which owns YouTube — have been the targets of numerous lawsuits in recent years from parents alleging the platforms caused their teens to develop eating disorders. Last year, the Seattle-based Social Media Victims Law Center filed three lawsuits — two against Meta and one against TikTok — alleging that the apps caused young girls to develop chronic eating disorders .
To be sure, the companies that run these social media apps have taken some measures to cut down the potentially harmful eating disorder content on their platforms, including adding warning labels or age restrictions to some posts and taking others down altogether.
Recently, YouTube wrote in an update to its community guidelines , “On April 18, 2023, we updated our Eating disorders policy to better protect the community from sensitive content that may pose a risk to some audiences. We may remove imitable content, age-restrict content, or show a crisis resource panel on videos about eating disorders or self-harm topics.”
It’s unclear whether, and to what degree, these lawsuits and policy updates will lessen social media’s role in the teen eating disorder crisis.
Diagnosing eating disorders
A broader recognition of what it means to have an eating disorder could explain, to some extent, the sharp rise in teen eating disorders. With a shift in the way psychiatrists, psychologists and physicians diagnose them, it’s possible more cases are being recognized, rather than more teens developing new disorders.
In 2013, the American Psychiatric Association's manual of mental disorders — the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, or D.S.M.-5 — included binge eating as an official eating disorder for the first time. The D.S.M.-5 also eliminated a requirement that people lose their periods to be diagnosed with anorexia, and added “atypical anorexia” for people with anorexia who aren’t technically underweight.
This updated manual drove greater recognition of eating disorders, including among boys.
“With the broadening criteria, we were able to be more aware that males can have eating disorders, specifically anorexia, and that people who live in larger bodies can also develop anorexia,” Lin said. “So there’s definitely been better recognition and better acceptance.”
Dr. Walter Kaye, director of the eating disorders program at the University of California, San Diego, suspects broader criteria may have played into the increase.
“Insurance companies are more likely to support something with a diagnosis behind it," he said.
Similar to the crisis for mental health care for teens , demand for treatment has created a tremendous gap in access , with the number of young people in need of care outpacing the availability of doctors, mental health professionals and facilities.
“Eating disorder care is significantly harder to access right now because of the increase in volume, and that’s where we’re stuck,” Lin said. “For these teens to recover, they need to get into treatment as soon as possible, and we’re still a long way away from having enough providers to help the number of patients we have.”
CLARIFICATION (May 1, 2023: 11:32 a.m. ET): A previous version of this article omitted Dr. Jessica Lin’s job description. She is an adolescent medicine physician who specializes in eating disorders at Cincinnati Children’s.
NBC News contributor Caroline Hopkins is a health and science journalist who covers cancer treatment for Precision Oncology News. She is a graduate of the Columbia University Graduate School of Journalism.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 April 2023
The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice
- Yong Fan ORCID: orcid.org/0000-0003-4813-7017 1 na1 ,
- René Klinkby Støving 2 na1 ,
- Samar Berreira Ibraim 3 na1 ,
- Tuulia Hyötyläinen 4 ,
- Florence Thirion ORCID: orcid.org/0000-0002-2310-5548 3 ,
- Tulika Arora ORCID: orcid.org/0000-0001-8821-8191 1 ,
- Liwei Lyu ORCID: orcid.org/0000-0002-7387-8557 1 , 5 ,
- Evelina Stankevic ORCID: orcid.org/0000-0002-6433-6975 1 ,
- Tue Haldor Hansen ORCID: orcid.org/0000-0001-5948-8993 1 ,
- Pierre Déchelotte ORCID: orcid.org/0000-0001-5224-6166 6 ,
- Tim Sinioja 4 ,
- Oddny Ragnarsdottir 4 ,
- Nicolas Pons 3 ,
- Nathalie Galleron 3 ,
- Benoît Quinquis 3 ,
- Florence Levenez 3 ,
- Hugo Roume ORCID: orcid.org/0000-0003-2995-242X 3 ,
- Gwen Falony 7 , 8 , 9 , 10 ,
- Sara Vieira-Silva ORCID: orcid.org/0000-0002-4616-7602 7 , 8 , 9 , 10 ,
- Jeroen Raes ORCID: orcid.org/0000-0002-1337-041X 7 , 8 ,
- Loa Clausen ORCID: orcid.org/0000-0002-4559-8347 11 , 12 ,
- Gry Kjaersdam Telléus 13 , 14 ,
- Fredrik Bäckhed 1 , 15 , 16 ,
- Matej Oresic ORCID: orcid.org/0000-0002-2856-9165 17 , 18 ,
- S. Dusko Ehrlich 3 , 19 &
- Oluf Pedersen ORCID: orcid.org/0000-0002-3321-3972 1 , 5
Nature Microbiology volume 8 , pages 787–802 ( 2023 ) Cite this article
34k Accesses
28 Citations
363 Altmetric
Metrics details
- Metabolic disorders
- Neurological disorders
- Nutrition disorders
Anorexia nervosa (AN) is an eating disorder with a high mortality. About 95% of cases are women and it has a population prevalence of about 1%, but evidence-based treatment is lacking. The pathogenesis of AN probably involves genetics and various environmental factors, and an altered gut microbiota has been observed in individuals with AN using amplicon sequencing and relatively small cohorts. Here we investigated whether a disrupted gut microbiota contributes to AN pathogenesis. Shotgun metagenomics and metabolomics were performed on faecal and serum samples, respectively, from a cohort of 77 females with AN and 70 healthy females. Multiple bacterial taxa (for example, Clostridium species) were altered in AN and correlated with estimates of eating behaviour and mental health. The gut virome was also altered in AN including a reduction in viral–bacterial interactions. Bacterial functional modules associated with the degradation of neurotransmitters were enriched in AN and various structural variants in bacteria were linked to metabolic features of AN. Serum metabolomics revealed an increase in metabolites associated with reduced food intake (for example, indole-3-propionic acid). Causal inference analyses implied that serum bacterial metabolites are potentially mediating the impact of an altered gut microbiota on AN behaviour. Further, we performed faecal microbiota transplantation from AN cases to germ-free mice under energy-restricted feeding to mirror AN eating behaviour. We found that the reduced weight gain and induced hypothalamic and adipose tissue gene expression were related to aberrant energy metabolism and eating behaviour. Our ‘omics’ and mechanistic studies imply that a disruptive gut microbiome may contribute to AN pathogenesis.
Similar content being viewed by others
Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice
Mette Simone Aae Madsen, Jacob Bak Holm, … Henrik H. Hansen

Gut microbiota in human metabolic health and disease
Yong Fan & Oluf Pedersen
What next for eating disorder genetics? Replacing myths with facts to sharpen our understanding
Laura M. Huckins, Rebecca Signer, … Cynthia M. Bulik
Anorexia nervosa (AN) is a serious mental health condition and eating disorder characterized by distorted body image, obsessive thoughts about food, ritualistic patterns of behaviour including reduced food intake, loss of body weight, raised physical activity and emotional rigidity 1 . AN primarily affects women in about 95% of cases and has a population prevalence of about 1% 2 . It can be classified into two subtypes, the common restricting (AN-RS) type and the less prevalent binge-eating or purging (AN-BP) type 1 . The evidence base for treatment is lacking 3 and although specialized multidisciplinary treatment can reduce mortality 4 , less than half of AN cases achieve complete remission 5 . The aggregate mortality rate is estimated to be 5.6% per decade, much higher than in the general population 6 .
Despite research to determine the aetiology of AN, it remains a syndrome, that is, a collection of symptoms without a well-defined unifying cause. Twin studies have reported heritability estimates of 50–60% 7 and genome-wide association studies have identified eight genomic loci showing correlations with psychiatric disorders, physical activity, and metabolic and anthropometric traits. This is independent of common variants associated with body-mass index 8 , 9 . At the pathophysiological level, AN is characterized by multiple endocrine changes 10 and perturbed signalling of neurotransmitters in various parts of the brain 11 .
The human digestive tract contains complex assemblies of microorganisms that can impact host metabolism, immunity and neurobiology via metabolites and other pathways 12 . This may include the gut-microbiota-brain axis, which can affect brain functions including regulation of appetite, behaviour and emotions 13 . For example, the bacterial metabolite caseinolytic peptidase B (ClpB), predominantly produced by enterobacteria, is an antigenic mimic of α-melanocyte-stimulating hormone, which can exert anorexigenic effects 14 , 15 .
It has been hypothesized that an aberrant gut microbiota may be involved in the pathogenesis of AN. Several small studies that used amplicon sequencing to characterize the gut microbiota at the genus level in AN have been published 16 , 17 , 18 , 19 , showing dysbiosis of gut bacterial microbiota (see Supplementary Note 1 ). Moreover, in a mouse model of anorexia, changes in the gut microbiota have been shown to be associated with changes in eating behaviour and expression of hypothalamic neuropeptides 20 .
Here we explored the hypothesis that a perturbed intestinal gut microbiota and serum metabolome contribute to the complex pathogenesis of AN. To do so, we performed shotgun metagenomics on faecal samples from 77 female AN cases and 70 age-matched female controls allowing for in-depth analyses of the gut bacterial and archaeal microbiota at taxonomic, functional and genetics levels, as well as analyses of the viral gut microbiota. We also characterized the serum metabolome, which was analysed with the gut metagenome data in relation to individual markers of eating- and psychological behaviour. Causal mechanisms were explored in silico using bidirectional mediation analyses and in vivo through faecal microbiota transplantation (FMT) of gut microbiota from AN cases to female germ-free littermates. Our findings lend support to the hypothesis that a disrupted AN gut microbiota and associated bacterial metabolites contribute to AN pathogenesis (Extended Data Fig. 1 ).
Phenotypes of women with AN and controls
Summarized statistics of clinical characteristics of the 77 enrolled women with AN and 70 age-matched control women considered to be of healthy weight (HC) are given in Supplementary Table 1 . The validated Eating Disorder Inventory-3 (EDI-3) questionnaire was used to estimate levels of specific eating behaviour 21 and a detailed description of the EDI-3 subscale is shown in Supplementary Note 2 . As expected, women with AN were much leaner, had lower fasting serum concentrations of glucose and insulin, higher insulin sensitivity as estimated by homoeostatic model assessment for insulin resistance (HOMA-IR) and lower serum C-reactive protein. Detailed baseline characteristics of study participants are shown in Supplementary Table 2 . Additionally, within AN cases, AN-RS type cases were characterized by higher values of serum insulin and lower insulin sensitivity than AN-BP individuals (Supplementary Table 1 ). Comparing stool samples from AN and HC, there was no significant difference in bacterial cell counts between AN and HC or within subtypes of AN ( P Wilcoxon > 0.05, Supplementary Table 1 ).
Gut microbiota composition is altered in AN
At the phylum level, AN microbiota samples were characterized by a reduction in Bacteroidota and Actinobacteriota (Extended Data Fig. 2a ). At the family level, Bacteroidaceae was dominant in both groups (Extended Data Fig. 2b ). Among the top 20 most abundant families, the abundance of Christensenellales CAG-138 was higher in AN as a new observation for this cohort, whereas the abundance of Ruminococcaceae and Lachnospiraceae was higher in HC. Among the 89 identified bacterial families, Christensenellaceae was the most significantly enriched in AN (Extended Data Fig. 2c ). We observed higher β -diversity of AN microbiome at the genus level (Extended Data Fig. 2d ), with Bacteroides being the dominant phylotype in both groups (Extended Data Fig. 2e ). Among the top 30 genera, Faecalibacterium, Agathobacter, Gemmiger , unclassified Lachnospiraceae G, Ruminococcus 2, Roseburia, Dysosmobacter – Oscillibacter, Coprococcus , Oscillospirales 4 CAG-103 and Eisenbergiella were more abundant in HC, while unclassified Christensenellales CAG-138 was more abundant in AN (Extended Data Fig. 2e ). Additionally, among the 225 identified bacterial genera, Lactobacillus was the most significantly enriched in AN (Extended Data Fig. 2f ). Despite the difference in major genera between AN and HC, we found that richness of metagenomic species pangenomes (MSP, hereafter called species) was similar between the two groups (Extended Data Fig. 2g ). In enterotype analyses 22 , we found a higher prevalence of the Ruminococcacea-enterotype (R-enterotype) in AN compared with HC, and a higher prevalence of the same enterotype in AN-BP than in AN-RS subtype (Extended Data Fig. 2h ).
At the species level (Supplementary Table 3 ), we observed that the AN gut microbiota is characterized by higher β -diversity (Fig. 1a ). Within AN subgroups, the AN-BP subtype had a more heterogeneous bacterial community at the species level than the AN-RS subtype (Fig. 1b ). In the comparison between in- and out-patient AN cases, we found that β -diversity of in-patients was higher than that of out-patients at the species level (Supplementary Fig. 1 and Data ). Recent body weight change within 4 weeks was not associated with gut bacterial composition (Supplementary Table 4 ). The species that were significantly different in abundance distribution between AN and HC after deconfounding interferences of multiple medications (selective serotonin re-uptake inhibitors, antipsychotics and benzodiazepines, specified in Supplementary Table 2 ) are shown in Fig. 1c . Among the depleted species in AN were Roseburia intestinalis and Roseburia inulinivorans , species that have a high capacity for digesting plant polysaccharides and are considered to be part of the health-related gut microbiota 23 . In a co-abundance undirected network analysis (Extended Data Fig. 3 ), we identified a bacterial community consisting of Eisenbergiella , butyrate-producing bacterium SS3/4 - ( Clostridium ) sp. CAG:81, Faecalibacterium prausnitzii 3, ( Oscillibacter ) sp. ER4/ Firmicutes bacterium CAG: 129_59_24, Oscillibacter sp. 57_20, ( Clostridium ) sp. 2789STDY5834924 , unclassified Lachnospiraceae and unclassified Dysosmobacter – Oscillibacter , which was more abundant in HC. A community that was highly enriched in AN comprised Erysipelatoclostridium ramosum , Enterocloster bolteae , ( Clostridium ) innocuum and Blautia sp. CAG:257.
a , b Box plot (line, median; box, interquartile range (IQR); whiskers, 1.5× IQR) of β-diversity of AN ( n = 77) and HC ( n = 70) gut microbiota ( a ) and of two AN subtypes (AN-RS n = 56, AN-BP n = 21) and HC gut microbiota ( b ) at bacterial species level (Canberra distance). Statistical significance of differences between two groups was determined by Wilcoxon rank-sum test (two-sided). c , Significantly contrasted bacterial species between AN and HC. Differences in abundance were detected using the metadeconfoundR pipeline where covariates including age, BMI, smoking and multiple drug intake were corrected. Cliff’s delta values give estimates of effect size. For each contrasted MSP, prevalence in the whole cohort, HC, AN, and P adj are given next to the MSP annotation. d , Heat map showing that gut bacterial species are linked to eating disorder scores in AN cases, using a linear regression model where age, BMI, smoking and multiple drug intake were defined as covariates and adjusted for. Variables in specific eating disorder scale are marked in blue, and general psychological scale is marked in red. Right panel to the heat map indicates the direction of each variable. For each MSP, prevalence in AN is given next to the MSP annotation. +, P adj < 0.05 by Benjamini-Hochberg method (see for exact P values).
Source data
Associations between absolute bacterial abundance and bioclinical variables in an.
We investigated numerical covariations between the absolute abundance of bacteria at genus and species levels and bioclinical variables in the combined HC and AN cohort. We used a linear regression model adjusting for confounders including age, smoking and multiple drug intake (Methods and Supplementary Note 3 , Fig. 2 and Data ).
Interestingly, some bacterial taxa were linked to eating disorder scores and psychological conditions after adjusting for multiple confounding factors including age, body mass index (BMI), smoking history and medications. At the species level, we found that Clostridium species were positively correlated with eating disorder scores (Fig. 1d ), indicating a potential role of these species in the regulation of eating behaviour and neuropsychiatric symptoms 24 . Moreover, among the bacterial species that were inversely correlated with eating disorder scores, we found that the absolute abundances of Lactococcus acidophilus 25 and Faecalibacterium prausnitzii 26 , both of which are associated with depressive symptoms, were related to a score for interpersonal alienation (Fig. 1d ). Additionally, the absolute abundance of Parasutterella correlated positively with body dissatisfaction and absolute abundance of Bifidobacterium correlated with a marker of perfectionism. Despite a similar absolute abundance of Brachyspira in AN and HC, this genus was positively correlated with markers for ‘drive for thinness’ in AN (Extended Data Fig. 4 ). As shown in Extended Data Fig. 5 , we found no difference in circulating levels of anorexigenic ClpB 14 between AN and HC groups (Supplementary Note 4 ).
Predicted bacterial growth rates are altered in AN
We estimated growth dynamics of the bacterial gut microbiota from the metagenomic data by calculating the peak-to-trough ratio (PTR) 27 for 50 bacterial species. Thirty-five of these were present in more than 20 samples. The median PTR values differed markedly between AN and HC ( P Wilcoxon = 2.0 × 10 −4 , Extended Data Fig. 6 ), which might be related to the severe reduction in food intake in AN patients. Six bacteria were predicted to have significantly lower growth rate in AN ( P Wilcoxon < 0.05, Extended Data Fig. 6 ). These were Akkermansia muciniphila , Alistipes finegoldii , Coprococcus catus , Eubacterium siraeum , Odoribacter splanchnicus and butyrate-producing bacterium SS3/4.
Lactococcus phages and weakened trans-kingdom interactions in AN
We observed higher viral richness (Chao1, Fig. 2a ) and diversity (Shannon, Fig. 2b ) in AN faecal samples compared with HC. Recent body weight change within 4 weeks was not associated with gut viral composition (Supplementary Table 4 ). After deconfounding for covariates (age, smoking and drug intake), we identified 31 viral species that were enriched or decreased in AN (Fig. 2c ). Of major interest, 25 of the 30 increased viral species in AN were Lactococcus phages with known Lactococcus lactis hosts—bacteria that have been extensively used in the production of fermented food products.
a , b , Box plot (line, median; box, IQR; whiskers, 1.5× IQR) of changes in Chao1 richness ( a ) and Shannon diversity ( b ) of the viral gut microbiota between AN ( n = 77) and HC ( n = 70) at viral species level. Significance was examined by two-sided Wilcoxon rank-sum test ( a , b ). c , Cliff’s delta values of contrasted gut viral species between AN and HC with P adj < 0.05 by Benjamini-Hochberg correction (given next to the viral annotation). The differential species were identified by metadeconfoundR pipeline where impacts of cofactors including age, smoking and multiple drug intake were deconfounded. d , Difference in number of trans-kingdom ecologic correlations between the viral and bacterial gut microbiota in AN ( n = 77) compared to HC ( n = 70), and between two AN subtypes (AN-RS n = 56, AN-BP n = 21) using the SparCC algorithm.
Analysis of viral–bacterial correlations within the AN and HC gut microbiota revealed a remarkable decrease in the number of viral–bacterial interactions in AN (219 for HC versus 84 for AN, P Fisher’s exact test = 8.8 × 10 −15 , Fig. 2d ). This was primarily driven by weakened interactions between viral species and short-chain fatty acid bacterial producers, such as Roseburia inulinivorans , Faecalibacterium prausnitzii and Roseburia hominis (Supplementary Fig. 3 and Data ). We did not observe any interplay between Lactococcus phages and Lactococcus bacteria in the trans-kingdom analysis (Supplementary Figs. 3 and 4 and Data ).
In analyses of AN subgroups, principal coordinate analysis (PCoA) on the Canberra distance showed no remarkable alterations in gut viral composition ( P PERMANOVA = 0.571, Supplementary Fig. 5 and Data ). However, when comparing the number of viral–bacterial correlations in AN-RS and AN-BP gut microbiota, we found a reduction in the number and a much lower ratio of positive correlations in AN-RS (164 for AN-BP versus 44 for AN-RS, P Fisher’s exact test < 2.2 × 10 −16 ) (Fig. 2d and Supplementary Fig. 4 ). This suggests that the gut microbiota in AN-RS has weakened gut viral–bacterial interactions.
Predicted gut microbiota functions correlate with eating behaviours and metabolism
Using Gut Metabolic Modules (GMMs) 28 and Gut Brain Modules (GBMs) 29 to predict gut bacterial functional potentials, we identified 159 functional modules. Notably, the abundance of GBMs for serotonin biosynthesis and the degradation of dopamine, glutamate and tryptophan, which are metabolites with effects on mood and appetite, were enriched in AN (Fig. 3a ). Conversely, the abundance of the glutamate synthesis II and vitamin K2 pathways were higher in HC (Fig. 3a ) 30 . In addition, we found that serotonin synthesis and glutamate degradation pathways were inversely correlated with circulating concentrations of glucose and insulin, or insulin sensitivity (Fig. 3b ). While we observed differences in GBMs, we did not identify differences in GMMs between AN and HC (Supplementary Table 5 ).
a , Cliff’s delta effect size of contrasted functional modules between AN ( n = 77) and HC ( n = 70) using the metadeconfoundR pipeline where interferences from covariates including age, BMI, smoking and multiple drug intake were corrected. Gold bars, functional modules more abundant in AN; blue bars, functional modules more abundant in HC. For each contrasted module, P value after Benjamini-Hochberg correction is given next to the module annotation. b , Heat map of the associations between clinical variables and functional potentials of gut bacteriome by linear regression model where impacts of covariates including age, smoking and multiple drug intake were deconfounded. + indicates P < 0.05 by Benjamini-Hochberg correction (see for exact P values).
Bacterial genomes may have structural variants (SVs) that potentially interfere with functional genes impacting the interplay between microbes and their host 31 . Therefore, differences in the presence or abundance of SVs between otherwise identical bacterial strains may underlie critical phenotypic and functional differences 31 , 32 . Here we profiled SVs in all samples and identified 5,056 deletion SVs and 2,423 variable SVs across 56 bacterial species (Fig. 4a,b ). For some species, we observed marked differences in copy number variation. We identified 87 deletion SVs and 18 variable SVs in Bacteroides uniformis across 134 individuals, 78 deletions and 15 variable SVs in Faecalibacterium prausnitzii in 124 individuals, and 110 deletion SVs and 55 variable SVs in Parabacteroides distasonis in 121 individuals. For the archaeal microbiota, we only identified SVs in Methanobrevibacter smithii in 13 individuals (Fig. 4a , and Supplementary Fig. 6 and Data ). To explore potential differences in bacterial genetics, we further computed the Canberra distance of bacterial SV profiles between all 147 samples (Fig. 4c ). AN and HC samples were significantly different in the β -diversity of SV composition ( P Wilcoxon < 2.2 × 10 −16 ). Collectively, these results suggest the composition of bacterial SVs differs between the two groups.
a , Number of SVs of each bacterial or archaeal species in 147 (77 cases and 70 controls) study participants. For each species, the number of deletion and variable SVs are given. b , Pie chart showing the total identified SVs numbers. c , Box plot (line, median; box, IQR; whiskers, 1.5× IQR) of β -diversity (Canberra distance) of SV-based genetic composition in AN ( n = 77) and HC ( n = 70) bacteriome. P value was determined by two-sided Wilcoxon rank-sum test. d , Chord diagram showing significant associations between eating disorder scores and bacterial SVs after adjusting for age, BMI, smoking and multiple drug intake. e , Heat map showing the associations between SVs of Bacteroides uniformis and EDI-3 scores using linear regression model where impacts of age, BMI, smoking and multiple drug intake were deconfounded. + indicates P < 0.05 corrected by Benjamini-Hochberg (see for exact P values). In d and e , variables of eating disorder scale are coloured in blue, general psychological scale are in red, and bacterial SVs are in black. f , The deletion rate of the 10-kbp deletion SV harbouring thiamine-monophosphate kinase in B. uniformis genome in the AN group. g , Box plot (line, median; box, IQR; whiskers, 1.5× IQR) showing the EDI-3 scores in anorexia individuals with ( n = 49) and without ( n = 28) the 10-kbp deletion. Significance was determined by Wilcoxon rank-sum test (two-sided).
Exploring relationships between gut bacterial SVs and markers of eating behaviour, we found that in AN cases, bacterial SVs were significantly associated with eating disorder scores after deconfounding for multiple covariates (Fig. 4d ). As a noteworthy example, in the association analysis between SVs in the B. uniformis genome and host eating scores, we found that a 10-kbp deletion was directly associated with markers of bulimia and self-denial, indicating that this deletion SV may be involved in the regulation of eating disorder and psychological traits in AN (Fig. 4e ). Indeed, gene analysis showed that the beginning of this specific genomic region in B. uniformis encodes a thiamine-monophosphate kinase (Fig. 4f and Supplementary Table 6 ), which is the distal enzyme involved in the thiamine (vitamin B1) biosynthesis pathway. Thiamine deficiency has been associated with mental health including memory loss, anxiety, depression, irritability, insomnia as well as appetite loss and gastrointestinal complaints 33 . About one third of AN cases may suffer from thiamine deficiency 34 . We found that AN cases lacking this bacterial genomic region had higher scores for bulimia (a key characteristic for the AN-BP subtype) and self-denial (Fig. 4g ), a pattern also suggested by our correlation analyses (Fig. 4e ). Another example linking bacterial genetics to metabolism-related bioclinical variables is given in (Extended Data Fig. 7 ) and Supplementary Note 5.
Serum metabolites are associated with markers of appetite and food intake regulation
We performed untargeted metabolomics profiling of serum from AN cases and controls. This revealed a serum metabolome profile consisting of 28 polar metabolites and 35 microbiota-related metabolites, which was significantly different between AN and HC (Fig. 5a ), while only slightly altered between the two AN subtypes (Extended Data Fig. 8a ). We identified 25 serum metabolites with concentration differences between cases and controls after adjusting for confounders (adjusted P value ( P adj ) < 0.05) (Fig. 5b ).
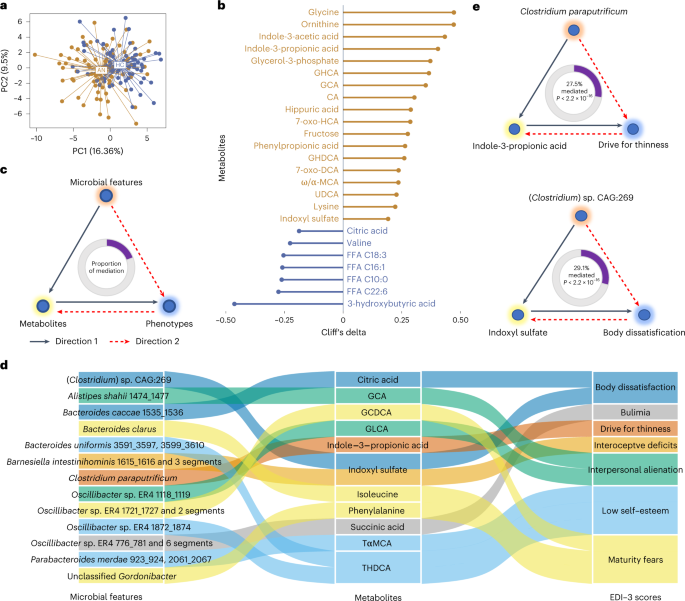
a , Principal component analysis (PCA) of the serum metabolome profile of AN cases and HC participants. b , Cliff’s delta values of contrasted metabolites between AN ( n = 77) and HC ( n = 70) after adjusting for age, BMI, smoking and multiple drug intake. Gold lollipops are metabolites enriched in AN, and blue lollipops show serum metabolites enriched in HC. c , Workflow for the bidirectional mediation analysis for gut microbial features, serum metabolites and host phenotypes. d , Sankey diagram showing the inferred causal relationship network of direction 1 where gut microbial features including bacterial species, gut brain/metabolic modules and bacterial genetics were treated as causal factors, metabolites are mediators, and EDI-3 scores are outcomes. e , Examples of inferred causal relationships between microbial features, metabolites and EDI-3 scores. Direction 1 means microbial features → eating disorder scores mediated by metabolites, illustrated with a black line; direction 2 means microbial features → metabolites mediated by EDI-3 scores, illustrated with a dashed red line. The proportions of mediation effects are shown at the centre of ring charts. FFA, free fatty acid.
Serum concentrations of both primary (cholic acid (CA), glycocholic acid (GCA)) and secondary bile acids (glycohyocholic acid (GHCA), 7-oxo-hyocholic acid (7-oxo-HCA), glycohyodeoxycholic acid (GHDCA), 7-oxo-deoxycholic acid (7-oxo-DCA), ω/α-muricholic acid (ω/α-MCA), ursodeoxycholic acid (UDCA)) were higher in AN, indicating a potential role of the gut microbiota in AN-related changes in secondary bile acid synthesis and metabolism, and satiety regulation 35 . Moreover, serum concentrations of indole-3-acetic acid and indole-3-propionic acid (IPA), two tryptophan metabolites, were higher in AN compared with HC. Interestingly, IPA is associated with the secretion of glucagon-like peptide 1, which can stimulate satiety and slow gastric emptying 36 , 37 . Dysregulation of valine, saturated and long-chain unsaturated fatty acids were also observed in the AN group (Fig. 5b and Supplementary Note 6 ).
Associations between serum metabolites, gut microbiota, and markers of appetite and mental health
To explore the role of serum metabolites in the interaction between gut microbiota and host phenotypes, we constructed bidirectional mediation models. For direction 1, we hypothesized that serum metabolites (including microbiota-related metabolites) as variables mediate the causal effect of gut microbial features (bacterial species, gut brain modules and bacterial genetics) on host phenotypes (Eating Disorder Inventory-3 (EDI-3) scores and metabolic traits). For direction 2, we treated phenotypes as mediators and alterations in metabolites as outcomes of changes in microbial features (Fig. 5c ). This in silico bidirectional analysis enabled us to quantify the extent to which a hypothesized mediator (a metabolite) participates in the interaction between a cause (microbial features) and its effect (host phenotypic traits).
We first performed the causal inference of bacterial features on EDI-3 questionnaire scores in AN cases (Extended Data Fig. 8b ). Inferred causal relationships in direction 1 consisted of 13 microbial features as initiators, 11 metabolites as mediators and 7 EDI-3 scores as outcomes (Fig. 5d ). As a noteworthy example, we identified ‘drive for thinness’ as an outcome of the AN-enriched bacterial species C. paraputrificum (Fig. 1c ), which was linked to changes in serum IPA levels, also enriched in the AN group. C. paraputrificum is a producer of multiple tryptophan catabolites, including IPA, indoleacrylic acid, indoleacetic acid and tryptamine, all of which are involved in regulating appetite and mental health 24 (Fig. 5e ).
In another example, indoxyl sulfate, which was enriched in AN patients, was identified as a mediator of AN-enriched ( Clostridium ) sp. CAG:269 in the score for body dissatisfaction (Fig. 5e ). Indoxyl sulfate is a cardio- and uraemic toxin, and has been shown to induce anxiety- or depression-like behaviours in humans and animal models 38 , 39 . Clostridium is a genus of indole-producing bacteria that can encode tryptophanase 40 , the critical enzyme converting tryptophan to indole, pyruvate and ammonia 41 .
Next, we analysed causal inference between microbial features and host metabolic traits across the whole cohort (Extended Data Fig. 8c ). The causal network in direction 1 consisted of 14 microbial features, 10 metabolites and 5 metabolic traits as causal treatments, mediators and outcomes, respectively (Extended Data Fig. 8d ). Notably, we found that the serotonin synthesis module causally affected host BMI via the secondary bile acid glycoursodeoxycholic acid, which is upregulated by serotonin 42 (Extended Data Fig. 8e ). Finally, consistent with our previous findings, serum leucine mediated the impact of B. vulgatus on glucose homoeostasis 43 (Extended Data Fig. 8e ). We observed no unidirectional causal relationship between changes in eating behaviour and psychological status, gut microbial features and metabolites (Supplementary Table 7 ).
Gut microbiota induces reduced weight gain and altered energy metabolism in mice
To investigate potential causal relationships between an altered gut microbiota in AN and relevant phenotypes, we transplanted faecal microbiota from three randomly chosen AN-RS cases (to achieve uniformity as AN-BP has a more heterogeneous phenotype than the AN-RS subtype) and three age-matched HC participants to three independent litters of female germ-free (GF) mice (Extended Data Fig. 9a ). To minimize variations in genetic background, we included littermates as control mice. In each litter study, 8, 6 and 6 littermates, respectively, were randomly assigned as recipients of AN or HC faecal microbiota. Following stool transplantation, recipient mice were singly housed and received a 30% calorie restricted diet for 3 weeks to mimic reduced food intake in human AN (Extended Data Fig. 9b ). Ad libitum chow diet feeding did not generate any phenotypic alterations in GF mice 44 , an observation consistent with a previous report on kwashiorkor 45 (Extended Data Fig. 10a ).
After 21 d, GF mice transplanted with stools from AN cases showed a larger initial decrease in body weight and a slower weight gain over time compared with mice that received HC FMT (Fig. 6a and Extended Data Fig. 10b ; see Supplementary Note 7 for discussion).
a , Body weight (BW) change compared to the body weight at day 0 after energy-restricted diet (AN-T n = 10, HC-T, n = 10 examined over 3 independent experiments). Significance was calculated by two-way analysis of variance (ANOVA), followed by Benjamini-Hochberg post hoc test. b , c , mRNA levels of the indicated mice genes in hypothalamus ( b ) and inguinal white adipose tissue ( c ) in the faecal microbiota mouse recipients (AN-T n = 10, HC-T, n = 10, examined over 3 independent experiments). Significance between the two groups was tested using unpaired two-tailed Student’s t -test. Data are presented as mean ± s.e.m. ( a – c ). d , Venn diagram of the identified and transferred ASVs between human donors and GF mouse recipients. e , Left: heat map of the 84 conserved ASVs in human donors. Middle: differences in the 84 conserved ASVs derived from the caecal content between AN-T and HC-T GF mouse recipients (AN-T n = 10, HC-T, n = 10, examined over 3 independent experiments). Transferred microbial alterations are marked in blue. Right: taxonomic information of ASVs.
We performed hypothalamic gene expression analysis following FMT. AN-transplanted and HC-transplanted recipients differed in expression of several hypothalamic genes involved in the control of eating behaviour and energy expenditure (Fig. 6b ). This included increased expression of the appetite suppressors Bdnf 46 and Cartpt 47 , and the receptor for serotonin, Htr1b (involved in the downstream regulation of serotonin), in AN FMT recipients. Expression of Snca , which encodes the neuronal protein alpha-synuclein associated with several neurodegenerative diseases, was higher in AN-transplanted mice 48 . In addition, we analysed messenger RNA (mRNA) levels of genes encoding proteins regulating adipose tissue thermogenesis. We found that abundances of Ucp1 , Elovl3 and Pgc1α mRNA were increased in inguinal fat of AN-transplanted mice, indicating enhanced adipose tissue thermogenesis in this group of mice (Fig. 6c ).
16S ribosomal ribonucleic acid (rRNA) gene amplicon sequencing of human donor stools and GF mouse recipient caecal contents identified 85 overlapping amplicon sequence variants (ASVs; Fig. 6d ), of which 45 (53%) altered ASVs in donors were transferred to recipients (Fig. 6e ). Serum metabolome profiling detected 31 conserved metabolites between donors and recipients, and alterations of 19 of these (61%) were transferred from human to mouse (Extended Data Fig. 10c ). We identified three ASVs: ASV_021, ASV_229 and ASV_002 that were annotated as Bacteroides at the genus level. The relative abundance of these ASVs and the expression of browning genes, including Ucp1 (Extended Data Fig. 10d ), were strongly positively correlated, indicating a potential role of these ASVs in body weight loss or lower weight gain through enhanced adipose browning. The inverse correlation between the relative abundance of ASV_122, annotated as genus Akkermansia , and the hypothalamic appetite-suppressing gene Htr1b is also notable and suggests a potential role of ASV_122 in appetite regulation (Extended Data Fig. 10d ). Taken together, alterations in hypothalamic and adipose tissue gene expression and changes in body weight over time in mice suggest that a disrupted gut microbiota in human AN may contribute to some of the elements in the complex pathogenesis of AN.
Using a combination of sequencing and metabolomics to characterize the gut microbiome and metabolome in humans and mice, we show that the bacterial and viral components of the microbiome and the serum metabolome are altered in those with AN compared with healthy individuals. We also find that bacterial species SVs are different between AN and healthy controls, and in silico causal inference analyses imply that bacterial metabolites mediate some effects of an altered gut microbiota on AN behaviour. Finally, GF mice transplanted with AN stools on an energy-restricted diet initially lose more weight and have slower weight gain over time compared with mice transplanted with stools from healthy individuals. This was associated with higher expression of appetite suppressor genes in the hypothalamus and higher expression of thermogenesis-related genes in adipose tissue of AN-transplanted mice.
In both FMT experiments and in silico inference analyses, we observed changes in circulating levels of glycine-chenodeoxycholic acid, indole-3-propionic acid, taurine-α-muricholic acid and taurine-hyodeoxycholic acid. We propose that these metabolites may act as potential mediators of some of the AN phenotypes. For example, indole-3-propionic acid is a tryptophan metabolite, which is implicated in serotonin activity 49 , and hyodeoxycholic acid is a 6α-hydroxylated bile acid, also called muricholic acid, which reduces body weight gain 50 . This bile acid is involved in satiety regulation 51 . Serotonin activity as well as appetite regulation could be implicated in development and/or maintenance of the AN syndrome. Future studies will need to explore the individual and combined effects of these metabolites on energy metabolism. Still, many more gut bacterial species and derived metabolites may mediate the observed AN traits in humans and mice, as reported in an activity-based anorexia model 20 , 52 .
Analyses of deletion and variable SVs in gut bacterial species indicated that bacterial genetics may influence AN-relevant behavioural traits and pathophysiology. Of special interest is a 10-kbp deletion in the B. uniformis genome that was associated with estimates of bulimia and self-denial. We predicted that this deletion results in the loss of a thiamine-monophosphate kinase encoding gene, which may result in a relative deficiency of microbiota-produced thiamine. This is of interest in the context of AN pathology since various mental and intestinal health issues are known to be linked to thiamine deficiency 33 .
Our studies of the viral gut microbiota in AN showed a partial uncoupling of the ecological interactions between viral species and short-chain producing bacterial species with impact on brain biology 53 . In addition, AN samples were enriched in Lactococcus phages with known bacterial L. lactis hosts. The AN-associated enrichment in Lactococcus phages may result in disruption in food fermentation and suggest the possible use of fermented food items in future treatment of AN. The reason for this enrichment of Lactococcus phages is unsettled but our finding may justify testing a multistrain probiotic containing L. lactis in AN adolescents 54 .
Our study has limitations: (1) the use of a cross-sectional Danish AN cohort limits generalizability of findings to other ethnicities; (2) the same limitation holds for the use of AN patients in treatment in a specialized centre, as these patients may not be representative of milder forms of AN; and (3) although effects of multiple covariates were deconfounded, we had no information on diet and physical activity—behaviours that impact the gut microbiota.
In conclusion, the present multi-omics study uncovers profound and complex disruptions of the gut microbiota in individuals with AN, with functional implications and altered serum metabolites. These compounds may act via the blood circulation or via gut-microbiota-brain neuronal signalling pathways affecting brain regulation of appetite, emotions and behaviour. FMT from human AN donors to GF mice under energy-restricted feeding resulted in lower body weight gain and a number of changes in expression of hypothalamic and adipose tissue genes involved in controlling behaviour and energy homoeostasis. The combination of multi-omics and in vivo experiments complement our causal inference analyses to allow the identification of specific bacterial metabolites that potentially mediate human host AN traits. Our findings lend support to the hypothesis that a severely disrupted intestinal microbiota contributes to some of the stages in the pathogenesis of AN.
Study participants
The AN patients were all diagnosed by an experienced consultant psychiatrist and they met the DSM-5 criteria for the restricting or binge/purging subtypes of AN 1 . Since 90–95% of individuals with diagnosed AN are females, we decided to include only women in the present study and since ethnicity may influence gut microbiota, we only included Danish Caucasian women with AN cases, recruited from three specialized centres in Denmark from 1 September 12014 to 31 July 2016. The centres were: Center for Eating Disorders (Odense University Hospital), Child and Adolescent Psychiatric Unit (Aarhus University Hospital) and Unit for Psychiatric Research (Aalborg University Hospital). Exclusion criteria comprised antibiotic or antifungal treatment within the previous 3 months, any acute or chronic somatic diseases or infections. All the included patients were treated in specialized centres, and they were interviewed by an experienced and specialized psychologist or psychiatrist at the start of their treatment. The validated Eating Disorder Inventory (EDI, details given in Supplementary Note 2 ) was used for the interview and as a questionnaire filled out by trained health professional specialists 21 . The exclusion criteria for the age-matched healthy control women were BMI below 18.5 or above 25 kg m −2 , regular medication of any kind apart from birth control pills, and antibiotics within the last 3 months. The control participants were recruited via public advertisement and via direct contact to health staff, medical students and their relatives. BMI and other clinical characteristics of the healthy controls are listed in Supplementary Table 2 .
The study protocol was registered at ClinicalTrials.gov (NCT02217384) and the study was conducted in accordance with the Helsinki declaration and approved by the Regional Scientific Ethical Committee for Southern Denmark (file no 42053 S-20140040). All participants involved in this study provided written informed consent.
In-patients. All the below-described measurements were conducted during routine treatment in the specialized unit for somatic and psychological stabilization of patients with severe AN. Safe and effective weight restoration of 2.0–3.0% per week is the goal of the treatment in the inpatient unit. Care was given by a multidisciplinary team and is in accordance with international guidelines. Individually customized meals were given under the supervision of trained nurses or dietitians at scheduled times. If the meals could not be consumed within the predefined timeframes (15 min for a snack and 30 min for a main meal), supplemental nutrition drinks were added either orally or via a duodenal tube. To account for individual preferences, a choice of three different meals was offered. The macronutrient content was consistent and within recommended energy percent ranges: 40–50% carbohydrate (maximum of 10% sugar), 30–40% fat and 20–25% protein. The daily energy intake was individualized according to the weight course. If a patient failed to reach 2% of weekly weight gain, the energy content of the daily menu was increased. All meals were followed by a supervised rest varying from 30 to 60 min in a seated position. Between the rests, light physical activity such as a walk was allowed; however, no forms of exercise training were allowed. For patients with an urge for excessive exercise or otherwise a lack of compliance with behaviour rules, behaviour supervision was extended and if needed, to 24 h a day.
Out-patients. AN patients paid regular visits to outpatient units where care was given by a multidisciplinary team involving medical doctors, nurses, psychologists and health behaviour educators.
They were given an individual diet plan, which had the same macronutrient composition as mentioned above for in-patients. As with the in-patients, all out-patients were also enrolled in cognitive behavioural therapeutic treatment courses.
Height was measured on a wall-mounted stadiometer and weight was measured in the morning before breakfast on a calibrated platform scale. BMI was calculated as weight divided by the square of height (=kg/m 2 ).
Biochemical analyses
Blood samples were taken in the morning after an overnight fast. The blood samples were collected on ice and processed to obtain serum and plasma, and subsequently stored at −80 °C. Serum concentrations of sodium, potassium, albumin and creatinine were measured by enzymatic assays on a Roche/Hitachi cobas c system. Serum concentrations of total cholesterol, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol were determined using the phosphotungstic acid magnesium chloride precipitation method. Serum immunoreactive insulin levels were measured using an enzyme-linked immunosorbent assay, while serum concentration of alanine aminotransferase was analysed with an enzymatic coulometric method including pyridoxal phosphate activation.
Analysis of plasma ClpB was performed as previously described 55 .
Faecal sample collection and DNA extraction
Stools were collected according to International Human Microbiome Standards (IHMS) guidelines (SOP 03 V1) in kits by AN cases and HC at home and immediately frozen at −20 °C until they were transported on dry ice and frozen 4–24 h later at −80 °C in plastic tubes at the biobanks. DNA extraction from aliquots of faecal samples was performed following IHMS SOP P7 V2 56 .
Bacterial cell counting
For bacterial cell counting (Supplementary Fig. 7 ), 0.08–0.12 g of frozen (−80 °C) faecal samples were diluted 15 times in pH 7.2 Dulbecco’s phosphate-buffered saline (DPBS) (Sigma-Aldrich), mechanically homogenized using tissue lyser (40 min, 12.5 agitations per second; QIAGEN) and fixed with 2% paraformaldehyde (10 min, room temperature; Biotum). Then the samples were diluted 120 times in filtered staining buffer (1 mM EDTA, 0.01% Tween20, pH 7.2 DPBS, 1% BSA (Sigma-Aldrich)). To minimize clumps, the samples were filtered through a cell strainer (pore size 5 μm; pluriSelect), pre-wet in the staining buffer. Next, the bacterial cell suspension was stained with SYBR Green I (1:200,000; Thermo Fisher) in DMSO (Sigma-Aldrich) and incubated in the dark for 30 min. For accurate determination of bacterial cell counts, a known concentration of 123count eBeads (Invitrogen) was added to the samples before the analysis. Measurements were performed using a BD Fortessa LSRII flow cytometer (BD Biosciences) and data were acquired using BD FACSDiVaTM software. A threshold value of 200 was applied on the FITC (530/30 nm) channel. Fluorescence intensity at green (530/30 nm, FITC), blue (450/50 nm, Pacific Blue), yellow (575/26 nm, PE) and red (695/40 nm, PerCP-Cy5-5) fluorescence channels as well as forward- and side-scattered (FSC and SSC) light intensities were collected. Measurements were performed at a pre-set flow rate of 0.5 μl s −1 . Data were processed in R using flowcore package (v1.11.20) 57 in R (v4.1.2). Fixed gating strategy separated the microbial fluorescent events from the faecal sample background.
Shotgun sequencing
DNA was quantitated using Qubit fluorometric quantitation (Thermo Fisher) and qualified using DNA size profiling on a fragment analyser (Agilent). High molecular weight DNA (>10 kbp; 3 µg) was used to build the library. Shearing of DNA into fragments of approximately 150 bp was performed using an ultrasonicator (Covaris) and DNA fragment library construction was performed using the Ion Plus Fragment Library and Ion Xpress Barcode Adapters kits (Thermo Fisher). Purified and amplified DNA fragment libraries were sequenced using the Ion Proton Sequencer (Thermo Fisher), with a minimum of 20 million high-quality reads of 150 bp (on average) generated per library.
Construction of a gene count table
To construct the gene count table, METEOR software was used 58 : first, reads were filtered for low quality by AlienTrimmer 59 . After removal of low-quality reads and human DNA reads, 75.7% ± 2.7% high-quality metagenomics sequencing reads of faecal DNA were mapped onto the Integrated Gut Catalog 2 (IGC2) 60 , comprising 10.4 million genes, using Bowtie2 (ref. 61 ). Reads mapped to a unique gene in the catalogue were attributed to their corresponding genes. Then, reads that mapped with the same alignment score to multiple genes in the catalogue were attributed according to the ratio of their unique mapping counts to the captured genes. The resulting count table was further processed using the R package MetaOMineR v1.31 62 . It was downsized at 14 million mapped reads to take into account differences in sequencing depth and mapping rate across samples. Then the downsized matrix was normalized for gene length and transformed into a frequency matrix by fragments per kilobase of transcript per million fragments mapped normalization. Gene count was computed as the number of genes present (abundance strictly positive) in the frequency matrix.
Profiling and annotation of MSPs and gut enterotypes
IGC2 was previously organized into 1,990 MSPs with MSPminer 63 using a publicly available updated MSP dataset 64 . Relative abundance of MSP was computed as the mean abundance of its 100 ‘marker’ genes (that is, the genes that correlate the most altogether). If less than 10% of ‘marker’ genes were seen in a sample, the abundance of the MSP was set to 0. This approach was used in the MetaHIT 62 and Metacardis 65 consortia. For the 4 MSPs with less than 100 core genes, all available core genes were used.
Abundances at higher taxonomical ranks were computed as the sum of the MSP that belong to a given taxa. MSP count was assessed as the number of MSPs present in a sample (that is, whose abundance is strictly positive). Enterotypes profiling was performed as previously demonstrated 66 .
Estimating functional modules of gut bacteriome
Genes from the IGC2 catalogue were mapped with diamond 67 onto KEGG orthologues (KO) from the KEGG database 68 (v8.9). Each gene was assigned to the best-ranked KO among hits with e -value < 10 × 10 −5 and bit score >60. Then we assessed presence and abundance of GMMs 28 and GBMs 29 in a metagenomic sample by the pipeline implemented in the R package omixerRpm (v0.3.2) as previously described 28 , 29 .
Estimation of dynamic growth rate of bacteria from metagenomic samples
We used the computational pipeline to infer gut bacterial growth dynamics from metagenomic samples as previously described 27 . Sequencing reads were mapped to a database that contains complete genomic references of 2,991 strains belonging to 1,509 microbial species. For each bacterial species, a reference strain with prevalence of 100% across the samples was selected. A coverage map was then assembled on the basis of aligned reads to the reference genome. Genomic segments were binned into 10-kbp regions and coverage of the resulting bins was calculated and smoothed. The location of the origin and terminus of replication was predicted by fits of the same strain across multiple samples. Lastly, PTRs were calculated for each bacterial species in every sample as the smoothed sequencing coverage of the representative strain at the predicted peak location, divided by that at the predicted trough location.
Studies of bacterial structural variations
Before the SVs classification, the pipeline with iterative coverage-based read assignment algorithm was performed to reassign the ambiguous reads to the most likely reference with high accuracy 31 , 32 . The reference genomes provided in the proGenomes database ( http://progenomes1.embl.de/ ) were concatenated and then divided into genomic 1-kbp bins and applied for the detection of highly variable genomic segments. The SGV-Finder pipeline 31 was used to detect the SVs that are either (1) with deletion percentage of the genomic segment across the population of <25% (variable SVs, vSVs), (2) with deletion percentage between 25% and 75% (deletion SVs, dSVs; the absence or presence of this particular genomic segment was kept) or (3) with deletion percentage of >75% (this genomic segment was excluded from the analysis). All bacterial species with SV calling were present in at least 10% of the total samples and were used for subsequent analysis.
Mediation analysis
The R package ‘mediation’ 69 (v4.5.0) was used to infer causal relationships between gut microbial features, polar and microbiota-related metabolites, and metabolic traits and eating disorder scores. To reduce the testing numbers, we only kept the candidate groups consisting of variables that were strongly associated with each other; that is, for a candidate microbial feature-metabolite-phenotypic variable causal group, the association between gut microbial feature and serum metabolite was significant ( P adj < 0.1); the association between metabolite and phenotypic variable was significant ( P adj < 0.1); and the association between microbial feature and phenotypic variable was significant ( P adj < 0.1). After performing mediation analysis, only candidate groups with significance in direction 1 were kept for Sankey diagram visualization.
Profiling and analysis of viral gut microbiota
We profiled the viral gut microbiota using MiCoP 70 , as this method is optimized to call viruses directly from bulk metagenomics sequencing reads and compute relative abundance within the virome dataset. As a reference dataset, MiCoP draws upon the NCBI’s RefSeq Viral database 71 . We identified a total of 209 viral species with prevalence of > and =10% and relative abundance of > and =0.01% for 147 (77 AN versus 70 HC) individuals included in the dataset. Richness, alpha and beta diversity were calculated with the R package ‘fossil’ 72 and ‘vegan’ 73 . Two-tailed Wilcoxon’s rank-sum test was used to determine statistically significant differences in richness and alpha diversity indices between groups. Permutational multivariate analysis of variance (PERMANOVA) at n = 999 was performed for Canberra distance. The viral–bacterial interactions in both AN-RS and AN-BP microbiome data were computed using the Sparse Correlations for Compositional (SparCC) 74 algorithm. Before the SparCC analysis, the AN bacterial and viral microbiota datasets were subset to AN-RS and AN-BP datasets, which were then separately submitted for SparCC analysis.
Analysis of serum polar metabolites by gas chromatography–time-of-flight mass spectrometry
The metabolites listed as gut microbiota-related metabolites were based on literature mining 75 , 76 . Serum samples were randomized and sample preparation was done as described previously 43 , 77 . Briefly, 400 μl of methanol (MeOH) containing internal standards (heptadecanoic acid, deuterium-labelled dl -valine, deuterium-labelled succinic acid and deuterium-labelled glutamic acid, 1 µg ml −1 ) was added to 30 µl of the serum samples, which were then vortex mixed and incubated on ice for 30 min. Samples were then centrifuged (9,400 × g , 3 min) and 350 μl of the supernatant was collected after centrifugation. The solvent was evaporated to dryness, 25 μl of MOX reagent was added and the sample was incubated for 60 min at 45 °C. N -Methyl- N -(trimethylsilyl)trifluoroacetamide (25 μl) was added and after 60 min incubation at 45 °C, 25 μl of the retention index standard mixture (n-alkanes, 10 µg ml −1 ) was added.
The analyses were done using an Agilent 7890B gas chromatograph coupled to an Agilent 7200 quadrupole time-of-flight mass spectrometer. The following parameters were used: injection volume was 1 µl with 100:1 split on PTV at 70 °C, heating to 300 °C at 120 °C min −1 ; column: Zebron ZB-SemiVolatiles with length of 20 m, inner diameter of 0.18 mm, film thickness of 0.18 µm, with initial helium flow of 1.2 ml min −1 , increasing to 2.4 ml min −1 after 16 min. Oven temperature programme: 50 °C (5 min), then to 270 °C at 20 °C min −1 and then to 300 at 40 °C min −1 (5 min). EI source: 250 °C, 70 eV electron energy, 35 µA emission, solvent delay 3 min. Mass range 55 to 650 amu, acquisition rate 5 spectra per second, acquisition time 200 ms per spectrum. Quad at 150 °C, 1.5 ml min −1 N 2 collision flow, aux-2 temperature 280 °C.
Calibration curves were constructed using alanine, citric acid, fumaric acid, glutamic acid, glycine, lactic acid, malic acid, 2-hydroxybutyric acid, 3-hydroxybutyric acid, linoleic acid, oleic acid, palmitic acid, stearic acid, cholesterol, fructose, glutamine, indole-3-propionic acid, isoleucine, leucine, proline, succinic acid, valine, asparagine, aspartic acid, arachidonic acid, glycerol-3-phosphate, lysine, methionine, ornithine, phenylalanine, serine and threonine purchased from Sigma-Aldrich at a concentration range of 0.1–80 μg ml −1 . An aliquot of each sample was collected, pooled and used as quality-control samples, together with National Institute of Standards and Technology (NIST) CRM1950 serum sample, an in-house pooled serum sample. The relative standard deviation of the concentrations was on average 16% for the pooled quality-control samples and 10% for the NIST samples.
Analysis of serum bile acids and serum semipolar metabolites
The sample preparation procedure was performed as described previously 78 . The plate was preconditioned with 450 µl acetonitrile before the addition of 100 µl of sample and 10 µl of polyfluoroalkyl substances (PFAS) and bile acids (BAs) internal standard mixture (200 ng ml −1 and 1,000 ng ml −1 , respectively). Thereafter, 450 µl of acetonitrile containing 1% formic acid were added to each well and the samples extracted using a 10″ vacuum manifold. The eluate was evaporated to dryness under nitrogen gas flow and reconstituted to 80 µl of MeOH/2 mM aqueous ammonium ethanoate.
Chromatographic separation was carried out using an Acquity UPLC BEH C18 column (100 mm × 2.1 mm inner diameter, 1.7 µm particle size), fitted with a C18 precolumn (Waters). Mobile phase A consisted of H 2 O:MeOH (v/v 70:30) and mobile phase B of MeOH with both phases containing 2 mM ammonium acetate as an ionization agent. The flow rate was set at 0.4 ml min −1 with the elution gradient as follows: 0–1.5 min, mobile phase B was increased from 5% to 30%; 1.5–4.5 min, mobile phase B was increased to 70%; 4.5–7.5 min, mobile phase B was increased to 100% and held for 5.5 min. A post-time of 5 min was used to regain the initial conditions for the next analysis. The total run time per sample was 18 min. The dual electrospray ionization source settings were as follows: capillary voltage was 4.5 kV, nozzle voltage 1,500 V, N 2 pressure in the nebulizer was 21 psi and the N 2 flow rate and temperature as sheath gas were 11 l min −1 and 379 °C, respectively. To obtain accurate mass spectra in the mass spectrometry (MS) scan, the m / z range was set to 100–1,700 in negative ion mode. MassHunter B.06.01 software (Agilent) was used for all data acquisition.
Identification of compounds was done with an in-house spectral library using MS (and retention time), tandem mass spectrometry information. Quantitation was based on a matrix-matched calibration curve spiked with native compounds. The calibration curve consisted of concentrations ranging from 0–1,600 ng ml −1 for BAs. The relative standard deviation for the BAs was on average 17.8% for the quality-control samples and 19.4% for the NIST samples.
Animal experiments
Animal protocols were approved by the Science Ethics Committees of the Capital Region of Copenhagen, Denmark. Female germ-free Swiss Webster mice were bred and maintained in flexible film gnotobiotic isolators until the start of experiments at the Department of Experimental Medicine, University of Copenhagen. The mice were fed autoclaved chow diet (7% simple sugars, 3% fat, 50% polysaccharide, 15% protein (w/w), energy 3.5 kcal g −1 ) and water ad libitum under a 12 h light/12 h dark cycle (lights on at 7:30 a.m.) and constant temperature (21–22 °C) and humidity (55 ± 5%).
Faecal samples from randomly selected subsets of three patients with AN (females aged 20, 22 and 20 yr with BMI of 10.3, 11.5 and 11.7 kg m −2 , respectively) and three HC (females aged 20, 22 and 21 yr with BMI of 22.6, 21.1 and 21.2 kg m −2 , respectively) who were representatives of cases and controls were used to colonize 6-week-old female GF littermates. Briefly, 250 mg of faecal samples were suspended with 5 ml of LYBHI media (supplemented with 0.05% Cysteine and 0.2% Hemin as reducing agents) diluted in 20% glycerol (20 ml g −1 of faeces) in an anaerobic cryovial; these inoculum samples were then vortexed for 5 min, followed by 5 min standing to precipitate particles. The faecal slurries were then transferred to 1 ml cryovials and immediately frozen at −80 °C. At day one, both groups of mice were housed in autoclaved individually ventilated cages where they received the first dose (200 µl) of faecal slurries. The mice were then given autoclaved chow diet and water ad libitum for 2 d and their food intake was recorded. At day 3, mice were gavaged with a second dose of faecal material from the same matched AN and HC donors as before. Thereafter, mice in both groups were singly housed and subjected to 30% energy-restricted autoclaved chow diet (amount of given food was set at 70% of ad libitum food intake for each mouse) for 3 weeks where water was given ad libitum. Both the anorexia-transplanted (AN-T) and the normal control-transplanted (HC-T) mice were weighed every 5 d after the start of energy-restricted dieting.
At the end of the study, mice were anaesthetized with isoflurane and blood from the vena cava was collected in tubes containing EDTA. Blood samples were centrifuged for 6 min at 4,032 g at 4 °C. Plasma was isolated and stored at −80 °C for subsequent biochemical testing. Inguinal subcutaneous white adipose tissue, the caecum and the hypothalamus of each mouse were precisely dissected and collected for quantitative PCR analysis. No animal or data points were excluded from the present study.
Total RNA was extracted from tissues using Trizol reagent (Invitrogen) according to the manufacturer’s instructions, followed by concentration measurement. One µg of RNA was transcribed to complementary DNA using the Reverse Transcription System (Promega). Real-time PCR was performed using the LC480 detection system (Roche Diagnostics) and SYBR Green I Supermix (Takara). All qPCRs were run on the thermal cycles at 95 °C for 10 min, followed by 45 cycles of 0.01 s at 95 °C and of 20 s at 60 °C. Data were normalized to the housekeeping Rpl36 gene for adipose tissue and Rplp0 gene 79 for hypothalamus and analysed according to the delta-delta CT method. Sequences of oligonucleotides used in this study are provided in Supplementary Table 8 .
DNA extraction, 16S rRNA sequencing and data analyses in mice experiments
Microbial DNAs were isolated and purified from stool samples (~250 mg) of human donors and mouse recipients by using NucleoSpin soil mini kit (MACHEREY‑NAGEL). The DNA was then amplified using Phusion High-Fidelity PCR master mix (New England Biolabs) by PCR targeting the V3-V4 region of the 16S rRNA gene (primer sequences provided in Supplementary Table 8 ). The following PCR programme was used: 98 °C for 30 s, 25× (98 °C for 10 s, 55 °C for 20 s, 72 °C for 20 s), 72 °C for 5 min. Amplification was verified by running the products on an agarose gel. Indices were added in a subsequent PCR using an Illumina Nextera kit with the following PCR programme: 98 °C for 30 s, 8× (98 °C for 10 s, 55 °C for 20 s, 72 °C for 20 s), 72 °C for 5 min. Attachment of indices was verified by running the products on an agarose gel. Products from the nested PCR were pooled on the basis of band intensity and the resulting library cleaned with magnetic beads. The DNA concentration of pooled libraries was measured fluorometrically. Sequencing was done on an Illumina MiSeq desktop sequencer using the MiSeq reagent kit V3 (Illumina) for 2 × 300 bp paired-end sequencing. Paired-end reads were subsequently trimmed, merged and analysed using the DADA2 (v1.16.0) pipeline 80 .
Statistical analysis
No data were excluded before the statistical analysis in the present study. No allocation and randomization were included as the study is observational. This study includes all available samples ( n = 147) of patients with anorexia and healthy individuals. Although no statistical methods were used to predetermine sample sizes, our sample sizes are similar to those reported in previous publications 43 , 81 . Samples were randomly distributed across metagenomics and metabolomics batches. Investigators were blinded to group allocation during data collection in metagenomic, biochemical and metabolomics analyses. All analyses of human samples were performed using R (v4.1.2). Gene expression analyses and body weight comparisons for animal studies were performed using GraphPad Prism (v9.3.0).
Differential analysis
We carried out the differential analysis using the metadeconfoundR pipeline implemented in the R package metadeconfoundR (v0.1.8; see https://github.com/TillBirkner/ metadeconfoundR or https://doi.org/10.5281/zenodo.4721078 ) where we assessed the extent to which the observed differences between AN and HC participants in microbiome or metabolome analyses are confounded by covariates including age, BMI, smoking and medication. This pipeline initially used univariate statistics to find associations between microbiome features and disease status, followed by nested linear model comparison post hoc testing to check for the confounding effects of potential covariates and finally, returning a status label.
Association analysis
For the association and mediation analysis between omics features and eating behaviour and psychological traits within the AN group, we first checked the normality of continuous variables with Shapiro-Wilk normality test, finding most variables not to be normally distributed. Therefore, before association analysis, we standardized the continuous variables using empirical normal quantile transformation to follow a standard normal distribution (N ~ (0, 1)). Then we implemented a linear regression model to assess the associations between omics features and eating behaviour and psychological traits using the following formula where confounding factors were added as covariates.
Medication included selective serotonin re-uptake inhibitors, antipsychotics and benzodiazepines.
For the association analysis between omics features and host metabolic traits, a normality check and data standardization were also performed before the linear regression analysis. In the linear regression model, the above-mentioned confounding factors were included as covariates, except for BMI as the extremely low BMI is the most remarkable phenotypic change for AN patients compared with HC individuals.
Differences in gut microbial diversity (gene richness, species count, taxonomic composition) between AN and HC were calculated using Wilcoxon tests, and Kruskal-Wallis test was used for assessing the significance of differences between multiple groups. Unless otherwise stated, all P values were corrected using the Benjamini-Hochberg method and P adj < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Anonymized clinical data that are stored in Sharepoint via Odense Patient Data Explorative (file no OP_153) can be accessed by contacting [email protected] or can be found in Supplementary Table 2 . Raw shotgun sequencing data and 16s rRNA gene amplicon sequencing data that support the findings of this study have been deposited in the European Nucleotide Archive with accession numbers PRJEB51776 and PRJEB60103 , respectively. Metabolomics data are available from Metabolomics Workbench repository under the link https://doi.org/10.21228/M8KT5B . The KEGG Database is available at https://www.genome.jp/kegg/ . Source data are provided with this paper.
Code availability
Codes associated with the data analysis and visualization are available at https://github.com/fjw536/AnorexiaGutMicrobiome.git .
American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Publishing, Inc., 2013).
Smink, F. R., Van Hoeken, D. & Hoek, H. W. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 14 , 406–414 (2012).
Article PubMed PubMed Central Google Scholar
Bulik, C. M. The challenges of treating anorexia nervosa. Lancet 383 , 105–106 (2014).
Article PubMed Google Scholar
Winkler, L. A.-D., Bilenberg, N., Hørder, K. & Støving, R. K. Does specialization of treatment influence mortality in eating disorders?—A comparison of two retrospective cohorts. Psychiatry Res. 230 , 165–171 (2015).
Støving, R. K. et al. Purging behavior in anorexia nervosa and eating disorder not otherwise specified: a retrospective cohort study. Psychiatry Res. 198 , 253–258 (2012).
Sullivan, P. F. Mortality in anorexia nervosa. Am. J. Psychiatry 152 , 1073–1074 (1995).
Article CAS PubMed Google Scholar
Trace, S. E., Baker, J. H., Peñas-Lledó, E. & Bulik, C. M. The genetics of eating disorders. Annu. Rev. Clin. Psychol. 9 , 589–620 (2013).
Paolacci, S. et al. Genetic contributions to the etiology of anorexia nervosa: new perspectives in molecular diagnosis and treatment. Mol. Genet. Genom. Med. 8 , e1244 (2020).
CAS Google Scholar
Watson, H. J. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51 , 1207–1214 (2019).
Article CAS PubMed PubMed Central Google Scholar
Støving, R. K. Mechanisms in endocrinology: anorexia nervosa and endocrinology: a clinical update. Eur. J. Endocrinol. 180 , R9–R27 (2019).
Bailer, U. F. et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 211 , 160–168 (2013).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 , 55–71 (2021).
Morais, L. H., Schreiber, H. L. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19 , 241–255 (2021).
Tennoune, N. et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 4 , e458 (2014).
Sobrino Crespo, C., Perianes Cachero, A., Puebla Jimenez, L., Barrios, V. & Arilla Ferreiro, E. Peptides and food intake. Front. Endocrinol. 5 , 58 (2014).
Article Google Scholar
Morita, C. et al. Gut dysbiosis in patients with anorexia nervosa. PLoS ONE 10 , e0145274 (2015).
Kleiman, S. C. et al. The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med . 77 , 969 (2015).
Armougom, F., Henry, M., Vialettes, B., Raccah, D. & Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 4 , e7125 (2009).
Mack, I. et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 6 , 26752 (2016).
Breton, J. et al. Gut microbiota alteration in a mouse model of anorexia nervosa. Clin. Nutr. 40 , 181–189 (2021).
Clausen, L., Rosenvinge, J. H., Friborg, O. & Rokkedal, K. Validating the Eating Disorder Inventory-3 (EDI-3): a comparison between 561 female eating disorders patients and 878 females from the general population. J. Psychopathol. Behav. Assess. 33 , 101–110 (2011).
Costea, P. I. et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 3 , 8–16 (2018).
La Rosa, S. L. et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 10 , 905 (2019).
Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9 , 3294 (2018).
Smith, C. J. et al. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 307 , G793–G802 (2014).
Hao, Z., Wang, W., Guo, R. & Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 104 , 132–142 (2019).
Korem, T. et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 349 , 1101–1106 (2015).
Vieira-Silva, S. et al. Species–function relationships shape ecological properties of the human gut microbiome. Nat. Microbiol. 1 , 16088 (2016).
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4 , 623–632 (2019).
Maresz, K. Growing evidence of a proven mechanism shows vitamin K2 Can impact health conditions beyond bone and cardiovascular. Integr. Med. 20 , 34–38 (2021).
Google Scholar
Zeevi, D. et al. Structural variation in the gut microbiome associates with host health. Nature 568 , 43–48 (2019).
Wang, D. et al. Characterization of gut microbial structural variations as determinants of human bile acid metabolism. Cell Host Microbe 29 , 1802–1814.e5 (2021).
Dhir, S., Tarasenko, M., Napoli, E. & Giulivi, C. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front. Psychiatry 4 , 207 (2019).
Winston, A., Jamieson, C., Madira, W., Gatward, N. & Palmer, R. L. Prevalence of thiamin deficiency in anorexia nervosa. Int. J. Eat. Disord. 28 , 451–454 (2000).
Perino, A. et al. Central anorexigenic actions of bile acids are mediated by TGR5. Nat. Metab. 3 , 595–603 (2021).
Germain, N. et al. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am. J. Clin. Nutr. 85 , 967–971 (2007).
Kamal, N. et al. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology 101 , 1320–1324 (1991).
Sun, C.-Y. et al. Indoxyl sulfate caused behavioral abnormality and neurodegeneration in mice with unilateral nephrectomy. Aging 13 , 6681 (2021).
Brydges, C. R. et al. Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature. Sci. Rep. 11 , 21011 (2021).
Lee, J.-H. & Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 34 , 426–444 (2010).
Newton, W. A. & Snell, E. E. Formation and interrelationships of tryptophanase and tryptophan synthetases in Escherichia coli . J. Bacteriol. 89 , 355–364 (1965).
Yanchuk, P. et al. Role of serotonin in the regulation of respiration and bile secretory function of the liver. Fiziol. Zh. 61 , 102–110 (2015).
Article CAS Google Scholar
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535 , 376–381 (2016).
Glenny, E. M. et al. Gut microbial communities from patients with anorexia nervosa do not influence body weight in recipient germ-free mice. Gut Microbes 13 , 1897216 (2021).
Smith, M. I. et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339 , 548–554 (2013).
Unger, T. J., Calderon, G. A., Bradley, L. C., Sena-Esteves, M. & Rios, M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 27 , 14265–14274 (2007).
Yang, S.-C., Shieh, K.-R. & Li, H.-Y. Cocaine-and amphetamine-regulated transcript in the nucleus accumbens participates in the regulation of feeding behavior in rats. Neuroscience 133 , 841–851 (2005).
Hashimoto, M. & Masliah, E. Alpha-synuclein in Lewy body disease and Alzheimer’s disease. Brain Pathol. 9 , 707–720 (1999).
Konopelski, P. et al. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, increases blood pressure via cardiac and vascular mechanisms in rats. Am. J. Physiol. Regul. 321 , R969–R981 (2021).
Makki, K. et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 72 , 314–324 (2023).
Higuchi, S. et al. Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice. Gut 69 , 1620–1628 (2020).
Breton, J. et al. Characterizing the metabolic perturbations induced by activity-based anorexia in the C57Bl/6 mouse using 1H NMR spectroscopy. Clin. Nutr. 39 , 2428–2434 (2020).
Braniste, V. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6 , 263ra158 (2014).
Gröbner, E. M. et al. The effects of probiotics administration on the gut microbiome in adolescents with anorexia nervosa—a study protocol for a longitudinal, double‐blind, randomized, placebo‐controlled trial. Eur. Eat. Disord. Rev. 30 , 61–74 (2022).
Breton, J. et al. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 49 , 805–808 (2016).
Costea, P. I. et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 35 , 1069–1076 (2017).
Hahne, F. et al. flowCore: a Bioconductor package for high throughput flow cytometry. BMC Bioinformatics 10 , 106 (2009).
Pons, N. et al. METEOR, a platform for quantitative metagenomic profiling of complex ecosystems. JOBIM http://www.jobim2010.fr/sites/default/files/presentations/27Pons.pdf (2010).
Criscuolo, A. & Brisse, S. AlienTrimmer: a tool to quickly and accurately trim off multiple short contaminant sequences from high-throughput sequencing reads. Genomics 102 , 500–506 (2013).
Wen, C. et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 18 , 142 (2017).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 , 357–359 (2012).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500 , 541–546 (2013).
Plaza Oñate, F. et al. MSPminer: abundance-based reconstitution of microbial pan-genomes from shotgun metagenomic data. Bioinformatics 35 , 1544–1552 (2019).
Plaza Oñate, F. P. et al. Updated Metagenomic Species Pan-genomes (MSPs) of the Human Gastrointestinal Microbiota (Recherche Data Gouv, 2021); https://doi.org/10.15454/FLANUP
Fromentin, S. et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 28 , 303–314 (2022).
Vieira-Silva, S. et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581 , 310–315 (2020).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12 , 59–60 (2015).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44 , D457–D462 (2016).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R package for causal mediation analysis. J. Stat. Softw. 59 , 1–38 (2014).
LaPierre, N. et al. MiCoP: microbial community profiling method for detecting viral and fungal organisms in metagenomic samples. BMC Genomics 20 , 423 (2019).
Brister, J. R., Ako-Adjei, D., Bao, Y. & Blinkova, O. NCBI viral genomes resource. Nucleic Acids Res. 43 , D571–D577 (2015).
Vavrek, M. J. Fossil: palaeoecological and palaeogeographical analysis tools. Palaeontol. Electronica 14 , 1–16 (2011).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14 , 927–930 (2003).
Friedman, J. & Alm, E. J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 8 , e1002687 (2012).
Nicholson, J. K. et al. Host-gut microbiota metabolic interactions. Science 336 , 1262–1267 (2012).
Krautkramer, K. A., Fan, J. & Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 19 , 77–94 (2021).
Castillo, S., Mattila, I., Miettinen, J., Oresic, M. & Hyötyläinen, T. Data analysis tool for comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chem. 83 , 3058–3067 (2011).
Salihović, S. et al. Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra-high-performance liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 412 , 2251–2259 (2020).
Li, B. et al. Identification of optimal reference genes for RT-qPCR in the rat hypothalamus and intestine for the study of obesity. Int. J. Obes. 38 , 192–197 (2014).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13 , 581–583 (2016).
Thirion, F. et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 15 , 1 (2023).
Download references
Acknowledgements
Y.F. received the Marie Skłodowska-Curie Individual Fellowship (797267) dedicated to the present study. The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent research institution at the University of Copenhagen, partially funded by an unrestricted donation from the Novo Nordisk Foundation. R.K.S. was supported by Odense University Hospital Research Fund (R15-A800).
Author information
These authors contributed equally: Yong Fan, René Støving, Samar Berreira Ibraim.
Authors and Affiliations
Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Science, University of Copenhagen, Copenhagen, Denmark
Yong Fan, Tulika Arora, Liwei Lyu, Evelina Stankevic, Tue Haldor Hansen, Fredrik Bäckhed & Oluf Pedersen
Center for Eating Disorders, Odense University Hospital, and Research Unit for Medical Endocrinology, Mental Health Services in the Region of Southern Denmark, Open Patient data Explorative Network (OPEN) and Clinical Institute, University of Southern Denmark, Odense, Denmark
René Klinkby Støving
Université Paris-Saclay, INRAE, MGP, Jouy-en-Josas, France
Samar Berreira Ibraim, Florence Thirion, Nicolas Pons, Nathalie Galleron, Benoît Quinquis, Florence Levenez, Hugo Roume & S. Dusko Ehrlich
School of Science and Technology, Örebro University, Örebro, Sweden
Tuulia Hyötyläinen, Tim Sinioja & Oddny Ragnarsdottir
Department of Medicine, University of Copenhagen and Herlev-Gentofte University Hospital, Copenhagen, Denmark
Liwei Lyu & Oluf Pedersen
INSERM U1073 Research Unit and TargEDys, Rouen University, Rouen, France
Pierre Déchelotte
Laboratory of Molecular bacteriology, Department of Microbiology and Immunology, Rega Institute Ku Leuven, Leuven, Belgium
Gwen Falony, Sara Vieira-Silva & Jeroen Raes
Center for Microbiology, VIB, Leuven, Belgium
Institute of Medical Microbiology and Hygiene and Research Center for Immunotherapy (FZI), University Medical Center of the Johannes Gutenberg-University Mainz, Mainz, Germany
Gwen Falony & Sara Vieira-Silva
Institute of Molecular Biology (IMB), Mainz, Germany
Department of Child and Adolescent Psychiatry, Aarhus University Hospital, Aarhus, Denmark
Loa Clausen
Department of Clinical Medicine, Faculty of Health, Aarhus University, Aarhus, Denmark
Unit for Psychiatric Research, Aalborg University Hospital, Aalborg, Denmark
Gry Kjaersdam Telléus
Department of Communication and Psychology, The Faculty of Social Sciences and Humanities, Aalborg University, Aalborg, Denmark
The Wallenberg Laboratory, Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Fredrik Bäckhed
Department of Clinical Physiology, Sahlgrenska University Hospital, Region Västra Götaland, Gothenburg, Sweden
School of Medical Sciences, Örebro University, Örebro, Sweden
Matej Oresic
Turku Bioscience Centre, University of Turku and Åbo Akademi University, Turku, Finland
Department of Clinical and Movement Neurosciences, University College London, London, UK
S. Dusko Ehrlich
You can also search for this author in PubMed Google Scholar
Contributions
O.P. conceived and designed the study concept and detailed the protocol and supervised all phases of the study protocol including paper writing. Y.F., R.K.S., S.B.I. and F.T. performed the data analysis, interpreted the results and contributed to drafting the paper. T.H., T.S. and O.R. supported the metabolome profiling. Y.F. designed and undertook all animal experiments with support from T.A. and L.L. L.L. and E.S. performed bacterial cell counting. R.K.S. and T.H.H. performed phenotyping of study participants. P.D, N.P., N.G., B.Q., F.L., H.R., G.F., S.V.-S., J.R., L.C., G.K.T, F.B, M.O. and S.D.E. contributed to technical project development and supervision and revised the drafts of the paper. All authors contributed to the review and editing of the paper.
Corresponding author
Correspondence to Oluf Pedersen .
Ethics declarations
Competing interests.
F.B. is a shareholder in Implexion Pharma. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Microbiology thanks Tom Hildebrandt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 graphical abstract of the study workflow and findings..
Workflow was created with BioRender.com.
Extended Data Fig. 2 Taxonomic differences at phylum, family and genus level and differences in enterotypes in cases with AN compared with healthy women.
a , b , e Box plot (line, median; box, interquartile range (IQR); whiskers, 1.5× IQR) of comparison between AN (gold, n = 77) and HC (blue, n = 70) of relative abundance of the 12 bacterial phyla detected in at least 10% of individuals ( a ), the 20 most abundant bacterial families ( b ), and the top 30 genera ( e ). Features are sorted by decreasing mean abundance. Zero values are set to 1e-10. Features colored in blue are enriched in HC group, and gold are enriched in AN group. Significance was determined by two-sided Wilcoxon rank-sum test, followed by drug-deconfounding and multiple testing correction by Benjamini-Hochberg method on all features ( a , b ,and e , see Source Data for exact p values). c , f , Absolute values of Cliff’s Delta effect size of families ( c ) and genera ( f ) contrasted between AN and HC after drug-deconfounding (adjusted p-value ≤ 0.1). Gold barplots indicate features more abundant in AN; blue barplots indicate features more abundant in HC ( c , f ). d , g , Box plot (line, median; box, IQR; whiskers, 1.5× IQR) showing β-diversity (Canberra distance) of gut bacteriome at genus level ( d ) and richness of Metagenomic Species Pan-genomes (MSPs) ( g ) between AN (gold, n = 77) and HC (blue, n = 70). Significance was determined by two-sided Wilcoxon rank-sum test ( d , g ). h , Upper panel demonstrates enterotype prevalence in AN ( n = 77) and HC ( n = 70), lower panel shows enterotype prevalence in HC and AN patients split into AN-RS ( n = 56) and AN-BP ( n = 21) groups.
Extended Data Fig. 3 Co-occurrence network deducted from bacterial species enriched in AN cases (left) and healthy controls (right) after drug-deconfounding.
Node size and node colour represent the mean abundance and the genus of a given MSP, respectively. Genera represented by only one MSP are coloured in grey. Red and blue lines indicate positive and negative correlations, respectively. Line thickness represents the absolute correlation coefficient. Only correlations with an absolute coefficient above 0.4 are shown; MSP without any correlation above the threshold are hidden.
Extended Data Fig. 4 Heatmap showing that gut bacterial genera are linked to eating disorder scores in AN cases after drug de-confounding.
Variables in specific eating disorder scale are marked in blue, and in general psychological scale are marked in red. +, adjusted p < 0.05 (see Source Data for exact p values).
Extended Data Fig. 5 Fasting plasma concentration of Caseinolytic protease B (ClpB) in control subjects and AN subtypes.
a , Relative abundance of Enterobacteriaceae family in HC ( n = 70) and AN ( n = 77) groups. b , Log-scale transformed fasting plasma ClpB concentration between HC ( n = 70) and AN ( n = 77) groups. c , Log-scale transformed fasting plasma ClpB concentration between restrictive AN (AN-RS n = 56) and binge-purge AN (AN-BP n = 21) subtypes. Significance was calculated by two-sided Wilcoxon rank-sum test between two groups ( a - c ). Box plots indicate median and interquartile range (IQR) and whiskers represent 1.5× IQR ( a - c ).
Extended Data Fig. 6 Altered dynamic growth rates of bacterial species between AN ( n = 77) and control ( n = 70) groups.
Box plots indicate median and interquartile range (IQR), whiskers indicate 1.5× IQR. Significance was determined by two-sided Wilcoxon rank-sum test.
Extended Data Fig. 7 SVs in bacterial gut microbiota from AN cases and associations with anorexia-relevant traits.
a-c , Scatterplot showing, HOMA-IR, fasting plasma insulin and glucose in individuals harboring a 1-kbp variation in the A. putredinis genome ( n = 147). Significance determined by two-sided spearman correlation test corrected by false discovery rate using Benjamini and Hochberg method. Error band is linear regression line with 95% confidence band ( a - c ). Dots representing AN and HC individuals were colored in red and blue, respectively. d , Upper panel, standardized variability (y axis) along a genomic region of A. putredinis (x axis). Lower panel, locations (blue bar) of the gene of interest.
Extended Data Fig. 8 In silico analysis using bidirectional mediation inference.
a , Principal component analysis (PCA) plot of the serum metabolome of AN subtypes, AN-BP (binge/purge anorexia), AN-RS (restrictive anorexia). b , Summary number of inferred mediation relationship for direction 1 (gut microbial features → eating disorder scores mediated by serum metabolites), direction 2 (gut microbial features → serum metabolites mediated by eating disorder scores). c , Summary number of inferred mediation relationship for direction 1 (gut microbial features → phenotypes mediated by serum metabolites), direction 2 (gut microbial features → serum metabolites mediated by phenotypes) for the whole cohort. d , Sankey diagram showing the inferred causal relationship network of direction 1 where gut microbial features including bacterial species, gut brain and metabolic modules, and bacterial genetics were treated as causal factors, serum metabolites are mediators, and metabolic traits are outcomes. e , Examples of inferred causal relationships between gut microbial features, metabolites, and host metabolic traits. Direction 1that means microbial features → metabolic traits mediated by serum metabolites is illustrated with a black line while direction 2 that means microbial features → serum metabolites mediated by metabolic traits is illustrated with a stipulated red line. The proportion of mediation effect are shown at the center of ring charts. GDCA, glycodeoxycholic acid; GHCA, glycohyocholic acid; GHDCA, glycohyodeoxycholic acid; GUDCA, glycoursodeoxycholic acid; HCA, hyocholic acid; HOMA-IR homeostatic model assessment of insulin resistance; P-, plasma; S-, serum.
Extended Data Fig. 9 Workflow diagram of fecal microbiota transplantation from human donors to germ-free mice littermates.
a , Workflow for the preparation of fecal microbiota slurry. Stool (250 mg) from both anorexia (AN) and control (HC) was cut on dry ice, transferred to anaerobic chamber, and resuspended with 5 ml of LYBHI media diluted in 20% glycerol. The resuspended fecal slurries were aliquoted in cryotubes and refrozen back quickly at -80 °C until further use. All stool samples of matched AN and HC donors were prepared on the same day and frozen aliquots were stored frozen for gavage to mice. b , Experimental scheme for the GF mice transplantation study. In each independent litter study, GF female littermates at age of six weeks old were taken out of breeding isolator and were randomly assigned to receive 200 µl of fecal slurries from three AN cases or three HC subjects. Both groups of mice were housed in autoclaved individually ventilated cages and were given autoclaved chow diet and water ad libitum for two days. After two days, mice were gavaged with a second dose of fecal material from the same matched AN and HC donors as before. Thereafter, mice in both groups were single housed and subjected to 30% calorie restricted chow diet for three weeks. Water was given ad libitum during this period. Both the anorexia-transplanted (AN-T) and the normal control-transplanted (HC-T) mice were weighed every five days after the start of energy-restricted diet. Created with Biorender.com.
Extended Data Fig. 10 Effects of FMT from AN and controls to female GF mouse littermates.
a , Body weight change and fat percentage of germ-free (GF) mouse littermates ( n = 21) fed with ad libitum chow diet and transplanted with microbiota from anorexia patients. b , Body weight change compared to the body weight at day 0 after energy-restricted diet for three independent experiments ( n = 8, 6, and 6 for independent batches, respectively). Data are expressed as mean ± s.e.m.( a , b ). Significance was calculated by two-way analysis of variance (ANOVA), followed by Benjamini-Hochberg post hoc test. c , Serum metabolome profile in human donors and GF mouse recipients fed with 30% energy-restricted diet. Data were expressed as the log2-transformed fold changes (log2FC) between AN and control groups. Positive log2FC values indicate AN-enriched metabolites, while negative log2FC values indicate HC-enriched metabolites. Serum metabolites that were persistently changed between AN and HC groups in human donors and GF mouse recipients were marked with blue bars. CA, cholic acid; DCA, deoxycholic acid; FFA, free fatty acid; w/a-MCA, ω/α-muricholic acid; HDCA, hyodeoxycholic acid; TaMCA, taurine-α-muricholic acid; CDCA, chenodeoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid; G, glycine-conjugated bile acids; T, taurine-conjugated bile acids. d , Heat map on the left panel showing the correlation between ASVs and quantified genes in hypothalamus or subcutaneous white adipose tissue. Taxonomic information of ASVs is given on the right panel. +, p < 0.05 corrected by Benjamini-Hochberg method (see Source Data for exact p values).
Supplementary information
Supplementary information.
Supplementary Notes, Figs. 1–7 and References.
Reporting Summary
Supplementary table.
Supplementary Tables 1–8.
Supplementary Data
Source data for Supplementary Figs. 1–6.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Source data fig. 3, source data fig. 4, source data fig. 5, source data fig. 6, source data extended data fig. 2, source data extended data fig. 4, source data extended data fig. 5, source data extended data fig. 6, source data extended data fig. 7, source data extended data fig. 8, source data extended data fig. 10, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fan, Y., Støving, R.K., Berreira Ibraim, S. et al. The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Nat Microbiol 8 , 787–802 (2023). https://doi.org/10.1038/s41564-023-01355-5
Download citation
Received : 23 June 2022
Accepted : 03 March 2023
Published : 17 April 2023
Issue Date : May 2023
DOI : https://doi.org/10.1038/s41564-023-01355-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Sex-dependent circadian alterations of both central and peripheral clock genes expression and gut–microbiota composition during activity-based anorexia in mice.
- Colin Salaün
- Marine Courvalet
- Moïse Coëffier
Biology of Sex Differences (2024)
Systematic identification of the role of gut microbiota in mental disorders: a TwinsUK cohort study
- Julie Delanote
- Alejandro Correa Rojo
- Gökhan Ertaylan
Scientific Reports (2024)
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
- Jian Sheng Loh
- Kooi Yeong Khaw
Signal Transduction and Targeted Therapy (2024)
Neue Aspekte in der Ätiologie und Therapie der jugendlichen Anorexia nervosa – ein postuliertes biopsychosoziales Modell und die Auswirkungen der COVID-19-Pandemie
- Beate Herpertz-Dahlmann
- Brigitte Dahmen
- Jochen Seitz
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2024)
The gut microbiome in anorexia nervosa
- Tom Hildebrandt
- Deena Peyser
Nature Microbiology (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Psychol
Anorexia Nervosa and a Lost Emotional Self: A Psychological Formulation of the Development, Maintenance, and Treatment of Anorexia Nervosa
Anna oldershaw.
1 Salmons Centre for Applied Psychology, Canterbury Christ Church University, Canterbury, United Kingdom
2 Kent and Medway All Age Eating Disorder Service, North East London NHS Foundation Trust, London, United Kingdom
Helen Startup
3 Sussex Eating Disorders Service and Research and Development Department, Sussex Partnership NHS Foundation Trust, Sussex, United Kingdom
Tony Lavender
In this paper, we argue that Anorexia Nervosa (AN) can be explained as arising from a ‘lost sense of emotional self.’ We begin by briefly reviewing evidence accumulated to date supporting the consensus that a complex range of genetic, biological, psychological, and socio-environmental risk and maintenance factors contribute to the development and maintenance of AN. We consider how current interventions seek to tackle these factors in psychotherapy and potential limitations. We then propose our theory that many risk and maintenance factors may be unified by an underpinning explanation of emotional processing difficulties leading to a lost sense of ‘emotional self.’ Further, we discuss how, once established, AN becomes ‘self-perpetuating’ and the ‘lost sense of emotional self’ relentlessly deepens. We outline these arguments in detail, drawing on empirical and neuroscientific data, before discussing the implications of this model for understanding AN and informing clinical intervention. We argue that experiential models of therapy (e.g., emotion-focused therapy; schema therapy) be employed to achieve emergence and integration of an ‘emotional self’ which can be flexibly and adaptively used to direct an individual’s needs and relationships. Furthermore, we assert that this should be a primary goal of therapy for adults with established AN.
Introduction
Anorexia Nervosa (AN) is an eating disorder (ED) characterized by self-starvation driven by weight, shape and eating concerns and extreme dread of food, eating and normal body weight ( American Psychological Association [APA], 2013 ; Walsh, 2013 ; Treasure et al., 2015b ). The annual United Kingdom female incidence of AN is approximately 14 cases per 100,000 ( Micali et al., 2013 ), with up to 4% of women and 0.24% of men meeting the broad definition of AN in their lifetime ( Smink et al., 2013 ). The peak age of onset for girls is 15–25 years and for boys is 10–14 years ( Micali et al., 2013 ). AN is associated with poor prognosis and the highest mortality rates of all psychiatric disorders ( Smink et al., 2013 ).
The treatment of choice for AN is talking therapy ( National Institute for Health and Care Excellence [NICE], 2017 ). Yet the disorder has poor rates of remission and high levels of relapse. Current psychological interventions facilitate small change, with better interventions needed ( Bulik, 2014 ; Startup et al., 2015 ). Although early intervention is key to recovery, there is an average delay of 18 months from symptoms emerging to treatment, followed by multiple relapses even following treatment, each lasting 6 years ( PricewaterhouseCoopers, 2015 ). Costs of AN and other EDs to the individual, family and carers, and society are therefore substantial. Time spent caregiving for somebody with severe AN is almost twice that for somebody with a physical health disorder (e.g., cancer) or other mental health difficulty (e.g., psychosis; ( Viana et al., 2013 ). The annual cost to the United Kingdom economy is estimated to be £17.9 billion, offering a “compelling case for change” in services and treatment ( PricewaterhouseCoopers, 2015 , p. 9). There is now significant work underway in this vein; for example, the First Episode and Rapid Early Intervention for Eating Disorders (FREED; Brown et al., 2018 ).
Risk and Maintenance Factors Associated With AN
Anorexia Nervosa has been associated with numerous broad ranging risk and maintenance factors. Risk factors are variables which predict subsequent development of later pathology, in an individual currently disorder and symptom-free ( Stice, 2002 ). Risk factors can have effects mitigated by protective factors or amplified by potentiating factors . Maintenance factors predict symptom persistence versus remission over time in individuals already symptomatic for a disorder ( Stice, 2002 ). Clinical implications of risk and maintenance factors differ; risk factors are relevant to the development of preventative programs and maintenance factors to treatment interventions ( Stice, 2002 ). While we do not fully review evidence for risk and maintenance factors for AN herein; we briefly indicate those most well-accepted and summarize them diagrammatically in Figure 1 . This reveals complex interactions between genetic, biological, psychological, and socio-environmental factors in the development and maintenance of AN, with some factors proposed to represent both risk and maintenance factors.

Current Clinical Perspectives, Models, and Treatment for AN to Date
Psychological models to date use existing psychological theory to address relevant risk or maintaining factors within clinical treatment for AN. Recent guidance published by National Institute for Health and Care Excellence [NICE] (2017) recommends the following psychological interventions be considered for adults with AN: Eating Disorder Focused Cognitive-Behavioral Therapy (CBT-ED); Maudsley Anorexia Nervosa Treatment for Adults (MANTRA); Specialist Supportive Clinical Management (SSCM); and Focal Psychodynamic Therapy (FPT).
CBT-ED traditionally focuses on symptom-based accounts suggesting both control and overvaluation of weight/shape maintain AN. Later revisions include: clinical perfectionism; low self-esteem; mood intolerance; and, interpersonal difficulties as additional treatment foci ( Fairburn et al., 1999 , 2003 , 2009 , 2015 ). Using cognitive and behavioral techniques, it seeks to increase motivation to change, directly enhance weight gain while tackling concerns about weight and shape and prepare for set-backs to maintain gains made ( Fairburn et al., 2009 ).
MANTRA outlines a broader range of putative maintenance factors as treatment targets ( Schmidt and Treasure, 2006 ; Wade et al., 2011 ; Schmidt et al., 2012 , 2015 ; Treasure and Schmidt, 2013 ). Four core factors are included: (1) Rigid, detail-focused, and perfectionist information processing style; (2) Socioemotional difficulties (e.g., avoiding experience and expression of emotions within close relationships); (3) Positive beliefs about the value of AN; and (4) Close others exhibiting high expressed emotion or accommodating/enabling AN behaviors. These factors intensify once in a starved state, further maintaining them. The authors argue that CBT models miss important factors because key difficulties underlying EDs rarely concern ED-related themes (only 1%); with issues of interpersonal difficulties being more significant, including rejection and abandonment (42%); negative self-perception (22%); and emotional experience (20%; Sternheim et al., 2012 ).
Focal Psychodynamic Therapy employs three phases of treatment focussing upon relationships and breaking pro-anorexic belief and behavior patterns ( Zipfel et al., 2014 ). Firstly, it concentrates upon relationship building, therapeutic alliance, identifying pro-anorectic behavior/beliefs and self-esteem. Secondly, relevant relationships are examined and links with AN beliefs/behaviors made. Finally, this is transferred to everyday life and a therapeutic ending.
Specialist SSCM ( McIntosh et al., 2006 ) was originally developed as a comparison ‘treatment as usual’ for use in clinical trials. It combines clinical management and supportive psychotherapy to provide practical support and is not formulation-based; rather it focuses on psycho-education, resumption of eating and normalization of weight.
These treatment developments since the publication of previous NICE guidelines ( National Institute for Health, and Care Excellence [NICE], 2004 ) represent obvious innovation in the application of empirical research to treatment models and clinical practice. However, of these, there remains no clear front runner and it is uncertain which treatment best suits which sufferer of AN. Results from randomized controlled trials (RCTs) indicate that these speciality out-patient treatments do not out-perform each other or control comparisons post-therapy or at follow-up ( Carter et al., 2011 ; Schmidt et al., 2012 , 2015 ; Zipfel et al., 2014 ; Fairburn et al., 2015 ). They demonstrate only small non-significant effect sizes of change ( Watson and Bulik, 2013 ), with around 20% of people weight-restored after 1 year ( Schmidt et al., 2012 , 2015 ; Zipfel et al., 2014 ). Therefore, non-specific control interventions seeking to manage clinical symptoms (e.g., SSCM) appear as effective as complex, empirically driven models, prompting the inclusion of SSCM in NICE guidelines. This falls short of advances made in outcomes from interventions developed for other Axis I disorders, including CBT for depression, generalized anxiety disorder, panic disorder, obsessive compulsive disorder, and Bulimia Nervosa ( Butler et al., 2006 ).
Anorexia Nervosa is notoriously considered ‘difficult to treat’ and, as described, treatment outcomes indicate an unexplained discrepancy between theoretical models based on empirical data findings and clinical application. It may be that even where causal models are available and appear robust, it cannot be assumed that derived interventions effectively manipulate targets. Evidence of the impact of interventions upon proposed maintenance factors is absent in the field and not understanding how change is facilitated is a barrier to developing evidence-based interventions for AN ( Pennesi and Wade, 2016 ). Furthermore, speciality interventions developed to date tend to have complex hypotheses with many diverse target variables. This potentially falls into the trap of an unhelpful ‘everything is relevant’ approach common in mental health research and results in the inclusion of many possible risk or maintenance factors into a causal model ( Kendler and Campbell, 2009 ). It produces heterogeneity across the delivery of an intervention creating difficulty in drawing links between outcomes and causal processes. In addition, while earlier models have sought to describe and address the current clinical presentation of AN, it is imperative that models offer strong theoretical bases and robust consideration of how AN has arisen; a paradigm that the ED field has not optimally employed ( Pennesi and Wade, 2016 ). Previous models may fail to sufficiently consider and adequately account for etiology and phenomenology of EDs ( Fox, 2009 ; Waller et al., 2010 ). Like others, we therefore propose that any explanation for AN must include reference to phenomenological and interpretive aspects of the presentation ( Amianto et al., 2016 ). Moreover, the definition and extrication of risk and maintenance factors is complex, especially for AN which is compounded due to its mixed psychological and physical presentation, and there is a paucity of research examining risk and maintenance factors by differential ED diagnoses (cf. Stice, 2002 ; Jacobi et al., 2004 ; Jacobi et al., 2011 ). Risk factors can be potentiated once in the ill state, or mediated by other variables and maintenance factors can be generated as a consequence of AN, perpetuating the disorder ( Wonderlich et al., 2005 ). Thus, key foci for clinical interventions are difficult to discern and may alter as the disorder progresses.
Building an explanatory developmental framework attempting to understand how factors link together to cause and maintain AN makes it unnecessary to distinguish between risk and maintenance factors and is therefore desirable. We propose that an integrative account of the emergence of risk and maintenance factors and their interplay (including how this is potentiated once AN is established) is required to gain the necessary depth of understanding of the development and presentation of AN to develop and inform interventions.
Aims of the Current Paper
The current paper offers an account of AN integrating risk and maintenance factors by proposing their influence upon development and course of AN may be explained and potentiated by an underpinning unifying explanatory variable of emotional difficulties, giving rise to AN as a disorder of a ‘lost sense of emotional self.’
First, we describe the difficulties with emotion experienced by people with AN forming the basis of our argument of AN as a ‘lost sense of emotional self’ (see the section “Emotional Difficulties in AN”). We present how emotional difficulties link several known risk and maintenance factors for AN throughout development (see the section “Development of AN”). We describe how, once developed, AN becomes self-perpetuating within this context adding to persistence and complexity of disorder and treatment (see the section “Perpetuation of AN: The Ever Decreasing ‘Self”’). Once this core presentation to treatment is outlined (a ‘lost sense of emotional self’), we offer considerations for clinical conceptualization and practice and hypothesis testing (see the sections “Clinical Conceptualization and Practice” and “Future Directions in Testing the ‘Lost Emotional Self’ Hypothesis”).
Emotional Difficulties in AN
Emotions evolved as adaptive processes: they are learnt or instinctive responses to external or internal stimuli, informing about immediate environments, relationships and our needs, with critical impact upon physiology, behavior and cognition, including memory and decision-making capabilities ( Dolan, 2002 ). They evolved to organize and direct human cognitions and behavior ( Cosmides and Tooby, 2000 ). Emotions act as a super-ordinate system; we describe them as the conductor of an orchestra comprised of cognitive, behavioral, physiological and social functions ( Oldershaw et al., 2015 ).
Dysfunctional emotional processing and regulation are proposed to underpin many psychological disorders ( Aldao et al., 2010 ), including EDs ( Aldao et al., 2010 ; Hatch et al., 2010b ; Arcelus et al., 2013 ; Lavender et al., 2015 ; Oldershaw et al., 2015 ; Mallorqui-Bague et al., 2018 ). They play a significant role in the development and maintenance of AN ( Schmidt and Treasure, 2006 ; Haynos and Fruzzetti, 2011 ; Treasure and Schmidt, 2013 ; Wildes et al., 2014 ; Racine and Wildes, 2015 ). Indeed, even earliest descriptions recognize emotional experience as a factor in AN development and maintenance. In 1871, a women with AN was reported to “suffer from some emotions she avows or conceals” (Charles Lasègue cited in Vandereycken and Van Deth, 1990 ). A century later, women with AN were described as having underlying deficiencies in the identification of emotional states and responses ( Bruch, 1962 ). A link between emotional experience and the behavioral expression of AN is clear: potential emotion is avoided by eliciting predictable and controllable behavioral patterns from others ( Treasure et al., 2016 ); focus on food, eating, weight, and shape, as well as cognitive processes such as worry and rumination, affords cognitive distraction from negative thoughts/emotion ( Sternheim et al., 2011 ; Startup et al., 2013 ). Once starved, suppressed physiological experience numbs emotions and is valued ( Miller et al., 2003 , 2009 ; Serpell et al., 2004 ); while starvation and emaciation enables a maladaptive expression of distress ( Serpell et al., 2004 ) and ever-narrowing interpersonal life fuels greater reliance on AN ( Schmidt and Treasure, 2006 ).
Systematic reviews of emotional experience in AN expand on this understanding by providing summaries of experimental and self- report data ( Oldershaw et al., 2011 , 2015 ; Lavender et al., 2015 ), integrating pre-existing theory from the field of emotion regulation ( Aldao et al., 2010 ; Gross, 2013 ). The reviews indicate that, relative to healthy controls, people with AN experience difficulties in emotional awareness (including alexithymia and poor emotional clarity), alongside high self-reported levels of disgust and shame specifically. Difficulties in meta-level processes are also evident, such as elevated maladaptive schema, particularly around defectiveness, dependence/incompetence, social isolation and subjugation, and negative beliefs about having or expressing emotion.
Engagement in many emotion regulation strategies is evident, indicative of a pattern of emotion over-regulation ( Oldershaw et al., 2015 ) ( Figure 2 ). Adaptive emotion regulation strategies are absent, and unhelpful strategies dominant, including: avoiding emotion triggers, such as situations or modifying social interaction (e.g., submissiveness); worry and rumination processes; and emotion suppression, particularly to avoid conflict. It is argued that people with AN are disproportionately reliant upon the feedback of others for reassurance and regulation of emotion (e.g., via social comparison). These findings support the notion that emotional avoidance and unhelpful over-regulation strategies play a central role in AN, including those achieved via anorexic behaviors ( Dolhanty and Greenberg, 2009 ; Wildes et al., 2010 ; Brockmeyer et al., 2012 ; Arcelus et al., 2013 ; Treasure and Schmidt, 2013 ). Indeed, emotional avoidance and submissive behaviors (and not social cognition or neurocognition) are more promising predictors of clinical outcomes following treatment ( Oldershaw et al., 2018 ).

Emotional Avoidance as a Maintenance Factor for AN
In summary, following many other authors highlighting the key role of emotional avoidance in AN (e.g., Wildes et al., 2010 , 2014 ; Treasure and Schmidt, 2013 ), experimental and self-report findings point to a maintenance model of AN as a disorder underpinned by difficulties with emotional experience promoting emotional avoidance and over-regulation ( Oldershaw et al., 2015 ). This model posits that early life factors develop schemata and beliefs that leave somebody vulnerable to experiencing emotion as overwhelming and confusing. Emotion regulation strategies, including ED behaviors, develop in this context as a means to control and prevent triggering emotion. Strategies developed are perceived as useful in the first instance, generating an initial reduction in emotion. Ultimately, however, they are maladaptive methods of controlling and regulating emotion serving only to trigger further negative emotional experience and reinforce negative beliefs and schemata, thereby increasing reliance upon (maladaptive) emotion regulation strategies: hence a vicious cycle develops ( Oldershaw et al., 2015 ).
Emotional Avoidance and the Impact on Development of Self in AN
In the current paper, we consider the implications of this persistent emotional avoidance cycle and seek to further this as an explanation by considering both how it arises and its consequences. We will argue that this picture of emotional avoidance and over-regulation is shaped by and further impacts development of self. As such, we will propose that it is not simply a difficulty with emotional experience, interpersonal experience or emotion regulation that is observed and should be tackled in treatment, but the consequential impact of this on development and awareness of a core ‘emotional self.’
We posit that difficulties with emotion are so pervasive due to their integral part of our existence: the basis of the self and emotion are shared and inextricably linked ( Damasio, 2003 ). Primary emotional experience emerges from physical arousal, including interoceptive awareness, in addition to complex processes such as neural activation ( Barrett et al., 2007 ) and emotional memory networks ( Greenberg, 2004 ). Interoceptive emotion signals afford us a mental representation of selfhood experienced within the body – “a material me” ( Seth, 2013 ). Through such processes, emotion becomes fundamental to the construction and organization of ‘self’ with largely bottom-up interoceptive hierarchies of emotional experience interacting to motivate and direct behavior ( Damasio, 2003 ; Greenberg, 2004 ). Thus, there is a process of non-conscious emotional experience, giving rise to conscious thinking and feeling states, which are self-regulated ( Hatch et al., 2010b ).
‘Self’ is the overarching concept used to describe the organization and integration of our many identities; who I am within each relationship and every social interaction ( Haworth-Hoeppner and Manies, 2005 ). It is argued that our identity is ‘rooted in emotion, emerging in relationships, developing as a dynamic self-organizing system’ ( Bosma and Kunnen, 2001 , p. 5). If we cannot access those bottom-up emotional hierarchies (“a material me”), we are without the conductor of the orchestra (our emotional sense of self). If the conductor is not functioning or appears absent, the orchestra descends into disorder. It must muddle through as best it can, desperate to conceal in-fighting, misattunement and confusion, while persistently reliant upon and sensitive to audience feedback to ascertain if it is doing an adequate job.
People with AN lack interoceptive perception of their own body and the internal bodily sensations which give rise to the basic form of self-awareness and emotion perception ( Gaudio et al., 2014 ; Stanghellini et al., 2015 ). It is suggested that AN emerges in this context of vague and overwhelming emotional experience both to regulate emotion ( Oldershaw et al., 2015 ) and to regain a sense of bodily self, defined by the other’s gaze and external appearance ( Monteleone et al., 2017 ). Indeed, shape and weight concerns are thought to arise as the result of a disturbance in experience of one’s own body (embodiment) and are influential in how personal identity is determined ( Stanghellini et al., 2012 ). People with AN have described the disorder as a means of forging a new identity ( Nordbo et al., 2006 ) which becomes inexplicably linked with, or replaces, the ‘true’ self ( Williams et al., 2016 ). As such, AN has been referred to as a “false self” ( Bruch, 1988 ), arising due to an otherwise unstable and fragile self ( Karwautz et al., 2001b ) unable to integrate the body ( Amianto et al., 2016 ). In this paper, we further define this difficulty with self by arguing that AN arises specifically from a lost emotional sense of self . That is, a person struggling to navigate the world, themselves and others, without an emotional conductor to guide them, increasingly reliant upon feedback of others.
Development of AN
Here, we build a picture of the development of AN, moving from infancy through to adolescence, seeking to offer an explanatory developmental framework for the emergence of AN in the context of a ‘lost emotional self,’ drawing on identified risk and maintenance factors, and highlighting how they are unified by being both influential upon and potentiated by emotional difficulties.
Infancy and Early Life
Attunement and attachment.
Difficulties with emotional awareness and regulation in EDs are hypothesized to have their origins in childhood attachment ( Arcelus et al., 2013 ). At the earliest stage of infancy, maternal reflective functioning and attunement are critical to emotional development. Reflective functioning is caregiver capacity to hold her child’s emotional and mental states in mind. Attunement is caregiver responsiveness and ability to identify, model and name emotional experiences for a child. They are core to attachment, which enables the capacity to identify the feelings of self and others and to integrate them into a felt emotional experience, forming a template for self and others in close relationships (‘internal working models’; Bowlby, 1969 ). The mother acts as container for her child’s unbearable feelings, such as anger and distress, with her responses shaping the child’s future responses to emotions ( Bion, 1962 ). Through this process, emerging emotional awareness enables a gradual articulation of self-experience ( de Groot and Rodin, 1994 ) and differentiation ( Mahler, 1963 ). This requires a “goodness-of-fit” between maternal and infant temperament ( Belsky, 1984 ). When present, adequate mutual regulation of interactions and communication of needs and attention are possible. Yet this is an extremely sensitive process; even incongruence as early as 8 months old can impair neural discrimination of emotional facial expressions ( Rajhans et al., 2016 ).
Over 35 years ago, Bruch advocated a role of attachment and early social experiences in the development of AN ( Treasure and Cardi, 2017 ). Insecure patterns of attachment of people with AN to primary caregivers have been reported in adults ( Zachrisson and Skarderud, 2010 ; Tasca and Balfour, 2014 ) and adolescents ( Gander et al., 2015 ; Jewell et al., 2016 ; Pace et al., 2016 ), of large effect ( Caglar-Nazali et al., 2014 ). Further, people with AN have particularly low levels of reflective functioning ( Tasca and Balfour, 2014 ). This matches a similar pattern of attachment in the mothers of those with AN ( Ward et al., 2001 ; Pace et al., 2015 ). Higher attachment anxiety is significantly related to greater ED severity and poorer treatment outcomes ( Illing et al., 2010 ). A discrepancy in the fit between environment and the temperament of the individual who develops AN is proposed to lead to an experience of social environments as invalidating or demanding ( Karwautz et al., 2001a , b ; Corstorphine et al., 2007 ; Oldershaw et al., 2015 ). Chronic misattunement (“empathic failure”) is argued to affect the development of any or all sense of self and relate to ED development ( de Groot and Rodin, 1994 ).
AN is more prevalent for females than for males with ratio of 1:8 ( Steinhausen and Jensen, 2015 ). Female preponderance of EDs is often hypothesized to reflect cultural ideals, particularly internalization of the thin ideal and objectification of women. Yet this fails to adequately address the complexity of gender and gender role socialization. Gender differences in emotion expression arise as a complex combination of biological determinants, socialization, social context and cultural expectations ( Chaplin, 2015 ). We argue that gender socialization and social construction of emotion expectations from infancy plays a role in women becoming particularly at risk of developing an ED.
From an early age, parents socialize emotional expression based on gender stereotypes or expectations; encouraging internalizing emotions such as sadness, fear, shame and guilt and discouraging anger expression in girls, while the opposite is observed for boys ( Zeman et al., 2012 ). Girls are expected to display a greater array of emotions than boys, including positive emotions ( Brody and Hall, 2008 ), yet, perhaps due to biological differences, need more encouragement to learn to express their emotion than boys ( Chaplin, 2015 ). Consequently, girls have been observed to display more internalizing emotions than boys, with the strongest differences found for fear and shame ( Chaplin and Aldao, 2013 ). High levels of internalizing difficulties and emotions (particularly shame) are prevalent for people with AN ( Oldershaw et al., 2015 ).
The ‘self-in-relation’ (capacity for identification with and relatedness to the mother) is considered more important to female identity than male ( Gilligan, 1982 ). Miscoordinated interactions with the caregiver leads to a sense of ineffectiveness more prone with mother–daughter than with mother–son dyads ( Tronick and Cohn, 1989 ). Mothers see daughters as a continuation of themselves more than they do sons, thus affecting the extent to which girls are experienced as separate and individual ( de Groot and Rodin, 1994 ). Further, girls in particular may base their emotional relatedness on parental feelings rather than their own emotion; facilitating intimacy with the mother, but interfering with girls’ experience of their own unique emotional world ( de Groot and Rodin, 1994 ). Indeed, women can find it difficult to define themselves without first contextualizing ‘self’ within the mother–daughter relationship ( Miller-Day, 2012 ). In general, girls feel less powerful in parental relationships than boys ( Buhl, 2008 ). Thus, females value interconnected social and emotional experience more than males, but at the expense of developing their own unique emotional self-awareness and experience. Such patterns are observed in higher levels with people with AN. They report lower perceived individual autonomy and higher perceived cohesion in their relationship with their mother than unaffected sisters, although have similar perceived emotional connectedness ( Karwautz et al., 2003 ).
Females have greater innate empathy than males, linked not only to socialization, but resulting from evolutionary processes creating neurobiological differences ( Christov-Moore et al., 2014 ). Girls are expected to offer more empathy and sympathy than boys, and are socialized to this end ( Knafo et al., 2008 ). Girls are more sensitive than boys to the responses of others, including both approval and rejection, with girls’ need for approval superseding their striving for autonomy ( McClure, 2000 ). As such, females may consider their own subjective experience or evaluations as less valid than those of others. Indeed, it has been argued that mothers (consciously and unconsciously) direct their daughters in “gender appropriate ways” including offering the message their own “needs” and certain emotions are unacceptable ( Orbach, 1993 cited in Richmond, 2002 ). This can include modeling for girls “feminine” emotion expression such as appearing cheerful, even where this is not felt ( Chaplin, 2015 ). Girls demonstrate greater suppression of emotions, particularly in relation to their own goals or needs (e.g., disappointment; Saarni, 1984 ).
This presents an image of girls as sensitive to the feelings of others, likely to suppress their own emotions and needs, and to experience the self via the other (mother). These features may be heightened for people who go on to develop AN. People with AN report low levels of their own emotional awareness, alexithymia (inability to identify and describe their own feelings), limited expression of their own emotion and submissiveness, especially to avoid conflict ( Oldershaw et al., 2015 ). They suppress facial expression of emotion and use fewer words when describing their emotional experiences as compared with healthy people or those recovered from AN ( Davies et al., 2011 , 2012 , 2013 ). They thus inhibit the expression of their own emotion ( Gramaglia et al., 2016 ) and autonomy ( Karwautz et al., 2003 ) while seeming able to acknowledge others’ emotions. Individuals with AN during both starvation and weight restoration report greater empathy than healthy controls ( Hambrook et al., 2008 ), including in the domain of personal distress (vicarious negative arousal to others’ suffering; Beadle et al., 2013 ). They appear highly motivated to understand the feelings of others, ‘hyperscanning’ stimuli relating to others while avoiding visually attending to salient features of their own facial image ( Phillipou et al., 2015 ). They have low self-directedness, scoring highly on ‘Other-Directedness’ ( Bachner-Melman et al., 2009 ). People currently with AN have significantly less self-focussed attention than those who are weight restored ( Zucker et al., 2015 ), while tending to self-attribute negative over positive social interactions ( McAdams et al., 2018 ). Perhaps unsurprisingly then, adolescents with disordered eating devalue personal subjective experience, and favor socially accepted externally validated ideals (e.g., “thin ideal”) as their own ( Steiner-Adair, 1990 , cited in de Groot and Rodin, 1994 ).
It is of note that the male incidence of AN has increased in recent decades, in the absence of any such increase in bulimia nervosa ( Steinhausen and Jensen, 2015 ). Attitudes toward gender roles have shifted substantially since the 1980s, including toward roles in relationships, parenting, and women’s participation in the labor market ( Park et al., 2013 ). It is possible that changes in gender role norms, as well as in emotion socialization may have ensued, including changes in mother–son dyad relationships; further research is required to investigate this. We propose that any such shifts could in part relate to this increase in male AN presentations.
Food and Communication
Effectively feeding one’s children is of innate importance, essential for well-being and survival. Up to 52% of infants and toddlers are viewed by caregivers as experiencing feeding problems ( Reau et al., 1996 ). Even feeding healthy children can be time-consuming, tedious and exasperating: up to 15 exposures of a new food can be required before it is trusted enough to even be tasted ( Wardle et al., 2005 ). Moreover, around age two, children undergo a developmental change, causing them to reject foods previously liked and accepted ( Cooke et al., 2003 ) triggering potential frustration and anxiety for parents. Emotions and mealtimes are strongly linked, and although largely positive in valence, negative emotions such as disgust and guilt are aroused in subgroups described as “indifferent restrictives” ( den Uijl et al., 2014 ). Indeed, adults’ food preferences and emotions can be traced back to early negative experiences such as pressure to eat ( Batsell et al., 2002 ), with less pressure predicting food enjoyment ( Webber et al., 2010 ). Moreover, EDs have been retrospectively linked to greater use of food for communication and emotion regulatory purposes, such as rewarding, comforting, or punishing ( Mazzeo and Bulik, 2009 ). Thus, even preverbally, children learn to associate food with feeling misunderstood, stress and anxiety. Further, using food and eating as communication and for emotion regulation may be fostered and is observed in girls as young as five ( Carper et al., 2000 ).
People who develop AN have increased early life experiences generating sensitivity to and preoccupation with somatic experience, including early gastrointestinal events and eating difficulties, with evidence pointing to digestive problems as a risk factor for AN ( Marchi and Cohen, 1990 ; Jacobi et al., 2004 ). Once people develop AN and caregivers become concerned, food offers a way to communicate needs valued by the person with AN ( Serpell et al., 2004 ). Emotion expressed around eating, and engagement with the ED by parents, may represent an emotional engagement that is reinforcing, but ultimately leaves the sufferer feeling overwhelmed and misunderstood in the moment serving only to remove them further from developing an awareness and acceptance of authentic internal experiences.
Infancy and Early Life: Summary
People who develop AN may be those with poor fit in their environment, leading to poor attunement and insecure attachment. Consequently they may feel invalidated as a separate, unique person, and be left with a sense that their own emotions and needs are less important than those of others and should be suppressed. It follows that this could result in poor emotional awareness and regulation, in the context of attempts at pleasing or meeting the emotional needs of others. Even from an early age, food and mealtimes may represent periods of heightened emotion and emotional communication for both parent and child.
Adolescence
Anorexia Nervosa can affect people of any age, gender, culture; however, adolescent and young adult females are most at risk ( Zipfel et al., 2014 ). AN typically emerges in early- to mid-adolescence ( Herpertz-Dahlmann, 2009 ), highlighting adolescence as a risk factor in its development. Adolescence is a challenging period emotionally and socially with consequences for emotion regulation and psychological adjustment ( Miller-Slough and Dunsmore, 2016 ). It is a critical period for developing identity ( Pfeifer and Berkman, 2018 ) associated with emergence of self-concept and enhanced self-awareness ( Weil et al., 2013 ). The ‘self’ develops driven by a key intersection of social, cognitive, affective, motivational, and regulatory processes ( Pfeifer et al., 2013 ). As such, adolescence is a phase of life representing an opportunity, yet also a vulnerability ( Fuhrmann et al., 2015 ).
Parents and Identity
Identity development during adolescence has roots in the parent–child relationship. Separation–individuation factors such as autonomy-supportive parenting, separateness from parents, and personal autonomy are crucial ( Luyckx et al., 2006 , 2007 ; Beyers and Goossens, 2008 ). Early developmental interactions with parents, and whether they met identity needs of parent or child, can have implications for identity development and separation–individuation in adolescence ( Koepke and Denissen, 2012 ). Autonomy supporting parents promote a belief in personal agency by enabling expression of identity and opinion without fear of parental rejection or engulfment ( Luyckx et al., 2007 ). To fully engage in adult-to-adult relationships with grown up children, parents need to let go of the part of their own identity of “omnipresent caretaker” ( Koepke and Denissen, 2012 ). And yet, emotion socialization continues to rely on parents. The need to offer appropriate emotional guidance is enhanced by new social demands and risks ( Garcia and Scherf, 2015 ). Thus, adolescence is a challenging time of adaptation for parents, alongside their child.
Separation–individuation processes during adolescence may be particularly problematic for people who develop AN, and may follow from interactions during early life described above. Women with AN identify boundary violations by their parents within the family ( Rowa et al., 2001 ). Adolescents with AN rate their families as less communicative, flexible, cohesive, and more disengaged, compared to control participants ( Laghi et al., 2017 ). Maternal criticism and emotional over-involvement links to ED severity ( Kyriacou et al., 2008 ; Duclos et al., 2014 ). High levels of expressed emotion is included in maintenance models of AN and reflects a description of families as critical, hostile and/or emotionally over involved and overprotective, particularly toward the person with AN ( Schmidt and Treasure, 2006 ; Treasure and Schmidt, 2013 ). Thus, there is a traditional view that families of people with AN are enmeshed and rigid in their style ( Minuchin et al., 1978 ). This fits with the presentation described: a person with AN unsure of their own internal world, seeking to meet the needs of others over their own. Indeed, adolescents with EDs have lower levels of self-differentiation, indicating high emotional reactivity, emotional cut-off, and greater fusion with others, causing confusion between one’s own emotional and mental states and those of others ( Doba et al., 2018 ). Consequently, people with AN report a longing for independence seen as a reaction to helplessness ( Karwautz et al., 2001b ). Taking such an oppositional stance by refusing to eat in the face of desperate parental persuasion may reflect a need for adversarial transference ( de Groot and Rodin, 1994 ), thus, enabling growth of the person with AN and a sense of separateness through assertion of opposition. The instinct for individuation may also be a factor in seeking external validity of their worth via social reinforcement from others.
Although, some of these relational patterns can be observed in earlier childhood, there may be increased consequences during the adolescent period, when identity is struggling to emerge, and there are also shifts in the most adaptive response to emotion. Parents therefore need to adjust to this developmental phase, shifting in their socialization of emotion and demonstrating flexibility in emotional responding. In infancy and early childhood, focussing on the child’s emotional experience functions to encourage recognition and labeling of emotion supporting basic emotion knowledge and self-regulation ( Sanders et al., 2015 ). By contrast, during adolescence, encouraging excessive focus on emotions, particularly via increased expression of parental reactive emotion or parental matching of emotion, can prolong a negative emotional state resulting in emotion dysregulation and psychopathological risk ( Brand and Klimes-Dougan, 2010 ; Moed et al., 2015 ). Reassurance or distraction to a positive activity is a more adaptive response better alleviating distress. Yet, magnifying is more commonly used for girls than boys and there is a more robust link between magnifying responses and adolescent psychological problems for girls over boys ( Klimes-Dougan et al., 2014 ). Indeed, girls show higher rates of depression and anxiety than boys, starting in adolescence, and which is associated with internalizing negative emotions of sadness, guilt, and fear ( Chaplin, 2015 ). High expressivity of parental negative emotion relates to internalizing symptoms, depression and anxiety in adolescents ( Suveg et al., 2008 ; Luebbe and Bell, 2014 ), all of which are associated with development of AN ( Adambegan et al., 2012 ). High levels of parental emotion dysregulation are associated with invalidation of adolescent’s emotional expression, and in turn results in adolescent’s emotion dysregulation ( Buckholdt et al., 2014 ). Equally, by being dismissive of emotion, parents can transmit beliefs that emotions are dangerous or invalid and need to be suppressed ( Morris et al., 2007 ). These beliefs are reported by adults with AN ( Hambrook et al., 2011 ). Therefore, parental emotion socialization is found to be crucial to emotion regulation and adolescent psychological outcomes ( Miller-Slough and Dunsmore, 2016 ), and appears to have a higher cost for female adolescents, and with direct relevance to the development of AN.
Parents enabling the emergence of a child’s own narrative voice is fundamental to development of healthy identity ( Fivush and Zaman, 2015 ). The stories adolescents know about their parents (intergenerational narratives) are critical for understanding self ( Fivush and Zaman, 2015 ). Story telling narratives offer a coherent sense of temporal, autobiographical self, within which discrete experiences can be embedded and understood ( Habermas and Bluck, 2000 ). This is scaffolded by story-telling with parents during early years ( McLean and Jennings, 2012 ). Development of narrative identity is also subject to gender differences. Mothers are especially important in helping their adolescent child to construct narratives around emotion and vulnerability (being overpowered by negative emotion) ( McLean and Mansfield, 2012 ). Mothers employ more emotion words and discuss the causes of emotion when reminiscing with their children than fathers ( Fivush et al., 2000 ). Moreover, both mothers and fathers use more emotion words when constructing narratives with preschool daughters than sons, focusing on elaboration of emotions such as sadness and social-relational themes, with mothers–daughter dyads being most emotionally expressive ( Reese and Newcombe, 2007 ). Meanwhile, adolescent boys receive more supportive scaffolding from mothers than girls do ( McLean and Mansfield, 2012 ). The ability of children and their parents to tell detailed stories about negative emotional past events, cognitive-processing and emotion words, are related to adolescent well-being ( Fivush et al., 2008 ).
It is possible that there is diminished parental scaffolding of emotional narratives and narrative identity is for those who develop AN. It is known that people with AN use fewer words when describing their emotional experiences compared with healthy people or those recovered from AN ( Davies et al., 2011 , 2012 , 2013 ). People with AN recall over-generalized autobiographical memories, reflecting lack in ability to integrate positive and negative emotional experiences, and which worsens with disorder duration ( Nandrino et al., 2006 ). Memories are associated with less negative emotion expression than healthy people, especially at a lower weight ( Brockmeyer et al., 2013 ). This may reflect poorer narrative identity within which emotions and emotional self cannot be embedded, further impacting difficulties in individuation and self-differentiation.
Peers and Identity
During adolescence, emotional and social pressures significantly increase, alongside volume and complexity of social experience. Friendships grow in salience as young people seek out peers for emotional support and explore their identity outside of the family context ( Jobe-Shields et al., 2014 ). Peer interactions become more influential and their quality reflects that of early attachments ( Lieberman et al., 1999 ). Adolescence is therefore considered a time of ‘social reorientation’ ( Meeus et al., 2005 ; Nelson et al., 2016 ). Influence of peers disproportionately affects behaviors such as risk-taking and relational reasoning during adolescence ( Wolf et al., 2015 ). Adolescents are highly attuned to peer evaluation ( Somerville, 2013 ). They are hypersensitive to social exclusion, particularly in younger adolescence ( Sebastian et al., 2010 ), with older adolescents especially fearful of peer evaluation ( Westenberg et al., 2004 ). There is also acceleration of gender-differentiation and an intensification of gender role expectations ( Hill and Lynch, 1983 ).
Early emotional difficulties outlined above, such as alexithymia, emotion acceptance and regulation, alongside suppression of own emotion and needs, may make an individual particularly vulnerable during adolescence when peer relationships and social acceptance become so vital. Social cognition (mental processes underlying human social behavior and interaction; ( Adolphs, 2001 ) is crucial in successfully negotiating complex social interactions and decisions ( Crone, 2013 ). However, the social brain network undergoes protracted development throughout adolescence before stabilizing in the mid-twenties ( Giedd et al., 1999 ; Gogtay et al., 2004 ; Sowell et al., 2004 ; Barnea-Goraly et al., 2005 ; Shaw et al., 2008 ). Capacity for introspection or ‘metacognition’ (reflection on our thoughts and behaviors) begins to slowly emerge and gradually improve throughout adolescence ( Weil et al., 2013 ). Younger adolescents are relatively focused on self-oriented choices; impulse control and perspective taking afford increased consideration of consequences for others later in adolescence and early adulthood ( Crone, 2013 ). Further, change in neural and hormonal activity impacts social cognitive abilities, with attachment and mentalization (identifying or inferring mental states of self and others) appearing to enter a state of flux ( Jewell et al., 2016 ). At puberty (around age 11), performance dips on some social cognitive abilities, such as facial emotion recognition and perspective taking, before gradually recovering; a process thought reflect the sudden proliferation of synapses at puberty which are pruned during adolescence ( Blakemore and Choudhury, 2006 ).
People with AN may have impaired emotion recognition and theory of mind (of small to medium effect), that precede AN development, and are further exacerbated by secondary consequences of starvation ( Oldershaw et al., 2011 ; Caglar-Nazali et al., 2014 ; Bora and Kose, 2016 ). Difficulties recognizing emotion from blended facial expressions ( Dapelo et al., 2016 ), tone of voice ( Kucharska-Pietura et al., 2004 ; Oldershaw et al., 2010 ), body movement ( Lang et al., 2015 ) and affective touch ( Crucianelli et al., 2016 ) are reported. A drop in these already potential (trait) difficulties during adolescence is likely to cause distress and increase reliance on external feedback, while simultaneously decreasing ability to define self as a ‘self-in-relation,’ increasing an already impoverished sense of self. Further, identity and other associated self-related processes become a source of information with which to shape decision-making and intrinsic motivations across adolescence ( Pfeifer and Berkman, 2018 ), in the context of strong extrinsic social forces ( Wolf et al., 2015 ). In the absence of a clear developing self, it is possible that extrinsic motivations (e.g., thin ideal; others’ needs) become further reinforced as the key driving force of decisions and behaviors. Thus eating, weight and shape cognitions begin to emerge and drive behavior.
Adolescence and Body Image
During adolescence physical shape changes, and awareness of and focus on the body heightens. Adolescents are influenced by and fearful of peer exclusion and evaluation, and this likely includes body and image valuations. EDs are associated with higher family standards on physical appearance ( Gunnard et al., 2012 ). Perfectionism, eating and weight concern are observed at higher levels in mothers of people with AN than in comparison groups ( Woodside et al., 2002 ). The link between insecure (both anxious and avoidant) attachment and EDs is largely mediated by emotion dysregulation, including social comparison ( Dias et al., 2011 ; Ty and Francis, 2013 ). High externalized self-perceptions and attributions about the importance of weight and shape for popularity and dating by adolescent girls predicts body esteem and eating behavior ( Lieberman et al., 2001 ).
As shape changes, an adolescent vulnerable to AN may receive comments from family or peers that are perceived as external validation. They may seek to manipulate their body through dieting resulting in its conditioned positive reinforcement ( Walsh, 2013 ). Adolescents are particularly sensitive to reward ( Steinberg, 2004 ), with sensitivity to reward and punishment even greater for those at risk of AN ( Harrison et al., 2010 ; Jappe et al., 2011 ). Initial weight loss is often met with compliments (extrinsic reinforcement); a validation that for a person with the early life experiences described above may finally feel like an achievable goal that is self-motivated (perceived intrinsic achievement).
Adolescence: Summary
Adolescence is a time of increased sensitivity to peer rejection and evaluation. It is expected that emotion socialization in infancy and early-mid childhood will afford a child with the foundational skills in emotion recognition, regulation, and expression with which to meet the social and emotional challenges of adolescence. Self-reflection and identity emerging during adolescence is built upon experience and abilities that have been developing since early childhood ( Reese et al., 2010 ). For somebody entering this life stage with poor emotion awareness and a need to please others, such challenges become magnified and may be further exacerbated by familial patterns. The emergence of identity within this context is especially likely to hinge on sources of external evaluation and validation. Thus, poorly integrated identity is built, which, in light of the pervasive ‘thin ideal’ within Western society and increased bodily attention due to puberty, may make somebody particularly drawn to a concrete externally validated sense of self. AN becomes a means to better oneself ( Bates, 2015 ) and to find validity and direction in the face of expectations of what is perceived others want and which is seen as functional and valued.
Perpetuation of AN: the Ever Decreasing ‘Self’
One of the challenges in working with people with AN is that, once established, there appears an ever-tightening vicious cycle. Increased severity links to increased positive egosyntonic beliefs about the value of AN ( Serpell et al., 2004 ). In many ways, AN becomes self-perpetuating due to its reinforcement of social, emotional and behavioral patterns and the impacts of starvation itself. We argue that these factors further distance an individual from an ‘emotional self’ and thus from recovery; hence AN becomes ‘self-perpetuating’ in and of itself, beyond risk and maintenance factors.
Emotion Over-Regulation and Perpetuation of Interpersonal Patterns
As discussed throughout the Section “Development of AN,” low levels of expression and high emotion suppression are observed amongst people with AN, including in the context of avoiding conflict ( Oldershaw et al., 2015 ). The impact of emotion suppression in healthy people is increased negative feeling, decreased positive feeling, and decreased emotion expressivity, cardiovascular activity, and oxygenation ( Gross and Levenson, 1997 ; Dan-Glauser and Gross, 2011 ). Thus, emotion suppression paradoxically leaves somebody with greater negative emotion to regulate, reinforcing their need for emotion regulation strategies. Suppression elicited in experiments with healthy participants reduces interpersonal responsiveness during face-to-face interaction, increasing negative partner-perceptions and hostile behavior ( Butler et al., 2007 ). When watching film clips, people with AN report stimuli incongruent emotions, with limited facial expression ( Lang et al., 2016 ). Inaccurate signaling predicts higher levels of depression and lower levels of well-being, mediated by social connectedness, since accurately signaling emotion states enhances social connectedness ( Mauss et al., 2011 ). Therefore, suppressing emotions, reducing emotion expression and displaying emotions incongruent with those felt, impedes the congruent responding of others. This could perpetuate ‘attunement’ difficulties, echoing those proposed in childhood, further invalidating a true sense of self. Indeed, even when others actively seek to attune to the person with AN, it may paradoxically decrease social connectedness. AN is associated with high levels of internal shame ( Grabhorn et al., 2006 ). People with high levels of internalized shame display shame even following positive task feedback intended to elicit positive emotion ( Claesson et al., 2007 ). Thus, people with AN may experience negative emotions in response to situations more commonly viewed as positive. Further, they have difficulty in distinguishing between positive and negative feedback and report social anhedonia that relates to disorder severity and alexithymia, even following recovery ( Wagner et al., 2007 ; Tchanturia et al., 2012 ).
There may therefore be numerous ways in which the expected response from others (on both sides) is misinterpreted; social interaction and connection becomes constantly further misattuned. This increases negative emotional experience, particularly shame, and only makes the need for emotional regulation strategies, including social reinforcement, feel more urgent: unhelpful cycles are strengthened, AN becomes embedded and helpful regulation strategies cannot be developed. This fits with reports that people with AN feel they have insufficient emotion regulation strategies available ( Harrison et al., 2009 ; Oldershaw et al., 2015 ). Actual success in emotion regulation follows expected success ( Bigman et al., 2016 ) further limiting potential emotion regulation abilities, stifling validation of felt emotional experience and cues.
Starvation Effects
Once AN has developed, starvation effects contribute to self-perpetuation of AN. Starvation reinforces existing psychological difficulties and underpins development of new difficulties. Starvation alone can trigger a reinforcing feedback loop arousing evolved physiological mechanisms, triggering urges to binge eat, increased metabolic efficiency and fat storage, reinforcing fear of fatness and generating renewed attempts at restriction ( Nesse, 2017 ).
Interoception
Starvation perpetuates difficulties via its influence on physiological feedback from the body. People with AN struggle to discriminate between bodily sensations ( Skǎrderud, 2007 ), with limited access to physiological experience of emotion when underweight ( Miller et al., 2003 ). Interoceptive cues are abnormally interpreted, resulting in erroneous judgments about internal bodily states ( Kaye et al., 2009 ) and an imbalance between external and internal perception of body relating to ED symptomatology severity ( Eshkevari et al., 2014 ). Thus, there is a disconnection between cognitive and physiological information ( Nandrino et al., 2012 ). There may also be high levels of internal incongruence, with reported emotion mismatching physiological arousal and reactivity ( Oldershaw et al., 2011 ; Nandrino et al., 2012 ). Low weight further lessens physiological experience of emotion, despite increased self-reported arousal ( Miller et al., 2009 ). This results in interoceptive confusion and the experience of emotions as vague and overwhelming. Such poor interoceptive awareness is increasingly recognized as a core feature of AN, contributing to its emergence and maintenance ( Kaye et al., 2009 ; Nandrino et al., 2012 ; Strigo et al., 2013 ).
Interoceptive awareness does not simply inform us of our emotion experience, but of our sense of self; thus disrupted interoceptive processes and feedback, deepened by starvation, further damages emotional sense of self for people with AN. This sense of self is generated, not only by the bottom-up process of interoceptive emotion signals, but also a counter flow of top-down feedback on predictions of their outcomes (such as emotional validation or reflection by others); this establishes accuracy of bottom-up signals and highlights prediction errors ( Seth, 2013 ). Where there is a discrepancy, for example, where what we feel and observe (external feedback) are not highly correlated, we are driven to reduce the discrepancy and revise our predictive model accordingly ( Talsma, 2015 ). This is achieved by altering our sense of internal or external perception. For example, by updating our internal sense of emotional experience to match the one mirrored back to us or updating predictions of the environment in future, with greater reliance on seemingly more precise data (e.g., visual over proprioception/interoception; Seth et al., 2011 ). It is argued that interoceptive signals in people with EDs are insufficient to accurately predict consequences ( Riva and Dakanalis, 2018 ) or integrate emotional information with sensory experience ( Nunn et al., 2008 ). Even where one does act on one’s felt emotional sense, as described, a lack of attunement may result in non-corresponding feedback. For these reasons, people with AN may be subject to large prediction errors, furthering disruption of interoceptive emotion information and encouraging adjusting or quietening interoceptive feedback (even if felt) in favor of external feedback or heightening desire to manipulate external feedback (e.g., via submissiveness). Inability to integrate or reconcile predictions and confirmations causes persistent anxiety and sense of uncertainty. In this case, minimizing the discrepancy can lead to incorporation of the external signal as part of the self-representation, especially for people with low interoceptive sensitivity ( Seth, 2013 ). Self-objectification (experiencing one’s body via the perspective of an external observer) significantly predicts onset and maintenance of EDs over other more commonly proposed factors (e.g., dieting, body dissatisfaction; Dakanalis et al., 2017 ).
This hypothesis fits with observed patterns of anxiety, intolerance of uncertainty and over reliance on emotion regulation strategies (via continual checking, worry/rumination processes, submissiveness and social reassurance seeking). It further invalidates internal emotional experience and sense of self. Disrupted interoceptive awareness due to suppression and starvation effects once AN is established may result in an inability to update bodily memory or representation, in part explaining persistent belief in ‘fatness’ or lack of ‘insight’ into the physical severity of the disorder, even when the body becomes dangerously emaciated ( Riva and Dakanalis, 2018 ).
The Starved Brain
Abnormalities in brain functioning are reported in AN while ill, and following recovery ( Schmidt and Campbell, 2013 ). Findings of altered brain structure and function demonstrate that the brain is affected by prolonged malnutrition ( Bang et al., 2017 ), increasing damage and functional disability over time ( Treasure and Russell, 2011 ; Treasure et al., 2015a ). Indeed, differences in gray matter volume are not observed between newly diagnosed adolescents and healthy comparisons ( Olivo et al., 2018 ). Here, we briefly review key observations in brain functioning for people with AN and how these become exacerbated by the starved state, linking to emotional processing and sense of self.
The amygdala is considered the brain’s ‘threat detector,’ central to fear conditioning ( Fossati, 2012 ) and highly responsive to emotional stimuli, particularly faces ( Sergerie et al., 2008 ). Amygdala hyperactivation is observed in adolescents compared with younger children and adults, and does not habituate in anxious individuals due to poorer connectivity with areas providing top-down emotion inhibition (ventromedial prefrontal cortex) ( Hare et al., 2008 ). Adolescents have elevated amygdala-hippocampal complex responses when anticipating social feedback; activation which persists following feedback of rejection for anxious individuals ( Lau et al., 2012 ). Hyperactivity in the amygdala is argued to have a pivotal role in AN ( Joos et al., 2011 ). When exposed to disorder related (e.g., food) and ‘non-disorder related’ stimuli (including emotion stimuli), people with AN exhibit greater amygdala activation ( Seeger et al., 2002 ; Miyake et al., 2010 ; Joos et al., 2011 ; Seidel et al., 2018 ), and heightened fear response ( Friederich et al., 2006 ), indicating heightened emotional arousal ( Seidel et al., 2018 ). Hatch et al. (2010a) argue that for people who develop AN, there is early non-conscious amygdala hypersensitivity to emotion cues, irrespective of weight or nutritional status. It is suggested that bias toward emotional stimuli, poor recognition of facial emotion expression and lack of soothing may be linked to the hyperactive amygdala in AN, persistent following recovery ( Oldershaw et al., 2011 ). Greater ability to tolerate emotion sensations may occur following recovery, in spite of continued increased amygdala activation ( Merwin et al., 2013 ). This suggests a trait amygdala hypersensitivity for people who go on to develop AN contributing to the experience of emotion as overwhelming and aversive, and which may become further heightened during adolescence corresponding with disorder onset.
Prefrontal cortex
During displays of negative emotion stimuli, increased activity is also found in the right and left dorsolateral and ventromedial prefrontal cortex ( Leppanen et al., 2017 ; Seidel et al., 2018 ). Inhibition of negative affect is associated with activation of dorsal anterior cingulate, dorsal medial prefrontal, and lateral prefrontal cortices, and attenuation of brain activity within limbic regions ( Phan et al., 2005 ). This pattern of activity is therefore argued to reflect active control mechanisms in response to emotion, considered particularly necessary in the context of high levels of amygdala activity ( Seidel et al., 2018 ). Studies using resting state and task-based fMRI also appear to support the use of prefrontal mediated cognitive control and self-control processes in AN ( Wierenga et al., 2014 ; Ehrlich et al., 2015 ; Boehm et al., 2018 ) with increased prefrontal activation also observed following AN recovery ( Wierenga et al., 2014 ; Ehrlich et al., 2015 ). Self-control is considered an important mechanism for emotion regulation ( Paschke et al., 2016 ); thus, fits with self-report evidence of emotion over-regulation by people with AN ( Oldershaw et al., 2015 ).
During adolescence, neural adaptations are crucial to the experience of emotion and development of emotion regulation. Self-reported embarrassment and activation in the medial prefrontal cortex are elevated ( Somerville et al., 2013 ). Increased dorsolateral prefrontal activation in girls particularly, coupled with decreased activation in the amygdala, may underpin development of the ability during adolescence to contextualize and regulate emotional experience ( Blakemore and Choudhury, 2006 ). Thus, increased activation of both dorsolateral and amygdala regions for people susceptible to AN ( Seidel et al., 2018 ) may result in a vicious circle of high emotion, poor emotion regulation and need for over-regulation. It is argued that development of orbital and dorsolateral prefrontal cortex regions during and after puberty and increased activity might contribute to excessive worry, perfectionism and strategizing in people with AN ( Kaye et al., 2009 ).
The insula is involved in affective processing and functionally connected with the anterior cingulate cortex, amygdala and ventral tegmental areas ( Ham et al., 2013 ). The insula receives input regarding internal body states ( Craig, 2002 ). It supports visceral, muscular and physiological bodily feedback ( Swick et al., 2008 ) and is involved in anxiety control, regulating disgust, hunger, taste, pain and experience of body image ( Suchan et al., 2013 ). It is implicated in interoception, integrating visual and body perception with emotion and emotional feeling states ( Nunn et al., 2008 ). Access to conscious awareness of interoceptive experience is supported by the posterior insula, while the anterior insula is involved in emotion processing, integration of interoceptive, emotional and cognitive input and contextual integration of interoceptive information ( Li et al., 2017 ). This highlights the role of the insula in emotional awareness and conscious self-representations ( Craig, 2002 ; Seth, 2013 ).
It is proposed that people with AN are unable to integrate emotional information with sensory experience due to disruptions within the insula ( Nunn et al., 2008 ). Indeed, impaired thalamo-insular circuits are thought to explain a failure of integration of visuospatial information (e.g., pertaining to body image) and homoeostatic (e.g., hunger) signals for people with AN ( Geisler et al., 2016 ). This lack in ability of the insula to integrate basic emotion detection for people with AN occurs in the backdrop of a hyperactive amygdala sending high levels of negative emotional threat information. It is argued that the anterior insula, alongside the amygdala, nucleus accumbens and orbitofrontal cortex are key to supporting our conscious awareness of emotion and integrating this with our sense of self ( Seth, 2013 ). Further, the posterior insula receives and encodes visceral interoceptive input, and increased right posterior insular volume correlates with disorder duration and severity in AN suggesting that difficulties in integrating bottom-up interoceptive information increase with length of disorder ( Zucker et al., 2017 ). Moreover, patterns of insula responding to unpleasant interoceptive states are significantly different for those in remission from AN versus healthy comparison participants ( Berner et al., 2018 ). Altered interoceptive processing within the insula is observed even following recovery from AN ( Strigo et al., 2013 ). It is argued that this may contribute to difficulties predicting and adapting to internal state fluctuation for people with AN, that relates to past AN severity ( Berner et al., 2018 ). These data highlight that disruptions within the insula might link to observations of poor interoceptive awareness for people with AN, and which are exacerbated by disorder duration or severity.
Starvation Effects: Summary
People with AN have poor interoceptive awareness exacerbated by low weight. This impedes development and awareness of a sense of self and may be exacerbated by a need to reduce discrepancy between internal and external perception via over-reliance and internalization of external feedback or concrete perceptive cues. People with AN appear to have hyperactive amygdala leading to overwhelming emotional experience and increased prefrontal control mechanisms reducing emotional awareness and clarity. Disruptions within the insula further impair the integration of internal experience with self-awareness and self-representation. This may become more entrenched as the disorder progresses.
AN as a Lost Sense of Emotional Self
In short, the argument provided outlines AN as a difficulty with attunement and differentiation, leading to a sense of overwhelming emotional experience, that cannot be fully integrated, and is unable to develop into a coherent sense of self during adolescence. This results in poor understanding or perceived value in the individual’s own needs and emotions, resulting in reliance upon external signaling and validation. AN emerges as a means of regulating and managing emotional experience while also providing a false sense of self, including a false sense of needs and a concrete means to meet those needs (e.g., weight and shape goals). Indeed, perhaps the most compelling indication that people with AN lack a known and integrated sense of self is the fact that disorder appears in part to be a quest for this; a “blind search for identity” ( Bruch, 1973 ). The disorder very quickly becomes conflated with actual self, yet at a simplistic and concrete level that can be measurably perfected (“self as weight”; Bates, 2015 ). The anorexic identity emerges within social interaction and, once established, becomes validated through others’ responses: it achieves basic needs by expressing, communicating and encouraging connectedness from others. In the context of the description of the disorder above, and the self-perpetuation of this cycle, the paradox of AN becomes clear: it is a search for a congruent self, yet one which only removes the sufferer further from an authentic sense of self.
Clinical Conceptualization and Practice
We have argued that current interventions may be improved if one core hypothesized maintenance factor is isolated and addressed in therapy, with robust assessment of whether the intervention successfully manipulates it. We outlined our argument that in fact several risk and maintenance factors for AN may exert their effects, at least in part, by a single underpinning difficulty with emotion leading to a poorly integrated sense of self. We propose focusing on this putative underpinning factor in a future intervention. This is in keeping with an ‘interventionist-causal model approach’ which allows for independent vetting of a proposed maintenance factor’s relevance, and of the ability of an intervention to manipulate it; achieved by directly assessing whether the intervention on it changes outcomes ( Kendler and Campbell, 2009 ). Using this approach, theoretical models can be successfully translated into practice building a treatment using one hypothesized factor at a time (cf. Freeman et al., 2015 ). This is consistent with the MRC framework for developing complex evaluations which argues better understanding of expected changes is necessary before embarking on evaluation studies of new complex interventions ( Craig et al., 2008 ).
Addressing Poor Integrated Sense of ‘Emotional Self’ in Psychological Therapy
In keeping with our hypothesis that a ‘lost sense of emotional self’ forms a core underpinning factor, we argue that working to change this will impact clinical outcomes. Emotion-focussed interventions are supported by empirical evidence and have recently been described as promising for people with AN ( Sala et al., 2016 ). Indeed, since their recognition as putative maintenance factors, interventions have sought to include addressing emotional difficulties (cf. MANTRA, CBT-ED). Yet despite this, as described, limited efficacy above control comparisons remains. In keeping with other authors ( Schmidt and Treasure, 2006 ; Williams et al., 2016 ), we do not emphasize addressing weight and shape difficulties directly as part of a therapeutic intervention. Indeed, our model is not inconsistent with goals of other treatments, such as developing a ‘non-AN’ identity outlined in MANTRA; yet we explicitly argue the meaning of establishing identity as establishing a core emotional sense of self, which can be flexibly and adaptively used to direct an individual’s needs and relationships. Further, we state that this should be the primary therapeutic goal.
Although emotion is itself inherently complex, maintaining this focus throughout therapy may afford greater opportunity for change. Moreover, we suggest that other interventions do not fully address dfficulties with emotion. It is notable that previous research and intervention development in this area tends to focus upon understanding how emotions arise (e.g., negative appraisals generating guilt or shame), increasing psychoeducation around emotion, and managing difficulties with consequences for emotional experience (i.e., improving regulation). Yet this does not sufficiently address the breadth of difficulties as we view it. Reflecting on her lifetime of work seeking to understand AN, Bruch (1988) describes the therapeutic task: “To help the anorexic patient in her search for autonomy and self-directed identity by evoking awareness of impulses, feelings and needs that originate within herself. The therapeutic focus needs to be on her failure in self-experience, on her defective tools and concepts for organizing and expressing needs, and on her bewilderment when dealing with others” ( Bruch, 1988 , p. 8). In the 30 years since, there has been considerable effort and attention paid to establishing what the “defective tools and concepts” are and how the “bewilderment when dealing with others” manifests. This has clarified the outward picture of somebody with AN and we have sought to describe herein how this becomes self-perpetuating. As discussed, tackling such factors (e.g., in cognitive-behavioral interventions) may have some benefits; yet we propose that this may become less effective once AN has become established and ‘self-perpetuating.’ We argue that it is the “failure in self experience,” in establishing a felt sense of self through awareness that would naturally lead to self-directed identity, which is missed.
Emotion-Focused Therapeutic Approaches
As with all therapeutic approaches, the goals and formulation surrounding the emotions at hand must be carefully considered before applying an intervention or technique. There is a distinction between “primary” emotion as an adaptive inherent “bottom-up” emotional response to a stimulus ( Damasio, 2003 ; Greenberg, 2004 ; Greenberg and Pascual-Leone, 2006 ; Barrett et al., 2007 ) and secondary “top-down” emotions in response to cognitive appraisal ( Greenberg and Pascual-Leone, 2006 ). It has been shown that cognitive reappraisal is effective when used to regulate emotions generated by a “top-down process” (those occurring in response to cognitive appraisal of a situation), but not when applied to those generated by a “bottom-up” process (inherent, often adaptive, emotional response to a stimulus; McRae et al., 2012 ). Indeed, applying cognitive reappraisal to emotions generated in a “bottom-up” process results in increased amygdala activity, suggesting increased arousal, not regulation ( McRae et al., 2012 ). Furthermore, in low-intensity negative situations, people demonstrate preference for reappraisal, while high-intensity negative emotions are preferably regulated by disengagement distraction, blocking emotional processing before it gathers force ( Sheppes et al., 2011 ). This highlights clear differences in how emotional experience can be most adaptively approached and understood across situations and contexts, dependent upon the emotional need. People with AN may have a persistent disruption in early automatic emotion cues – “primary emotion” ( Hatch et al., 2010a ). This seems supported by our review findings ( Oldershaw et al., 2015 ) that people with AN report clarity mostly in emotions of guilt and disgust. Such emotions are generally considered to be top-down emotional responses influenced using compassion-focused and cognitive behavioral techniques ( Goss and Allan, 2014 ). While this can improve ED outcomes ( Kelly et al., 2014 ), such treatment may also miss a core feature of AN as described in this article, namely the primary emotional experience.
By the account outlined here, models and interventions for AN should bring focus to core “primary,” “bottom-up” emotional experience. They should seek to embed and integrate this into a self, affording an internal information system of needs to motivate appropriate action, thereby increasing self-efficacy and autonomy. It cannot be that people with AN are not subject to events generating primary emotions. Indeed, as described, hyperactive amygdala suggests a large amount of unconscious early emotion not fully integrated into a felt experience of self; thus undifferentiated, loses clarity and experienced as overwhelming, vague, and negative. Yet, how can one work with something blocked so early that it is experienced only as a vague and confusing state and the continued denial and avoidance of which is highly valued by the experiencer?
Existing psychotherapeutic models may be of benefit when considering how to address this and promote experiential processing of emotion. In Emotion-Focused Therapy (EFT; Greenberg, 2011 ) the therapeutic process is viewed as co-constructive in which “therapist and client influence each other in non-imposing ways to deepen client experiencing and exploration and promote emotional processing.” (p. 65). The primary goal is to utilize this therapeutic relationship to apply key principles of emotion change (awareness, expression regulation, reflection, transformation and corrective emotional experience). Emotional processing is achieved by recognizing that “the only way out is through” ( Pascual-Leone and Greenberg, 2007 ). Similarly, in Young’s Schema Therapy (ST; Young et al., 2003 ) a key goal is to be with an individual’s primary emotions or ‘core pain’ sometimes described as being ‘housed’ within a ‘vulnerable child mode.’ Time is spent understanding how an individual learnt to cope with their ‘core pain’ through surrendering, avoiding or overcompensating in relation to core maladaptive schema and the costs of these coping styles to their developing sense of ‘self.’
In both EFT and ST, methods of working with emotion are relational and experiential and a core part of therapy relies on chair work ( Rice and Greenberg, 1984 ; Kellogg, 2014 ). In ST, chair work can map out ‘parts of the self’ and their relationship with core pain or vulnerable child self, with the goal to promote adaptive communication between ‘parts of the self.’ For example, to ‘by pass’ coping modes (or parts of the self) that have learnt to block or deflect primary emotion (such as by ‘cutting off’), such that emotion can be experienced and expressed safely with the therapist and responded to in new and attuned ways. In EFT, parts of the self are most commonly understood as having functions of coach, critic or guard and their relationship with the ‘experiencer’ (which can be adult or child parts of self) are explored to enable primary feelings to emerge and be expressed directly in dialog between the parts, with a process of resolution facilitated ( Elliott et al., 2015 ). One previous adaptation of identifying parts of self to AN is to consider the critical voice specifically as an ‘anorexic voice’ which criticizes using core anorexic cognitions around eating, weight and shape ( Dolhanty and Greenberg, 2009 ; Pugh and Waller, 2017 ), the power of which relates to disorder severity ( Pugh and Waller, 2016 ).
In both EFT and ST, chair work aims to put parts of the self into live contact, differentiating and intensifying emotion expression and facilitating the identification and expression of an unmet need ( Young et al., 2003 ; Arntz and Jacob, 2012 ; Elliott et al., 2015 ). In ST, accessing primary emotion enables the therapist to attune via a re-parenting stance (in a limited way) to some of the unmet needs underneath the core pain ( Arntz and Jacob, 2012 ). In EFT, a distinction is made between the emotional states accessed: those which are adaptive or maladaptive. Once accessed and expressed, primary maladaptive emotions (e.g., core shame; fear of abandonment) can be transformed by putting them in contact with (coactivating) a more adaptive emotional experience (e.g., empowering anger or compassion for the self) to forge a new emotion ( Greenberg, 2011 ) often via expression of needs.
In addition to chair work, further experiential therapeutic tasks and tools enable access to emotional processes and memories. EFT draws upon experiencing-based tasks to respond to in-session client markers, such as experiential focussing to clarify and facilitate feeling shifts (cf. Gendlin, 1981 ) or systematic evocative unfolding to draw links between stimuli and puzzling reactions to create meaning ( Rice and Saperia, 1984 ). Enactment and imagery can activate adaptive emotional experience for emotion transformation ( Greenberg, 2011 ). ST uses imagery to help access less verbal ‘emotional memories’ for expression and integration into the individuals developing sense of self ( Arntz and Jacob, 2012 ).
While it is beyond the scope of this review to outline these therapeutic models and adaptation to AN in full, we have sought to highlight the compatibility of the EFT and ST models and associated change techniques to working with those with AN, particularly within the context of the conceptualization of a ‘lost emotional self.’ The goal is that a healthy and known self emerges, with a capacity to become aware of, make sense of, regulate, accept, express, and transform emotional experience, thereby using this flexibly and adaptively to navigate themselves, relationships and the world (a reclaimed “conductor”).
Future Directions in Testing the ‘Lost Emotional Self’ Hypothesis
A clear direction in testing the hypothesis of a ‘lost emotional self’ is to develop a psychological intervention based on the theory, and assess whether: (i) these changes to emotion and self can be achieved, (ii) these changes occur via the proposed mechanisms of enhanced emotional processing, particularly in regard primary ‘bottom up’ emotion, along with less reliance on external cues (and therefore less prediction errors), and an enhanced experience of and belief in core needs and; (iii) this impacts on ED outcomes.
Individual aspects of the model could also be tested. For example, we assert that people with AN experience large perceived prediction errors ( Seth, 2013 ; see the section “Interoception”). This results in integration of external signals as part of self-representation, including ultimately the external perception of their own body as an object. We argue that large prediction errors would predict anxiety, intolerance of uncertainty and reinforce unhelpful emotion regulation strategies. Cross sectional designs could compare those currently ill and those with no illness history, to examine where interoceptive difficulties lie (i.e., objective accuracy, self-evaluated trait interoceptive sensibility or meta-cognitive awareness; Garfinkel et al., 2015 ). Reduced objective interoceptive sensitivity alongside elevated trait interoceptive sensibility indicates an ‘interoceptive trait prediction error’ ( Garfinkel et al., 2016 ; Seth and Friston, 2016 ). This could be furthered by comparing with those recovered from AN and examining relationships with anxiety processes and alexithymia.
We propose AN arises from and perpetuates a lost sense of emotional self; a person without the conductor of the orchestra (our emotional sense of self), persistently reliant upon and sensitive to audience feedback to ascertain if it is performing adequately. This model suggests working to improve awareness, acceptance and valuing of one’s own adaptive interoceptive emotional experience over exteroceptive feedback to achieve emotional validation, emotional self-efficacy and self-agency. Yet this is hampered by high valuing of AN by the sufferer and an increased lack of interoceptive and emotional experience as the disorder progresses, such that AN becomes ‘self-perpetuating,’ creating a ‘stuckness’ in therapy. We propose working in detail with this core putative underpinning maintenance factor and emphasize experiential therapies for psychological engagement with this presentation. This formulation is now to be directly tested, but provides a novel interpretation of existing data, grounded within previous empirical findings.
Author Contributions
All authors contributed to conception of ideas within the manuscript. AO wrote the manuscript. HS and TL read and gave critical feedback on drafts. All authors approved the final version for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer DV and handling Editor declared their shared affiliation at the time of review.
Funding. This manuscript is independent research arising from an Integrated Clinical Academic Fellowship-Clinical Lectureship awarded to AO (ICA-CL-2015-01-005) supported by the National Institute for Health Research and Health Education England. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, Health Education England or the Department of Health.
- Adambegan M., Wagner G., Nader I. W., Fernandez-Aranda F., Treasure J., Karwautz A. (2012). Internalizing and externalizing behaviour problems in childhood contribute to the development of anorexia and bulimia nervosa-a study comparing sister pairs. Eur. Eat. Disord. Rev. 20 116–120. 10.1002/erv.1152 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Adolphs R. (2001). The neurobiology of social cognition. Curr. Opin. Neurobiol. 11 231–239. 10.1016/S0959-4388(00)00202-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aldao A., Nolen-Hoeksema S., Schweizer S. (2010). Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 30 217–237. 10.1016/j.cpr.2009.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- American Psychological Association [APA] (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: APA. [ Google Scholar ]
- Amianto F., Northoff G., Abbate Daga G., Fassino S., Tasca G. A. (2016). Is anorexia nervosa a disorder of the self? A psychological approach. Front. Psychol. 7 : 849 . 10.3389/fpsyg.2016.00849 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arcelus J., Haslam M., Farrow C., Meyer C. (2013). The role of interpersonal functioning in the maintenance of eating psychopathology: a systematic review and testable model. Clin. Psychol. Rev. 33 156–167. 10.1016/j.cpr.2012.10.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arntz A., Jacob G. (2012). Schema Therapy in Practice: An Introductory Guide to the Schema Mode Approach. Chichester: Wiley-Blackwell. [ Google Scholar ]
- Bachner-Melman R., Zohar A. H., Kremer I., Komer M., Blank S., Golan M., et al. (2009). Self-monitoring in anorexia nervosa. Int. J. Soc. Psychiatry 55 170–179. 10.1177/0020764008094647 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bang L., Treasure J., Ro O., Joos A. (2017). Advancing our understanding of the neurobiology of anorexia nervosa: translation into treatment. J. Eat. Disord. 5 : 38 . 10.1186/s40337-017-0169-8 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A., et al. (2005). White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex 15 1848–1854. 10.1093/cercor/bhi062 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barrett L. F., Mesquita B., Ochsner K. N., Gross J. J. (2007). The experience of emotion. Annu. Rev. Psychol. 58 373–403. 10.1146/annurev.psych.58.110405.085709 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bates C. F. (2015). “I am a waste of breath, of space, of time”: metaphors of self in a pro-anorexia group. Qual. Health Res. 25 189–204. 10.1177/1049732314550004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Batsell W. R., Jr., Brown A. S., Ansfield M. E., Paschall G. Y. (2002). “You will eat all of that!”: a retrospective analysis of forced consumption episodes. Appetite 38 211–219. 10.1006/appe.2001.0482 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beadle J. N., Paradiso S., Salerno A., McCormick L. M. (2013). Alexithymia, emotional empathy, and self-regulation in anorexia nervosa. Ann. Clin. Psychiatry 25 107–120. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Belsky J. (1984). The determinants of parenting: a process model. Child Dev. 55 83–96. 10.2307/1129836 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berner L. A., Simmons A. N., Wierenga C. E., Bischoff-Grethe A., Paulus M. P., Bailer U. F., et al. (2018). Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychol. Med. 48 142–154. 10.1017/S0033291717001635 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beyers W., Goossens L. (2008). Dynamics of perceived parenting and identity formation in late adolescence. J. Adolesc. 31 165–184. 10.1016/j.adolescence.2007.04.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bigman Y. E., Mauss I. B., Gross J. J., Tamir M. (2016). Yes I can: expected success promotes actual success in emotion regulation. Cogn. Emot. 30 1380–1387. 10.1080/02699931.2015.1067188 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bion W. R. (1962). Learning from Experience. London: Heinemann. [ Google Scholar ]
- Blakemore S. J., Choudhury S. (2006). Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry 47 296–312. 10.1111/j.1469-7610.2006.01611.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Boehm I., King J. A., Bernardoni F., Geisler D., Seidel M., Ritschel F., et al. (2018). Subliminal and supraliminal processing of reward-related stimuli in anorexia nervosa. Psychol. Med. 48 790–800. 10.1017/S0033291717002161 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bora E., Kose S. (2016). Meta-analysis of theory of mind in anorexia nervosa and bulimia nervosa: a specific Impairment of cognitive perspective taking in anorexia nervosa? Int. J. Eat. Disord. 49 739–740. 10.1002/eat.22572 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bosma H. A., Kunnen E. S. (2001). Identity & Emotion: Development through Self-Organization. Cambridge: Cambridge University Press; 10.1017/CBO9780511598425 [ CrossRef ] [ Google Scholar ]
- Bowlby J. (1969). Attachment and Loss: Attachment. New York, NY: Basic Books. [ Google Scholar ]
- Brand A. E., Klimes-Dougan B. (2010). Emotion socialization in adolescence: the roles of mothers and fathers. New Dir. Child Adolesc. Dev. 2010 85–100. 10.1002/cd.270 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brockmeyer T., Grosse Holtforth M., Bents H., Herzog W., Friederich H. C. (2013). Lower body weight is associated with less negative emotions in sad autobiographical memories of patients with anorexia nervosa. Psychiatry Res. 210 548–552. 10.1016/j.psychres.2013.06.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brockmeyer T., Holtforth M. G., Bents H., Kammerer A., Herzog W., Friederich H. C. (2012). Starvation and emotion regulation in anorexia nervosa. Compr. Psychiatry 53 496–501. 10.1016/j.comppsych.2011.09.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brody L. R., Hall J. A. (2008). “Gender and emotion in context,” in Handbook of Emotions , eds Lewis M., Haviland-Jones J. M., Barrett L. F. (New York, NY: The Guildford press; ), 395–408. [ Google Scholar ]
- Brown A., McClelland J., Boysen E., Mountford V., Glennon D., Schmidt U. (2018). The FREED Project (first episode and rapid early intervention in eating disorders): service model, feasibility and acceptability. Early Interv. Psychiatry 12 250–257. 10.1111/eip.12382 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bruch H. (1962). Perceptual and conceptual disturbances in anorexia nervosa. Psychosom. Med. 24 187–194. 10.1097/00006842-196203000-00009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bruch H. (1973). Eating Disorders. Obesity, Anorexia Nervosa, and the Person within. New York, NY: Basic Books. [ Google Scholar ]
- Bruch H. (1988). Conversations with Anorexics. London: Jason Aronson, Inc. [ Google Scholar ]
- Buckholdt K. E., Parra G. R., Jobe-Shields L. (2014). Intergenerational transmission of emotion dysregulation through parental invalidation of emotions: implications for adolescent internalizing and externalizing behaviors. J. Child Fam. Stud. 23 324–332. 10.1007/s10826-013-9768-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Buhl H. M. (2008). Development of a model describing individuated adult child–parent relationships. Int. J. Behav. Dev. 32 381–389. 10.1177/0165025408093656 [ CrossRef ] [ Google Scholar ]
- Bulik C. M. (2014). The challenges of treating anorexia nervosa. Lancet 383 105–106. 10.1016/S0140-6736(13)61940-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Butler A. C., Chapman J. E., Forman E. M., Beck A. T. (2006). The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin. Psychol. Rev. 26 17–31. 10.1016/j.cpr.2005.07.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Butler E. A., Lee T. L., Gross J. J. (2007). Emotion regulation and culture: are the social consequences of emotion suppression culture-specific? Emotion 7 30–48. 10.1037/1528-3542.7.1.30 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caglar-Nazali H. P., Corfield F., Cardi V., Ambwani S., Leppanen J., Olabintan O., et al. (2014). A systematic review and meta-analysis of ’Systems for Social Processes’ in eating disorders. Neurosci. Biobehav. Rev. 42 55–92. 10.1016/j.neubiorev.2013.12.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carper J. L., Orlet Fisher J., Birch L. L. (2000). Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite 35 121–129. 10.1006/appe.2000.0343 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Carter F. A., Jordan J., McIntosh V. V., Luty S. E., McKenzie J. M., Frampton C. M., et al. (2011). The long-term efficacy of three psychotherapies for anorexia nervosa: a randomized, controlled trial. Int. J. Eat. Disord. 44 647–654. 10.1002/eat.20879 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chaplin T. M. (2015). Gender and emotion expression: a developmental contextual perspective. Emot. Rev. 7 14–21. 10.1177/1754073914544408 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chaplin T. M., Aldao A. (2013). Gender differences in emotion expression in children: a meta-analytic review. Psychol. Bull. 139 735–765. 10.1037/a0030737 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Christov-Moore L., Simpson E. A., Coude G., Grigaityte K., Iacoboni M., Ferrari P. F. (2014). Empathy: gender effects in brain and behavior. Neurosci. Biobehav. Rev. 46(Pt 4) , 604–627. 10.1016/j.neubiorev.2014.09.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Claesson K., Birgegard A., Sohlberg S. (2007). Shame: mechanisms of activation and consequences for social perception, self-image, and general negative emotion. J. Pers. 75 595–627. 10.1111/j.1467-6494.2007.00450.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cooke L., Wardle J., Gibson E. L. (2003). Relationship between parental report of food neophobia and everyday food consumption in 2-6-year-old children. Appetite 41 205–206. 10.1016/S0195-6663(03)00048-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Corstorphine E., Mountford V., Tomlinson S., Waller G., Meyer C. (2007). Distress tolerance in the eating disorders. Eat. Behav. 8 91–97. 10.1016/j.eatbeh.2006.02.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cosmides L., Tooby J. (2000). “Evolutionary psychology and the emotions,” in Handbook of Emotions , 2nd Edn, eds Lewis M., Haviland-Jones J. M. (New York, NY: Guildford Press; ), 91–115. [ Google Scholar ]
- Craig A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3 655–666. 10.1038/nrn894 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M., et al. (2008). Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 337 : a1655 . 10.1136/bmj.a1655 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Crone E. A. (2013). Considerations of fairness in the adolescent brain. Child Dev. Perspectives 7 97–103. 10.1111/cdep.12022 [ CrossRef ] [ Google Scholar ]
- Crucianelli L., Cardi V., Treasure J., Jenkinson P. M., Fotopoulou A. (2016). The perception of affective touch in anorexia nervosa. Psychiatry Res. 239 72–78. 10.1016/j.psychres.2016.01.078 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dakanalis A., Clerici M., Bartoli F., Caslini M., Crocamo C., Riva G., et al. (2017). Risk and maintenance factors for young women’s DSM-5 eating disorders. Arch. Womens Ment. Health 20 721–731. 10.1007/s00737-017-0761-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Damasio A. (2003). Feelings of emotion and the self. Ann. N. Y. Acad. Sci. 1001 253–261. 10.1196/annals.1279.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dan-Glauser E. S., Gross J. J. (2011). The temporal dynamics of two response-focused forms of emotion regulation: experiential, expressive, and autonomic consequences. Psychophysiology 48 1309–1322. 10.1111/j.1469-8986.2011.01191.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dapelo M. M., Surguladze S., Morris R., Tchanturia K. (2016). Emotion recognition in blended facial expressions in women with anorexia nervosa. Eur. Eat. Disord. Rev. 24 34–42. 10.1002/erv.2403 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davies H., Schmidt U., Stahl D., Tchanturia K. (2011). Evoked facial emotional expression and emotional experience in people with anorexia nervosa. Int. J. Eat. Disord. 44 531–539. 10.1002/eat.20852 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davies H., Schmidt U., Tchanturia K. (2013). Emotional facial expression in women recovered from anorexia nervosa. BMC Psychiatry 13 : 291 . 10.1186/1471-244X-13-291 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Davies H., Swan N., Schmidt U., Tchanturia K. (2012). An experimental investigation of verbal expression of emotion in anorexia and bulimia nervosa. Eur. Eat. Disord. Rev. 20 476–483. 10.1002/erv.1157 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- de Groot J. M., Rodin G. (1994). Eating disorders, female psychology, and the self. J. Am. Acad. Psychoanal. 22 299–317. 10.1521/jaap.1.1994.22.2.299 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- den Uijl L. C., Jager G., de Graaf C., Waddell J., Kremer S. (2014). It is not just a meal, it is an emotional experience - a segmentation of older persons based on the emotions that they associate with mealtimes. Appetite 83 287–296. 10.1016/j.appet.2014.09.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dias P., Soares I., Klein J., Cunha J. P., Roisman G. I. (2011). Autonomic correlates of attachment insecurity in a sample of women with eating disorders. Attach. Hum. Dev. 13 155–167. 10.1080/14616734.2011.554005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Doba K., Berna G., Constant E., Nandrino J. L. (2018). Self-differentiation and eating disorders in early and middle adolescence: a cross-sectional path analysis. Eat. Behav. 29 75–82. 10.1016/j.eatbeh.2018.03.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dolan R. J. (2002). Emotion, cognition, and behavior. Science 298 1191–1194. 10.1126/science.1076358 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dolhanty J., Greenberg L. S. (2009). Emotion-focused therapy in a case of anorexia nervosa. Clin. Psychol. Psychother. 16 366–382. 10.1002/cpp.624 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Duclos J., Dorard G., Berthoz S., Curt F., Faucher S., Falissard B., et al. (2014). Expressed emotion in anorexia nervosa: what is inside the “black box”? Compr. Psychiatry 55 71–79. 10.1016/j.comppsych.2013.10.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ehrlich S., Geisler D., Ritschel F., King J. A., Seidel M., Boehm I., et al. (2015). Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 40 307–315. 10.1503/jpn.140249 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Elliott R., Watson J. C., Goldman R. N., Greenberg L. S. (2015). Learning Emotion-Focused Therapy. Washington, DC: American Psychological Association. [ Google Scholar ]
- Eshkevari E., Rieger E., Longo M. R., Haggard P., Treasure J. (2014). Persistent body image disturbance following recovery from eating disorders. Int. J. Eat. Disord. 47 400–409. 10.1002/eat.22219 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fairburn C. G., Bailey-Straebler S., Basden S., Doll H. A., Jones R., Murphy R., et al. (2015). A transdiagnostic comparison of enhanced cognitive behaviour therapy (CBT-E) and interpersonal psychotherapy in the treatment of eating disorders. Behav. Res. Ther. 70 64–71. 10.1016/j.brat.2015.04.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fairburn C. G., Cooper Z., Doll H. A., O’Connor M. E., Bohn K., Hawker D. M., et al. (2009). Transdiagnostic cognitive-behavioral therapy for patients with eating disorders: a two-site trial with 60-week follow-up. Am. J. Psychiatry 166 311–319. 10.1176/appi.ajp.2008.08040608 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fairburn C. G., Cooper Z., Shafran R. (2003). Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav. Res. Ther. 41 509–528. 10.1016/S0005-7967(02)00088-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fairburn C. G., Shafran R., Cooper Z. (1999). A cognitive behavioural theory of anorexia nervosa. Behav. Res. Ther. 37 1–13. 10.1016/S0005-7967(98)00102-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fivush R., Brotman M. A., Buckner J. P., Goodman S. H. (2000). Gender differences in parent–child emotion narratives. Sex Roles 42 233–253. 10.1023/A:1007091207068 [ CrossRef ] [ Google Scholar ]
- Fivush R., McDermott Sales J., Bohanek J. G. (2008). Meaning making in mothers’ and children’s narratives of emotional events. Memory 16 579–594. 10.1080/09658210802150681 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fivush R., Zaman W. (2015). “Gendered narrative voices: sociocultural and feminist approaches to emerging identity in childhood and adolescence,” in The Oxford Handbook of Identity Development , eds McLean K. C., Syed M. (New York, NY: Oxford University Press; ), 33–52. [ Google Scholar ]
- Fossati P. (2012). Neural correlates of emotion processing: from emotional to social brain. Eur. Neuropsychopharmacol. 22(Suppl. 3) , S487–S491. 10.1016/j.euroneuro.2012.07.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox J. R. (2009). A qualitative exploration of the perception of emotions in anorexia nervosa: a basic emotion and developmental perspective. Clin. Psychol. Psychother. 16 276–302. 10.1002/cpp.631 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Freeman D., Dunn G., Startup H., Pugh K., Cordwell J., Mander H., et al. (2015). Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single-blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry 2 305–313. 10.1016/S2215-0366(15)00039-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friederich H. C., Kumari V., Uher R., Riga M., Schmidt U., Campbell I. C., et al. (2006). Differential motivational responses to food and pleasurable cues in anorexia and bulimia nervosa: a startle reflex paradigm. Psychol. Med. 36 1327–1335. 10.1017/S0033291706008129 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fuhrmann D., Knoll L. J., Blakemore S. J. (2015). Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 19 558–566. 10.1016/j.tics.2015.07.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gander M., Sevecke K., Buchheim A. (2015). Eating disorders in adolescence: attachment issues from a developmental perspective. Front. Psychol. 6 : 1136 . 10.3389/fpsyg.2015.01136 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garcia N. V., Scherf K. S. (2015). Emerging sensitivity to socially complex expressions: a unique role for adolescence? Child Dev. Perspect. 9 84–90. 10.1111/cdep.12114 [ CrossRef ] [ Google Scholar ]
- Garfinkel S. N., Seth A. K., Barrett A. B., Suzuki K., Critchley H. D. (2015). Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104 65–74. 10.1016/j.biopsycho.2014.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garfinkel S. N., Tiley C., O’Keeffe S., Harrison N. A., Seth A. K., Critchley H. D. (2016). Discrepancies between dimensions of interoception in autism: implications for emotion and anxiety. Biol. Psychol. 114 117–126. 10.1016/j.biopsycho.2015.12.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gaudio S., Brooks S. J., Riva G. (2014). Nonvisual multisensory impairment of body perception in anorexia nervosa: a systematic review of neuropsychological studies. PLoS One 9 : e110087 . 10.1371/journal.pone.0110087 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Geisler D., Borchardt V., Lord A. R., Boehm I., Ritschel F., Zwipp J., et al. (2016). Abnormal functional global and local brain connectivity in female patients with anorexia nervosa. J. Psychiatry Neurosci. 41 6–15. 10.1503/jpn.140310 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gendlin E. T. (1981). Focusing. New York, NY: Bantam. [ Google Scholar ]
- Giedd J. N., Blumenthal J., Jeffries N. O., Castellanos F. X., Liu H., Zijdenbos A., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2 861–863. 10.1038/13158 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gilligan C. (1982). In a Different Voice. Cambridge, MA: Harvard UniversityPress. [ Google Scholar ]
- Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101 8174–8179. 10.1073/pnas.0402680101 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Goss K., Allan S. (2014). The development and application of compassion-focused therapy for eating disorders (CFT-E). Br. J. Clin. Psychol. 53 62–77. 10.1111/bjc.12039 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Grabhorn R., Stenner H., Stanguer U., Kaufhold J. (2006). Social anxiety in anorexia and bulimia nervosa: the mediating role of shame. Clin. Psychol. Psychother. 13 12–19. 10.1002/cpp.463 [ CrossRef ] [ Google Scholar ]
- Gramaglia C., Ressico F., Gambaro E., Palazzolo A., Mazzarino M., Bert F., et al. (2016). Alexithymia, empathy, emotion identification and social inference in anorexia nervosa: a case-control study. Eat. Behav. 22 46–50. 10.1016/j.eatbeh.2016.03.028 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Greenberg L. S. (2004). Emotion-focused therapy. Clin. Psychol. Psychother. 11 3–16. 10.1002/cpp.388 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Greenberg L. S. (2011). Emotion-Focused Therapy. Washington, DC: American Psychological Association. [ Google Scholar ]
- Greenberg L. S., Pascual-Leone A. (2006). Emotion in psychotherapy: a practice-friendly research review. J. Clin. Psychol. 62 611–630. 10.1002/jclp.20252 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gross J. J. (2013). Emotion regulation: taking stock and moving forward. Emotion 13 359–365. 10.1037/a0032135 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gross J. J., Levenson R. W. (1997). Hiding feelings: the acute effects of inhibiting negative and positive emotion. J. Abnorm. Psychol. 106 95–103. 10.1037/0021-843X.106.1.95 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gunnard K., Krug I., Jimenez-Murcia S., Penelo E., Granero R., Treasure J., et al. (2012). Relevance of social and self-standards in eating disorders. Eur. Eat. Disord. Rev. 20 271–278. 10.1002/erv.1148 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Habermas T., Bluck S. (2000). Getting a life: the emergence of the life story in adolescence. Psychol. Bull. 126 748–769. 10.1037/0033-2909.126.5.748 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ham T., Leff A., de Boissezon X., Joffe A., Sharp D. J. (2013). Cognitive control and the salience network: an investigation of error processing and effective connectivity. J. Neurosci. 33 7091–7098. 10.1523/JNEUROSCI.4692-12.2013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hambrook D., Oldershaw A., Rimes K., Schmidt U., Tchanturia K., Treasure J., et al. (2011). Emotional expression, self-silencing, and distress tolerance in anorexia nervosa and chronic fatigue syndrome. Br. J. Clin. Psychol. 50 310–325. 10.1348/014466510X519215 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hambrook D., Tchanturia K., Schmidt U., Russell T., Treasure J. (2008). Empathy, systemizing, and autistic traits in anorexia nervosa: a pilot study. Br. J. Clin. Psychol. 47(Pt 3) , 335–339. 10.1348/014466507X272475 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hare T. A., Tottenham N., Galvan A., Voss H. U., Glover G. H., Casey B. J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry 63 927–934. 10.1016/j.biopsych.2008.03.015 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harrison A., O’Brien N., Lopez C., Treasure J. (2010). Sensitivity to reward and punishment in eating disorders. Psychiatry Res. 177 1–11. 10.1016/j.psychres.2009.06.010 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Harrison A., Sullivan S., Tchanturia K., Treasure J. (2009). Emotion recognition and regulation in anorexia nervosa. Clin. Psychol. Psychother. 16 348–356. 10.1002/cpp.628 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hatch A., Madden S., Kohn M. R., Clarke S., Touyz S., Gordon E., et al. (2010a). Emotion brain alterations in anorexia nervosa: a candidate biological marker and implications for treatment. J. Psychiatry Neurosci. 35 267–274. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hatch A., Madden S., Kohn M., Clarke S., Touyz S., Williams L. M. (2010b). Anorexia nervosa: towards an integrative neuroscience model. Eur. Eat. Disord. Rev. 18 165–179. 10.1002/erv.974 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Haworth-Hoeppner S., Manies D. (2005). A sociological account of the persistence of invalidated anorexic identities. Symb. Interact. 28 1–23. 10.1525/si.2005.28.1.1 [ CrossRef ] [ Google Scholar ]
- Haynos A. F., Fruzzetti A. E. (2011). Anorexia nervosa as a disorder of emotion dysregulation: evidence and treatment implications. Clin. Psychol. Sci. Pract. 18 183–202. 10.1111/j.1468-2850.2011.01250.x [ CrossRef ] [ Google Scholar ]
- Herpertz-Dahlmann B. (2009). Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 18 31–47. 10.1016/j.chc.2008.07.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hill J. P., Lynch M. E. (1983). “The intensification of gender-related role expectations during early adolescence,” in Girls at Puberty: Biological and Psychosocial Perspectives , eds Brooks-Gunn J., Petersen A. (Boston, MA: Springer; ), 201–228. [ Google Scholar ]
- Illing V., Tasca G. A., Balfour L., Bissada H. (2010). Attachment insecurity predicts eating disorder symptoms and treatment outcomes in a clinical sample of women. J. Nerv. Ment. Dis. 198 653–659. 10.1097/NMD.0b013e3181ef34b2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jacobi C., Fittig E., Bryson S. W., Wilfley D., Kraemer H. C., Taylor C. B. (2011). Who is really at risk? Identifying risk factors for subthreshold and full syndrome eating disorders in a high-risk sample. Psychol. Med. 41 1939–1949. 10.1017/S0033291710002631 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jacobi C., Hayward C., de Zwaan M., Kraemer H. C., Agras W. S. (2004). Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol. Bull. 130 19–65. 10.1037/0033-2909.130.1.19 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jappe L. M., Frank G. K., Shott M. E., Rollin M. D., Pryor T., Hagman J. O., et al. (2011). Heightened sensitivity to reward and punishment in anorexia nervosa. Int. J. Eat. Disord. 44 317–324. 10.1002/eat.20815 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jewell T., Collyer H., Gardner T., Tchanturia K., Simic M., Fonagy P., et al. (2016). Attachment and mentalization and their association with child and adolescent eating pathology: a systematic review. Int. J. Eat. Disord. 49 354–373. 10.1002/eat.22473 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jobe-Shields L., Parra G. R., Buckholdt K. E., Tillery R. N. (2014). Adolescent reactions to maternal responsiveness and internalizing symptomatology: a daily diary investigation. Pers. Relatsh. 21 335–348. 10.1111/pere.12034 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joos A. A., Saum B., van Elst L. T., Perlov E., Glauche V., Hartmann A., et al. (2011). Amygdala hyperreactivity in restrictive anorexia nervosa. Psychiatry Res. 191 189–195. 10.1016/j.pscychresns.2010.11.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karwautz A., Nobis G., Haidvogl M., Wagner G., Hafferl-Gattermayer A., Wober-Bingol C., et al. (2003). Perceptions of family relationships in adolescents with anorexia nervosa and their unaffected sisters. Eur. Child Adolesc. Psychiatry 12 128–135. 10.1007/s00787-003-0319-1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karwautz A., Rabe-Hesketh S., Hu X., Zhao J., Sham P., Collier D. A., et al. (2001a). Individual-specific risk factors for anorexia nervosa: a pilot study using a discordant sister-pair design. Psychol. Med. 31 317–329. [ PubMed ] [ Google Scholar ]
- Karwautz A., Volkl-Kernstock S., Nobis G., Kalchmayr G., Hafferl-Gattermayer A., Wober-Bingol C., et al. (2001b). Characteristics of self-regulation in adolescent patients with anorexia nervosa. Br. J. Med. Psychol. 74(Pt 1) , 101–114. 10.1348/000711201160830 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaye W. H., Fudge J. L., Paulus M. (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 10 573–584. 10.1038/nrn2682 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kellogg S. (2014). Transformational Chairwork: Using Psychotherapeutic Dialogues in Clinical Practice. Lanham, MD: Rowman & Littlefield. [ Google Scholar ]
- Kelly A. C., Carter J. C., Borairi S. (2014). Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int. J. Eat. Disord. 47 54–64. 10.1002/eat.22196 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kendler K. S., Campbell J. (2009). Interventionist causal models in psychiatry: repositioning the mind-body problem. Psychol. Med. 39 881–887. 10.1017/S0033291708004467 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Klimes-Dougan B., Pearson T. E., Jappe L., Mathieson L., Simard M. R., Hastings P., et al. (2014). Adolescent emotion socialization: a longitudinal study of friends’ responses to negative emotions. Soc. Dev. 23 395–412. 10.1111/jora.12434 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Knafo A., Zahn-Waxler C., Van Hulle C., Robinson J. L., Rhee S. H. (2008). The developmental origins of a disposition toward empathy: genetic and environmental contributions. Emotion 8 737–752. 10.1037/a0014179 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koepke S., Denissen J. J. (2012). Dynamics of identity development and separation-individuation in parente-child relationships during adolescence and emerging adulthood–A conceptual integration. Dev. Rev. 32 67–88. 10.1016/j.dr.2012.01.001 [ CrossRef ] [ Google Scholar ]
- Kucharska-Pietura K., Nikolaou V., Masiak M., Treasure J. (2004). The recognition of emotion in the faces and voice of anorexia nervosa. Int. J. Eat. Disord. 35 42–47. 10.1002/eat.10219 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kyriacou O., Treasure J., Schmidt U. (2008). Expressed emotion in eating disorders assessed via self-report: an examination of factors associated with expressed emotion in carers of people with anorexia nervosa in comparison to control families. Int. J. Eat. Disord. 41 37–46. 10.1002/eat.20469 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laghi F., Pompili S., Zanna V., Castiglioni M. C., Criscuolo M., Chianello I., et al. (2017). How adolescents with anorexia nervosa and their parents perceive family functioning? J. Health Psychol. 22 197–207. 10.1177/1359105315597055 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lang K., Dapelo M. M., Khondoker M., Morris R., Surguladze S., Treasure J., et al. (2015). Exploring emotion recognition in adults and adolescents with anorexia nervosa using a body motion paradigm. Eur. Eat. Disord. Rev. 23 262–268. 10.1002/erv.2358 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lang K., Larsson E. E., Mavromara L., Simic M., Treasure J., Tchanturia K. (2016). Diminished facial emotion expression and associated clinical characteristics in Anorexia Nervosa. Psychiatry Res. 236 165–172. 10.1016/j.psychres.2015.12.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lau J. Y., Guyer A. E., Tone E. B., Jenness J., Parrish J. M., Pine D. S., et al. (2012). Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. Int. J. Behav. Dev. 36 36–44. 10.1177/0165025411406854 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lavender J. M., Wonderlich S. A., Engel S. G., Gordon K. H., Kaye W. H., Mitchell J. E. (2015). Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: a conceptual review of the empirical literature. Clin. Psychol. Rev. 40 111–122. 10.1016/j.cpr.2015.05.010 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leppanen J., Cardi V., Paloyelis Y., Simmons A., Tchanturia K., Treasure J. (2017). FMRI study of neural responses to implicit infant emotion in anorexia nervosa. Front. Psychol. 8 : 780 . 10.3389/fpsyg.2017.00780 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li D., Zucker N. L., Kragel P. A., Covington V. E., LaBar K. S. (2017). Adolescent development of insula-dependent interoceptive regulation. Dev. Sci. 20 : e12438 . 10.1111/desc.12438 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lieberman M., Doyle A. B., Markiewicz D. (1999). Developmental patterns in security of attachment to mother and father in late childhood and early adolescence: associations with peer relations. Child Dev. 70 202–213. 10.1111/1467-8624.00015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lieberman M., Gauvin L., Bukowski W. M., White D. R. (2001). Interpersonal influence and disordered eating behaviors in adolescent girls: the role of peer modeling, social reinforcement, and body-related teasing. Eat. Behav. 2 215–236. 10.1016/S1471-0153(01)00030-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Luebbe A. M., Bell D. J. (2014). Positive and negative family emotional climate differentially predict youth anxiety and depression via distinct affective pathways. J. Abnorm. Child Psychol. 42 897–911. 10.1007/s10802-013-9838-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Luyckx K., Goossens L., Soenens B., Beyers W. (2006). Unpacking commitment and exploration: preliminary validation of an integrative model of late adolescent identity formation. J. Adolesc. 29 361–378. 10.1016/j.adolescence.2005.03.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Luyckx K., Soenens B., Vansteenkiste M., Goossens L., Berzonsky M. D. (2007). Parental psychological control and dimensions of identity formation in emerging adulthood. J. Fam. Psychol. 21 546–550. 10.1037/0893-3200.21.3.546 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mahler M. S. (1963). Thoughts about development and individuation. Psychoanal. Study Child 18 307–324. 10.1080/00797308.1963.11822933 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mallorqui-Bague N., Vintro-Alcaraz C., Sanchez I., Riesco N., Aguera Z., Granero R., et al. (2018). Emotion regulation as a transdiagnostic feature among eating disorders: cross-sectional and longitudinal approach. Eur. Eat. Disord. Rev. 26 53–61. 10.1002/erv.2570 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marchi M., Cohen P. (1990). Early childhood eating behaviors and adolescent eating disorders. J. Am. Acad. Child Adolesc. Psychiatry 29 112–117. 10.1097/00004583-199001000-00017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mauss I. B., Shallcross A. J., Troy A. S., John O. P., Ferrer E., Wilhelm F. H., et al. (2011). Don’t hide your happiness! Positive emotion dissociation, social connectedness, and psychological functioning. J. Pers. Soc. Psychol. 100 738–748. 10.1037/a0022410 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mazzeo S. E., Bulik C. M. (2009). Environmental and genetic risk factors for eating disorders: what the clinician needs to know. Child Adolesc. Psychiatr. Clin. N. Am. 18 67–82. 10.1016/j.chc.2008.07.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McAdams C. J., Harper J. A., Van Enkevort E. (2018). Mentalization and the left inferior frontal gyrus and insula. Eur. Eat. Disord. Rev. 26 265–271. 10.1002/erv.2580 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McClure E. B. (2000). A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol. Bull. 126 424–453. 10.1037/0033-2909.126.3.424 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McIntosh V. V., Jordan J., Luty S. E., Carter F. A., McKenzie J. M., Bulik C. M., et al. (2006). Specialist supportive clinical management for anorexia nervosa. Int. J. Eat. Disord. 39 625–632. 10.1002/eat.20297 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McLean K. C., Jennings L. E. (2012). Teens telling tales: how maternal and peer audiences support narrative identity development. J. Adolesc. 35 1455–1469. 10.1016/j.adolescence.2011.12.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McLean K. C., Mansfield C. D. (2012). The co-construction of adolescent narrative identity: narrative processing as a function of adolescent age, gender, and maternal scaffolding. Dev. Psychol. 48 436–447. 10.1037/a0025563 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McRae K., Misra S., Prasad A. K., Pereira S. C., Gross J. J. (2012). Bottom-up and top-down emotion generation: implications for emotion regulation. Soc. Cogn. Affect. Neurosci. 7 253–262. 10.1093/scan/nsq103 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meeus W., Iedema J., Maassen G., Engels R. (2005). Separation-individuation revisited: on the interplay of parent-adolescent relations, identity and emotional adjustment in adolescence. J. Adolesc. 28 89–106. 10.1016/j.adolescence.2004.07.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Merwin R. M., Moskovich A. A., Wagner H. R., Ritschel L. A., Craighead L. W., Zucker N. L. (2013). Emotion regulation difficulties in anorexia nervosa: relationship to self-perceived sensory sensitivity. Cogn. Emot. 27 441–452. 10.1080/02699931.2012.719003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Micali N., Hagberg K. W., Petersen I., Treasure J. L. (2013). The incidence of eating disorders in the UK in 2000-2009: findings from the General Practice Research Database. BMJ Open 3 : e002646 . 10.1136/bmjopen-2013-002646 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller S. P., Erickson S. J., Branom C., Steiner H. (2009). Habitual response to stress in recovering adolescent anorexic patients. Child Psychiatry Hum. Dev. 40 43–54. 10.1007/s10578-008-0112-y [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller S. P., Redlich A. D., Steiner H. (2003). The stress response in anorexia nervosa. Child Psychiatry Hum. Dev. 33 295–306. 10.1023/A:1023036329399 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Miller-Day M. A. (2012). “Two of me: mothers and daughters in connection,” in Mothers and Daughters: Complicated Connections Across Cultures , eds Deakins A. H., Lockridge R. B., Sterk H. M. (Lanham, MD: University Press of America; ), 89–104. [ Google Scholar ]
- Miller-Slough R. L., Dunsmore J. C. (2016). Parent and friend emotion socialization in adolescence: associations with psychological adjustment. Adolesc. Res. Rev. 1 287–305. 10.1007/s40894-016-0026-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Minuchin S., Rosman B. L., Baker L. (1978). Psychosomatic Families: Anorexia Nervosa in Context. Cambridge, MA: Harvard University Press; 10.4159/harvard.9780674418233 [ CrossRef ] [ Google Scholar ]
- Miyake Y., Okamoto Y., Onoda K., Kurosaki M., Shirao N., Okamoto Y., et al. (2010). Brain activation during the perception of distorted body images in eating disorders. Psychiatry Res. 181 183–192. 10.1016/j.pscychresns.2009.09.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moed A., Gershoff E. T., Eisenberg N., Hofer C., Losoya S., Spinrad T. L., et al. (2015). Parent-adolescent conflict as sequences of reciprocal negative emotion: links with conflict resolution and adolescents’ behavior problems. J. Youth Adolesc. 44 1607–1622. 10.1007/s10964-014-0209-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Monteleone A. M., Monteleone P., Esposito F., Prinster A., Volpe U., Cantone E., et al. (2017). Altered processing of rewarding and aversive basic taste stimuli in symptomatic women with anorexia nervosa and bulimia nervosa: an fMRI study. J. Psychiatr. Res. 90 94–101. 10.1016/j.jpsychires.2017.02.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morris A. S., Silk J. S., Steinberg L., Myers S. S., Robinson L. R. (2007). The role of the family context in the development of emotion regulation. Soc. Dev. 16 361–388. 10.1111/j.1467-9507.2007.00389.x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nandrino J. L., Berna G., Hot P., Dodin V., Latree J., Decharles S., et al. (2012). Cognitive and physiological dissociations in response to emotional pictures in patients with anorexia. J. Psychosom. Res. 72 58–64. 10.1016/j.jpsychores.2011.11.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nandrino J. L., Doba K., Lesne A., Christophe V., Pezard L. (2006). Autobiographical memory deficit in anorexia nervosa: emotion regulation and effect of duration of illness. J. Psychosom. Res. 61 537–543. 10.1016/j.jpsychores.2006.02.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- National Institute for Health, and Care Excellence [NICE] (2004). Eating Disorders in Over 8s: Management. London: National Institute for Health and Clinical Excellence. [ Google Scholar ]
- National Institute for Health, and Care Excellence [NICE] (2017). Eating Disorders: Recognition and Treatment: NICE Guideline [NG69]. London: National Institute for Health and Care Excellence. [ Google Scholar ]
- Nelson E. E., Jarcho J. M., Guyer A. E. (2016). Social re-orientation and brain development: an expanded and updated view. Dev. Cogn. Neurosci. 17 118–127. 10.1016/j.dcn.2015.12.008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nesse R. M. (2017). Anorexia: a perverse effect of attempting to control the starvation response. Behav. Brain Sci. 40 : e125 . 10.1017/S0140525X16001503 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nordbo R. H., Espeset E. M., Gulliksen K. S., Skarderud F., Holte A. (2006). The meaning of self-starvation: qualitative study of patients’ perception of anorexia nervosa. Int. J. Eat. Disord. 39 556–564. 10.1002/eat.20276 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nunn K., Frampton I., Gordon I., Lask B. (2008). The fault is not in her parents but in her insula–a neurobiological hypothesis of anorexia nervosa. Eur. Eat. Disord. Rev. 16 355–360. 10.1002/erv.890 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oldershaw A., Hambrook D., Stahl D., Tchanturia K., Treasure J., Schmidt U. (2011). The socio-emotional processing stream in Anorexia Nervosa. Neurosci. Biobehav. Rev. 35 970–988. 10.1016/j.neubiorev.2010.11.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oldershaw A., Hambrook D., Tchanturia K., Treasure J., Schmidt U. (2010). Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom. Med. 72 73–79. 10.1097/PSY.0b013e3181c6c7ca [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oldershaw A., Lavender T., Sallis H., Stahl D., Schmidt U. (2015). Emotion generation and regulation in anorexia nervosa: a systematic review and meta-analysis of self-report data. Clin. Psychol. Rev. 39 83–95. 10.1016/j.cpr.2015.04.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Oldershaw A., Lavender T., Schmidt U. (2018). Are socio-emotional and neurocognitive functioning predictors of therapeutic outcomes for adults with anorexia nervosa? Eur. Eat. Disord. Rev. 26 346–359. 10.1002/erv.2602 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Olivo G., Solstrand Dahlberg L., Wiemerslage L., Swenne I., Zhukovsky C., Salonen-Ros H., et al. (2018). Atypical anorexia nervosa is not related to brain structural changes in newly diagnosed adolescent patients. Int. J. Eat. Disord. 51 39–45. 10.1002/eat.22805 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Orbach S. (1993). Hunger Strike. Harmondsworth: Penguin. [ Google Scholar ]
- Pace C. S., Cavanna D., Guiducci V., Bizzi F. (2015). When parenting fails: alexithymia and attachment states of mind in mothers of female patients with eating disorders. Front. Psychol. 6 : 1145 . 10.3389/fpsyg.2015.01145 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pace C. S., Guiducci V., Cavanna D. (2016). A controlled study of attachment representations and emotion regulation in female adolescents with anorexia nervosa. Mediterr. J. Clin. Psychol. 4. 10.6092/2282-1619/2016.4.1187 [ CrossRef ] [ Google Scholar ]
- Park A., Bryson C., Clery E., Curtice J., Phillips M. (2013). British Social Attitudes: the 30th Report. London: NatCen. [ Google Scholar ]
- Paschke L. M., Dorfel D., Steimke R., Trempler I., Magrabi A., Ludwig V. U., et al. (2016). Individual differences in self-reported self-control predict successful emotion regulation. Soc. Cogn. Affect. Neurosci. 11 1193–1204. 10.1093/scan/nsw036 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pascual-Leone A., Greenberg L. S. (2007). Emotional processing in experiential therapy: why “the only way out is through”. J. Consult. Clin. Psychol. 75 875–887. 10.1037/0022-006X.75.6.875 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pennesi J. L., Wade T. D. (2016). A systematic review of the existing models of disordered eating: do they inform the development of effective interventions? Clin. Psychol. Rev. 43 175–192. 10.1016/j.cpr.2015.12.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pfeifer J. H., Berkman E. T. (2018). The development of self and identity in adolescence: neural evidence and implications for a value-based choice perspective on motivated behavior. Child Dev. Perspect. 12 158–164. 10.1111/cdep.12279 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pfeifer J. H., Kahn L. E., Merchant J. S., Peake S. J., Veroude K., Masten C. L., et al. (2013). Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. J. Neurosci. 33 7415–7419. 10.1523/JNEUROSCI.4074-12.2013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phan K. L., Fitzgerald D. A., Nathan P. J., Moore G. J., Uhde T. W., Tancer M. E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry 57 210–219. 10.1016/j.biopsych.2004.10.030 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phillipou A., Abel L. A., Castle D. J., Hughes M. E., Gurvich C., Nibbs R. G., et al. (2015). Self perception and facial emotion perception of others in anorexia nervosa. Front. Psychol. 6 : 1181 . 10.3389/fpsyg.2015.01181 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- PricewaterhouseCoopers (2015). The Costs of Eating Disorders: Social, Health and Economic Impacts. London: PricewaterhouseCoopers LLP. [ Google Scholar ]
- Pugh M., Waller G. (2016). The anorexic voice and severity of eating pathology in anorexia nervosa. Int. J. Eat. Disord. 49 622–625. 10.1002/eat.22499 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pugh M., Waller G. (2017). Understanding the ’Anorexic Voice’ in Anorexia Nervosa. Clin. Psychol. Psychother. 24 670–676. 10.1002/cpp.2034 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Racine S. E., Wildes J. E. (2015). Dynamic longitudinal relations between emotion regulation difficulties and anorexia nervosa symptoms over the year following intensive treatment. J. Consult. Clin. Psychol. 83 785–795. 10.1037/ccp0000011 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rajhans P., Jessen S., Missana M., Grossmann T. (2016). Putting the face in context: body expressions impact facial emotion processing in human infants. Dev. Cogn. Neurosci. 19 115–121. 10.1016/j.dcn.2016.01.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reau N. R., Senturia Y. D., Lebailly S. A., Christoffel K. K. (1996). Infant and toddler feeding patterns and problems: normative data and a new direction. Pediatric Practice Research Group. J. Dev. Behav. Pediatr. 17 149–153. 10.1097/00004703-199606000-00002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reese E., Newcombe R. (2007). Training mothers in elaborative reminiscing enhances children’s autobiographical memory and narrative. Child Dev. 78 1153–1170. 10.1111/j.1467-8624.2007.01058.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reese E., Yan C., Jack F., Hayne H. (2010). “Emerging identities: narrative and self from early childhood to early adolescence,” in Narrative Development in Adolescence , eds McLean K. C., Pasupathi M. (Boston, MA: Springer; ), 23–43. [ Google Scholar ]
- Rice L. N., Greenberg L. S. (1984). Patterns of Change: Intensive Analysis of Psychotherapy Process. New York, NY: Guilford. [ Google Scholar ]
- Rice L. N., Saperia E. P. (1984). “Task analysis of the resolution of problematic reactions,” in Patterns of Change , ed. Greenberg L. N. R. L. S. (New York, NY: Guilford; ), 29–66. [ Google Scholar ]
- Richmond S. (2002). Psychoanalysis and Feminism: Anorexia, the Social World, and the Internal World , Vol. 8 Baltimore, MD: Johns Hopkins UniversityPress, 1–12 [ Google Scholar ]
- Riva G., Dakanalis A. (2018). Altered processing and integration of multisensory bodily representations and signals in eating disorders: a possible path toward the understanding of their underlying causes. Front. Hum. Neurosci. 12 : 49 . 10.3389/fnhum.2018.00049 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rowa K., Kerig P. K., Geller J. (2001). The family and anorexia nervosa: examining parent–child boundary problems. Eur. Eat. Disord. Rev. 9 97–114. 10.1002/erv.383 [ CrossRef ] [ Google Scholar ]
- Saarni H. U. (1984). An observational study of children’s attempts to monitor their expressive behavior. Child Dev. 55 1504–1513. 10.2307/1130020 [ CrossRef ] [ Google Scholar ]
- Sala M., Heard A., Black E. A. (2016). Emotion-focused treatments for anorexia nervosa: a systematic review of the literature. Eat. Weight Disord. 21 147–164. 10.1007/s40519-016-0257-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sanders W., Zeman J., Poon J., Miller R. (2015). Child regulation of negative emotions and depressive symptoms: the moderating role of parental emotion socialization. J. Child Fam. Stud. 24 402–415. 10.1007/s10826-013-9850-y [ CrossRef ] [ Google Scholar ]
- Schmidt U., Campbell I. C. (2013). Treatment of eating disorders can not remain ’brainless’: the case for brain-directed treatments. Eur. Eat. Disord. Rev. 21 425–427. 10.1002/erv.2257 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schmidt U., Magill N., Renwick B., Keyes A., Kenyon M., Dejong H., et al. (2015). The Maudsley Outpatient Study of Treatments for Anorexia Nervosa and Related Conditions (MOSAIC): comparison of the Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA) with specialist supportive clinical management (SSCM) in outpatients with broadly defined anorexia nervosa: a randomized controlled trial. J. Consult. Clin. Psychol. 83 796–807. 10.1037/ccp0000019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schmidt U., Oldershaw A., Jichi F., Sternheim L., Startup H., McIntosh V., et al. (2012). Out-patient psychological therapies for adults with anorexia nervosa: randomised controlled trial. Br. J. Psychiatry 201 392–399. 10.1192/bjp.bp.112.112078 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schmidt U., Treasure J. (2006). Anorexia nervosa: valued and visible. A cognitive-interpersonal maintenance model and its implications for research and practice. Br. J. Clin. Psychol. 45(Pt 3) , 343–366. [ PubMed ] [ Google Scholar ]
- Sebastian C., Viding E., Williams K. D., Blakemore S. J. (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 72 134–145. 10.1016/j.bandc.2009.06.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seeger G., Braus D. F., Ruf M., Goldberger U., Schmidt M. H. (2002). Body image distortion reveals amygdala activation in patients with anorexia nervosa – a functional magnetic resonance imaging study. Neurosci. Lett. 326 25–28. 10.1016/S0304-3940(02)00312-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seidel M., King J. A., Ritschel F., Boehm I., Geisler D., Bernardoni F., et al. (2018). Processing and regulation of negative emotions in anorexia nervosa: an fMRI study. Neuroimage Clin. 18 1–8. 10.1016/j.nicl.2017.12.035 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sergerie K., Chochol C., Armony J. L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 32 811–830. 10.1016/j.neubiorev.2007.12.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Serpell L., Teasdale J. D., Troop N. A., Treasure J. (2004). The development of the P-CAN, a measure to operationalize the pros and cons of anorexia nervosa. Int. J. Eat. Disord. 36 416–433. 10.1002/eat.20040 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seth A. K. (2013). Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17 565–573. 10.1016/j.tics.2013.09.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seth A. K., Friston K. J. (2016). Active interoceptive inference and the emotional brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 271 : 20160007 . 10.1098/rstb.2016.0007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Seth A. K., Suzuki K., Critchley H. D. (2011). An interoceptive predictive coding model of conscious presence. Front. Psychol. 2 : 395 . 10.3389/fpsyg.2011.00395 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Shaw P., Kabani N. J., Lerch J. P., Eckstrand K., Lenroot R., Gogtay N., et al. (2008). Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28 3586–3594. 10.1523/JNEUROSCI.5309-07.2008 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sheppes G., Scheibe S., Suri G., Gross J. J. (2011). Emotion-regulation choice. Psychol. Sci. 22 1391–1396. 10.1177/0956797611418350 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Skǎrderud F. (2007). Eating one’s words, part I: ’Concretised metaphors’ and reflective function in anorexia nervosa–an interview study. Eur. Eat. Disord. Rev. 15 163–174. 10.1002/erv.777 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Smink F. R., van Hoeken D., Hoek H. W. (2013). Epidemiology, course, and outcome of eating disorders. Curr. Opin. Psychiatry 26 543–548. 10.1097/YCO.0b013e328365a24f [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Somerville L. H. (2013). Special issue on the teenage brain: sensitivity to social evaluation. Curr. Dir. Psychol. Sci. 22 121–127. 10.1177/0963721413476512 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Somerville L. H., Jones R. M., Ruberry E. J., Dyke J. P., Glover G., Casey B. J. (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 24 1554–1562. 10.1177/0956797613475633 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sowell E. R., Thompson P. M., Leonard C. M., Welcome S. E., Kan E., Toga A. W. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 24 8223–8231. 10.1523/JNEUROSCI.1798-04.2004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stanghellini G., Castellini G., Brogna P., Faravelli C., Ricca V. (2012). Identity and eating disorders (IDEA): a questionnaire evaluating identity and embodiment in eating disorder patients. Psychopathology 45 147–158. 10.1159/000330258 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stanghellini G., Trisolini F., Castellini G., Ambrosini A., Faravelli C., Ricca V. (2015). Is feeling extraneous from one’s own body a core vulnerability feature in eating disorders? Psychopathology 48 18–24. 10.1159/000364882 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Startup H., Lavender A., Oldershaw A., Stott R., Tchanturia K., Treasure J., et al. (2013). Worry and rumination in anorexia nervosa. Behav. Cogn. Psychother. 41 301–316. 10.1017/S1352465812000847 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Startup H., Mountford V., Lavender A., Schmidt U. (2015). “Cognitive behavioural case formulation in complex eating disorders,” in Case Formulation in Cognitive Behaviour Therapy: The Treatment of Challenging and Complex Cases , ed. Tarrier N. J. (Hove: Routledge; ), 239–264. [ Google Scholar ]
- Steinberg L. (2004). Risk taking in adolescence: what changes, and why? Ann. N. Y. Acad. Sci. 1021 51–58. 10.1196/annals.1308.005 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Steiner-Adair C. (1990). “The body politic: normal female adolescent development and the development of eating disorders,” in Making Connections , eds Gilligan C., Lyons N., Hanmer T. (Cambridge, MA: Harvard University Press; ), 162–182. [ PubMed ] [ Google Scholar ]
- Steinhausen H. C., Jensen C. M. (2015). Time trends in lifetime incidence rates of first-time diagnosed anorexia nervosa and bulimia nervosa across 16 years in a Danish nationwide psychiatric registry study. Int. J. Eat. Disord. 48 845–850. 10.1002/eat.22402 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sternheim L., Konstantellou A., Startup H., Schmidt U. (2011). What does uncertainty mean to women with anorexia nervosa? An interpretative phenomenological analysis. Eur. Eat. Disord. Rev. 19 12–24. 10.1002/erv.1029 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sternheim L., Startup H., Saeidi S., Morgan J., Hugo P., Russell A., et al. (2012). Understanding catastrophic worry in eating disorders: process and content characteristics. J. Behav. Ther. Exp. Psychiatry 43 1095–1103. 10.1016/j.jbtep.2012.05.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Stice E. (2002). Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol. Bull. 128 825–848. 10.1037/0033-2909.128.5.825 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Striegel-Moore R. H., Bulik C. M. (2007). Risk factors for eating disorders. Am. Psychol. 62 181–198. 10.1037/0003-066X.62.3.181 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Strigo I. A., Matthews S. C., Simmons A. N., Oberndorfer T., Klabunde M., Reinhardt L. E., et al. (2013). Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: evidence of interoceptive dysregulation. Int. J. Eat. Disord. 46 23–33. 10.1002/eat.22045 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Suchan B., Bauser D. S., Busch M., Schulte D., Gronemeyer D., Herpertz S., et al. (2013). Reduced connectivity between the left fusiform body area and the extrastriate body area in anorexia nervosa is associated with body image distortion. Behav. Brain Res. 241 80–85. 10.1016/j.bbr.2012.12.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Suveg C., Sood E., Barmish A., Tiwari S., Hudson J. L., Kendall P. C. (2008). “I’d rather not talk about it”: emotion parenting in families of children with an anxiety disorder. J. Fam. Psychol. 22 875–884. 10.1037/a0012861 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Swick D., Ashley V., Turken A. U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 9 : 102 . 10.1186/1471-2202-9-102 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Talsma D. (2015). Predictive coding and multisensory integration: an attentional account of the multisensory mind. Front. Integr. Neurosci. 9 : 19 . 10.3389/fnint.2015.00019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tasca G. A., Balfour L. (2014). Attachment and eating disorders: a review of current research. Int. J. Eat. Disord. 47 710–717. 10.1002/eat.22302 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tchanturia K., Davies H., Harrison A., Fox J. R., Treasure J., Schmidt U. (2012). Altered social hedonic processing in eating disorders. Int. J. Eat. Disord. 45 962–969. 10.1002/eat.22032 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treasure J., Cardi V. (2017). Anorexia nervosa, theory and treatment: where are we 35 years on from Hilde Bruch’s foundation lecture? Eur. Eat. Disord. Rev. 25 139–147. 10.1002/erv.2511 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treasure J., Russell G. (2011). The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br. J. Psychiatry 199 5–7. 10.1192/bjp.bp.110.087585 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treasure J., Schmidt U. (2013). The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J. Eat. Disord. 1 : 13 . 10.1186/2050-2974-1-13 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treasure J., Smith G., Crane A. (2016). Skills-Based Caring for a Loved One with an Eating Disorder: The New Maudsley Method. Hove: Routledge; 10.4324/9781315735610 [ CrossRef ] [ Google Scholar ]
- Treasure J., Stein D., Maguire S. (2015a). Has the time come for a staging model to map the course of eating disorders from high risk to severe enduring illness? An examination of the evidence. Early Interv. Psychiatry 9 173–184. 10.1111/eip.12170 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Treasure J., Zipfel S., Micali N., Wade T., Stice E., Claudino A., et al. (2015b). Anorexia nervosa. Nat. Rev. Dis. Primers 1 : 15074 . 10.1038/nrdp.2015.74 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tronick E. Z., Cohn J. F. (1989). Infant-mother face-to-face interaction: age and gender differences in coordination and the occurrence of miscoordination. Child Dev. 60 85–92. 10.2307/1131074 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ty M., Francis A. J. (2013). Insecure attachment and disordered eating in women: the mediating processes of social comparison and emotion dysregulation. Eat. Disord. 21 154–174. 10.1080/10640266.2013.761089 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vandereycken W., Van Deth R. (1990). A tribute to Lasègue’s description of anorexia nervosa (1873), with completion of its English translation. Br. J. Psychiatry 157 902–908. 10.1192/bjp.157.6.902 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Viana M. C., Gruber M. J., Shahly V., Alhamzawi A., Alonso J., Andrade L. H., et al. (2013). Family burden related to mental and physical disorders in the world: results from the WHO World Mental Health (WMH) surveys. Braz. J. Psychiatry 35 115–125. 10.1590/1516-4446-2012-0919 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wade T. D., Treasure J., Schmidt U. (2011). A case series evaluation of the Maudsley Model for treatment of adults with anorexia nervosa. Eur. Eat. Disord. Rev. 19 382–389. 10.1002/erv.1078 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wagner A., Aizenstein H., Venkatraman V. K., Fudge J., May J. C., Mazurkewicz L., et al. (2007). Altered reward processing in women recovered from anorexia nervosa. Am. J. Psychiatry 164 1842–1849. 10.1176/appi.ajp.2007.07040575 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waller G., Kennerley H., Ohanian V. (2010). “Schema-focused cognitive behaviour therapy with the eating disorders,” in Cognitive Schemas and Core Beliefs in Psychological Problems: A Scientist-Practitioner Guide , eds Riso L. P., du Tiot P. L., Stein D. J., Young J. E. (Washington DC: American Psychological Association; ), 139–175. [ Google Scholar ]
- Walsh B. T. (2013). The enigmatic persistence of anorexia nervosa. Am. J. Psychiatry 170 477–484. 10.1176/appi.ajp.2012.12081074 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward A., Ramsay R., Turnbull S., Steele M., Steele H., Treasure J. (2001). Attachment in anorexia nervosa: a transgenerational perspective. Br. J. Med. Psychol. 74(Pt 4) , 497–505. 10.1348/000711201161145 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wardle J., Carnell S., Cooke L. (2005). Parental control over feeding and children’s fruit and vegetable intake: how are they related? J. Am. Diet. Assoc. 105 227–232. 10.1016/j.jada.2004.11.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watson H. J., Bulik C. M. (2013). Update on the treatment of anorexia nervosa: review of clinical trials, practice guidelines and emerging interventions. Psychol. Med. 43 2477–2500. 10.1017/S0033291712002620 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Webber L., Cooke L., Hill C., Wardle J. (2010). Associations between children’s appetitive traits and maternal feeding practices. J. Am. Diet. Assoc. 110 1718–1722. 10.1016/j.jada.2010.08.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Weil L. G., Fleming S. M., Dumontheil I., Kilford E. J., Weil R. S., Rees G., et al. (2013). The development of metacognitive ability in adolescence. Conscious. Cogn. 22 264–271. 10.1016/j.concog.2013.01.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Westenberg P. M., Drewes M. J., Goedhart A. W., Siebelink B. M., Treffers P. D. (2004). A developmental analysis of self-reported fears in late childhood through mid-adolescence: social-evaluative fears on the rise? J. Child Psychol. Psychiatry 45 481–495. 10.1111/j.1469-7610.2004.00239.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wierenga C., Bischoff-Grethe A., Melrose A. J., Grenesko-Stevens E., Irvine Z., Wagner A., et al. (2014). Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PLoS One 9 : e92017 . 10.1371/journal.pone.0092017 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wildes J. E., Marcus M. D., Cheng Y., McCabe E. B., Gaskill J. A. (2014). Emotion acceptance behavior therapy for anorexia nervosa: a pilot study. Int. J. Eat. Disord. 47 870–873. 10.1002/eat.22241 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wildes J. E., Ringham R. M., Marcus M. D. (2010). Emotion avoidance in patients with anorexia nervosa: initial test of a functional model. Int. J. Eat. Disord. 43 398–404. 10.1002/eat.20730 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Williams K., King J., Fox J. R. (2016). Sense of self and anorexia nervosa: a grounded theory. Psychol. Psychother. Theory Res. Pract. 89 211–228. 10.1111/papt.12068 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wolf L. K., Bazargani N., Kilford E. J., Dumontheil I., Blakemore S. J. (2015). The audience effect in adolescence depends on who’s looking over your shoulder. J. Adolesc. 43 5–14. 10.1016/j.adolescence.2015.05.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wonderlich S. A., Lilenfeld L. R., Riso L. P., Engel S., Mitchell J. E. (2005). Personality and anorexia nervosa. Int. J. Eat. Disord. 37(Suppl.) , S68–S71. 10.1002/eat.20120 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Woodside D. B., Bulik C. M., Halmi K. A., Fichter M. M., Kaplan A., Berrettini W. H., et al. (2002). Personality, perfectionism, and attitudes toward eating in parents of individuals with eating disorders. Int. J. Eat. Disord. 31 290–299. 10.1002/eat.10032 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Young J. E., Klosko J. S., Weishaar M. E. (2003). Schema Therapy: A Practitioner’s Guide. New York, NY: Guildford Press. [ Google Scholar ]
- Zachrisson H. D., Skarderud F. (2010). Feelings of insecurity: review of attachment and eating disorders. Eur. Eat. Disord. Rev. 18 97–106. 10.1002/erv.999 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zeman J., Cassano M., Adrian M. C. (2012). “Socialization influences on children’s and adolescents’ emotional self-regulation processes,” in Handbook of Self-Regulatory Processes in Development: New Directions and International Perspectives , eds Barrett K. C., Fox N. A., Morgan G. A., Fidler D. J., Daunhauer L. A. (New York, NY: Psychology Press; ), 79–101. [ Google Scholar ]
- Zipfel S., Wild B., Gross G., Friederich H. C., Teufel M., Schellberg D., et al. (2014). Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet 383 127–137. 10.1016/S0140-6736(13)61746-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zucker N., Wagner H. R., Merwin R., Bulik C. M., Moskovich A., Keeling L., et al. (2015). Self-focused attention in anorexia nervosa. Int. J. Eat. Disord. 48 9–14. 10.1002/eat.22307 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zucker N. L., Kragel P. A., Wagner H. R., Keeling L., Mayer E., Wang J., et al. (2017). The clinical significance of posterior insular volume in adolescent anorexia nervosa. Psychosom. Med. 79 1025–1035. 10.1097/PSY.0000000000000510 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Anorexia nervosa is an eating disorder defined by restriction of energy intake relative to requirements, leading to a significantly low body weight. Patients will have an intense fear of gaining weight and distorted body image with the inability to recognize the seriousness of their significantly low body weight.[1][2][3]
Anorexia (an-o-REK-see-uh) nervosa — often simply called anorexia — is an eating disorder characterized by an abnormally low body weight, an intense fear of gaining weight and a distorted perception of weight. People with anorexia place a high value on controlling their weight and shape, using extreme efforts that tend to significantly ...
Anorexia nervosa is the name of a mental health condition. It is a serious disease, but, with the right treatment, recovery is possible. It is part of a potentially life threatening mental health ...
Anorexia nervosa is associated with a high incidence of coexisting psychiatric conditions, marked treatment resistance, frequent medical complications, and a substantial risk of death. Several psyc...
Anorexia nervosa through the lens of a severe and enduring experience: 'lost in a big world' Severe and enduring anorexia nervosa (SE-AN), is a serious and persistent illness, despite 'state of the art' treatment. Criteria have been theoretically proposed, but not tested, and may not adequately captur...
Anorexia nervosa is an eating disorder characterized by weight loss (or lack of appropriate weight gain in growing children); difficulties maintaining an appropriate body weight for height, age, and stature; and, in many individuals, distorted body image. People with anorexia nervosa generally restrict the number of calories and the types of ...
Anorexia nervosa increases anxiety about gaining weight and eating high-calorie foods — and yet this is necessary for thoughts, mood, and body dissatisfaction to improve in patients with the disorder. Key goals of treatment are weight restoration and eating a variety of foods of differing calorie densities at regular meals. We know the ...
Notably, the publication of DSM-5 in 2013 and DSM-5-TR in 2022 resulted in broader diagnostic criteria for both anorexia nervosa and bulimia nervosa as well as the additions of binge-eating disorder and avoidant/restrictive food intake disorder (ARFID) categories. Binge-eating and avoidant/restrictive food intake disorders are both referenced ...
Diagnosis. If your doctor suspects that you have anorexia nervosa, he or she will typically do several tests and exams to help pinpoint a diagnosis, rule out medical causes for the weight loss, and check for any related complications. These exams and tests generally include: Physical exam. This may include measuring your height and weight ...
Anorexia nervosa is a serious eating disorder characterized by a refusal to maintain a healthy body weight, an intense fear of gaining weight, and a distorted body image. Anorexia can result in unhealthy, often dangerous weight loss. In fact, the desire to lose weight may become more important than anything else.
DSM-IV (2) lists four criteria for the diagnosis of anorexia nervosa: 1. Refusal to maintain body weight at or above a minimally normal weight for age and height. 2. Intense fear of gaining weight or becoming fat, even though underweight. 3. Disturbance in the way in which one's body weight or shape is experienced, undue influence of body ...
Anorexia nervosa (AN) is an eating disorder that is difficult to treat, and relapse is common. This article addresses management strategies and nursing interventions for adolescents diagnosed with AN. Figure. DX, 16, WAS ADMITTED with anorexia nervosa (AN) after unsuccessful outpatient treatment. She had intentionally lost 30 lb over 6 months ...
Anorexia nervosa (AN) is a disorder that predominantly affects women in early adolescence 1. The characteristic features of AN include severe weight loss and secondary problems associated with ...
Anorexia nervosa is a psychiatric disorder characterized by altered body image, persistent food restriction and low body weight 1.In 2013, the American Psychiatric Association revised the ...
Visits related to anorexia nervosa, which has the highest death rate of any mental illness, jumped 129.26%. From 2018 through mid-2022, visits among people younger than 17 jumped 107.4% across all ...
Anorexia nervosa (AN) is an eating disorder with a high mortality. About 95% of cases are women and it has a population prevalence of about 1%, but evidence-based treatment is lacking. The ...
Anorexia Nervosa (AN) is a serious psychiatric disorder characterised by significant medical complications, disordered eating, weight loss, and body image distortion [1]. It has the highest fatality rate of all mental disorders [1,2], and is common amongst young Western women and adolescents below the age of 15 [3-5].
Risk and Maintenance Factors Associated With AN. Anorexia Nervosa has been associated with numerous broad ranging risk and maintenance factors. Risk factors are variables which predict subsequent development of later pathology, in an individual currently disorder and symptom-free (Stice, 2002).Risk factors can have effects mitigated by protective factors or amplified by potentiating factors.