Additional Info
Barry funck, bethany p. pridgen, mfs, carol murren, charles bruce williams, iii, ph.d., chris trevey, christopher michael (mike) bailiff, dr. frederic whitehurst, edward g. brown, ph.d., guy oldaker, ph.d., lyle liechty, patra watson.
- Foundations of Forensics
- Blood & Bodily Fluids
- Child Abuse Allegations
- Crime Scene Investigation
- Death Investigation
- Detection Dogs
- Digital Evidence

Drug Analysis
- Drug Recognition Experts
- Eyewitness ID
- Fingerprints
- Forensic/Sexual Assault Exams
- Measurement Uncertainty
- Mental Health
- Trace Evidence
- Forensic Consultations
Featured Articles
- Legislation
- Discovery Motions
- Funding for Experts
- Motions for Appropriate Relief
- Motions to Exclude Expert Testimony
- Motions for Independent Testing
- Motions to Preserve Evidence
- Motions to Suppress
- Analyst Certification Motions
- Reports & Publications
- Forensic Terminology
- Online Research Tools
- General Information
- NC State Crime Lab Procedures
- Charlotte Mecklenburg Crime Lab
- CCBI Lab Procedures
- NC OCME Toxicology Lab
- Pitt Co. Sheriff’s Forensic Services
- Sec. of State Digital Forensic Lab
- Wilmington Police Dept Crime Lab
- Private and Out-of-State Labs
- News Articles
- Browse All Experts
- Working with Experts
- Expert Services Project
- Add or Update Expert Records
- Find a Private Investigator
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

North Carolina Office of Indigent Defense Services
Reports and Publications
From the blog, motions and briefs.
- In the News

Drug analysis is the testing of a suspected controlled substance to determine its composition. For information about forensic toxicology, or the testing of bodily fluids for controlled substances, click here .
Understanding Test Results
Every analysis of a suspected controlled substance should consist of at least two tests. The first is a presumptive or screening test which indicates if the sample could be a controlled substance. The State Crime Lab’s procedures stated in 2008 that presumptive tests are “used to evaluate evidence in determining the possible presence of controlled substances into general categories.” (That language has since been removed from lab procedures.) In some cases, crime labs perform only a screening test and then write in the lab report that further testing will be conducted at the request of the District Attorney. Substances other than controlled substances may produce positive results with these tests (false positives). Therefore, confirmatory tests that are substance-specific must be performed in order to positively identify the substance. The State Crime Lab’s procedures in 2008 stated that confirmatory tests are “used to conclusively identify the identity of a controlled substance.” (This language has since been removed from lab procedures.) The State Crime Lab’s procedures divide drug testing techniques into three categories and describe what combinations of tests must be performed. (p. 8) The State Crime Lab’s Technical Procedure for Drug Chemistry Analysis provides helpful flow charts that show which test are used to analyze suspected controlled substances.
Explanation of Testing Procedures
Detailed descriptions of the tests used to analyze suspected controlled substances are contained in the expandable sections below. Information including links to the State Crime Lab’s testing procedures, explanations of how tests are performed, and limitations of the tests are contained in the expandable sections.
Presumptive Tests
- The Home Scientist – Forensic Presumptive Drug Testing
- Ohio University Forensic Chemistry Lab – Chemical Color Tests
- Concerns for color tests: (1) False positives. Some over the counter cold medication or other substances may test positive for illegal substances using presumptive color tests. Because some drugs behave similarly, a confirmatory test is needed. (2) Color tests may only give an indication of a class of drug present. (3) A color test cannot conclusively identify the presence of a substance.
- Case Law: In State v. Carter (2014), the NC Court of Appeals found the trial court abused its discretion by allowing an officer to testify that a narcotics indicator field test kit indicated the presence of cocaine on items of evidence where the State failed to demonstrate the reliability of the kit.
- To reduce the variability in results due to subjective analysis, good laboratory practices include running a positive control for visual comparison with the evidence sample.
- Typically a lab report from the State Crime Lab will only include the name of the test and the resulting color (or may simply state that the expected color change occurred).
- Link to the State Crime Lab’s procedure – Note the quality control check that is required on p. 2.
- Law Enforcement and Corrections Standards and Testing Program – National Institute of Justice standards for color test reagents and kits for the preliminary identification of controlled substances.
- Helpful questions to consider: Was the required quality control check performed? The reagent lasts for only X days – do you know the age of the reagent? How was the resulting color measured – was it compared to a color standard or simply eye-balled? Was the resulting color recorded (photographed)? What precautions were taken to avoid cross-contamination? If performed by a law enforcement officer, what training does he/she have to perform this test?
- Marquis Reagent
- Cobalt Thiocyanate Reagent (Scott’s Test)
- Duquenois-Levine
- Ferric Chloride
- Potassium Permanganate
- Ehrlich’s, Van Urk’s, or p-dimethylaminobenzaledhyde (PDMAB)
- Froehde’s
- Mecke’s
- Other tests
Includes techniques (visual examination and microscopic examination) to understand the physical properties and characteristics of substances.
- Visual Examination – for pills, NCSCL’s procedure is for analysts to identify a pharmaceutical preparation by physical characteristics and markings. This means the pill is compared to pictures and descriptions in reference materials such as Micromedex, The Physician’s Desk Reference, The Logo for Tablets and Capsules or websites. Visual examination is to be used for “preliminary examination only.” (p. 7) In State v. Ward the NC Supreme Court held that visual identification of controlled substances is not reliable enough to be admitted in criminal trials, and that a chemical analysis is required. For further discussion see the various posts on the subject on the NC Criminal Law blog . SWGDRUG allows pharmaceutical indicators as a class B identification of pills. This means that 2 other identification methods are necessary in order to positively identify a pill as a controlled substance.
- Polarized light microscopy – contrast-enhancing technique that improves the quality of the image obtained by equipping these microscopes with a polarizer and an analyzer (second polarizer).
- Microcrystalline test – a substance reacts with a reagent forming an insoluble crystal. The shape of the crystal suggests the type of drug. These tests are rapid and do not require the isolation of the drug prior to testing.
- Heroin and caffeine: mercuric chloride, hydrochloric acid
- Barbiturates: Wagenaar reagent (cupric sulfate), sodium hydroxide, sulfuric acid
- Cocaine and phencyclidine (PCP): gold chloride in acetic acid, hydrochloric acid
- Plant particles from marijuana, hashish: concentrated sodium hydroxide, petroleum ether, chloroform, chloral hydrate
- Amphetamines and methamphetamines: gold chloride in water, gold chloride in phosphoric acid, sodium hydroxide
- Excipients and diluents: hydrochloric acid, methanol, distilled water
- Propoxyphene: acetic acid, gold chloride in acetic acid
- Concerns: (1) Impurities may cause unusual crystal formation. (2) Check reagents with known standard of drug before performing an actual procedure. (3) Proper storage of reagents.
- DEA Resource for Parents – provides images of drugs of abuse and information about the effects and legal status of drugs.
- DEA Resource Guide – provides images of drugs of abuse as well as a list of federally scheduled drugs and descriptive information about the major classes of drugs.
- The University of Texas at Arlington – Pictures of Commonly Abused Drugs
- NCSCL Technical Procedure for the Identification of Marijuana
- Polarized Light Microscopy – for viewing suspected hashish/THC samples with the polarized microscope.
Confirmatory Tests
- Link to the State Crime Lab’s procedure
- Considered the “gold standard” of drug identification
- Most definitive and reliable of the confirmatory tests
- Video demonstration of how a GC column works.
- Video of a scientist performing the GC procedure.
- The amount of time it takes for a molecule to move through the entire machine and be detected by the detector is called the retention time. Analysts may use these retention times to differentiate between different compounds. However, the retention times alone are not reliable indicators of what compound is present. A specific compound will have a known retention time, but multiple compounds may have the same retention time in the same chromatogram. In order to distinguish and identify which compounds made the peaks, a mass spectrometer is used.
- A mass spectrometer will determine the exact molecular weight of a molecule or compound. A previously separated sample (flowing directly from GC after detection) or a pure sample (injected) must be ionized for mass spectrometry. The sample, now broken down into molecules, is subjected to an ionization source , a beam of steadily flowing electrons, which blasts the molecules and causes them to break apart into positively charged particles, or ions. Only positively charged ions will continue through the machine. Next, the ions travel through the mass analyzer, or quadrupole filter. The mass analyzer uses an electromagnetic field to separate the ions based on molecular weight. In a routine drug analysis performed by the laboratory, the analyst will already know the molecular weights of the drugs that are frequently detected. It is unnecessary and impractical to know the molecular weight of every compound in a substance. Therefore, to isolate these specific drugs at their known molecular weights, the scientist will manipulate the experimental environment (such as flow rate, type of gas used, temperature, etc.) in previously tested and scientifically accepted ways to produce the range of molecular weights required for analysis. This manipulation will allow only ions between the desired molecular weights to pass through the machine. Ions that flow through the mass analyzer without being filtered will reach the mass detector. The mass detector will count the number of ions with a specific mass. These detections will be translated by a computer program into a mass spectrum.
- A chromatogram (from the GC) and a spectrum (from the MS) can be used together to determine a molecule’s identity. Solely using GC results to identify a compound may lead to inaccurate conclusions. As mentioned earlier, a molecule will have a specific retention time, but a specific retention time does not identify a specific molecule. The analyst will use the spectra to determine the molecular weight of the molecule that generated the peak at a specific retention time. The molecular weight at that retention time will eliminate incorrect identities and narrow down the potential identities to one. The retention time, molecular weight, and patterns of both the chromatogram and spectrum must all match a known standard to be conclusively identified. Differences should not be disregarded unless specifically permitted in the lab procedures.
- A mass spectrometer cannot distinguish between compounds in mixed samples. A sample must be separated prior to MS testing. A gas chromatograph is used to separate the impurities out of a sample in preparation for mass spectrometry. The mass spectrometer will measure the atomic weight of the compounds that were separated by the gas chromatograph.
- MS cannot differentiate between molecules that have the same molecular weight but have chemical structures that are mirror images (also known as enantiomers). This is the case specifically with pseudoephedrine and ephedrine. Subsequent FTIR testing may be necessary to distinguish enantiomers.
- GC/MS will detect the presence of methamphetamine, however, it will not differentiate between methamphetamine stereoisomers . L-Methamphetamine is the active ingredient in the over-the-counter cold medication Vick’s VapoInhaler. This ingredient has different effects on the human body and is completely legal. It is commonly labeled as levmetamfetamine to signify that is the legal form of methamphetamine. The other isomer, D-Methamphetamine, is not legal, but will create the exact same peaks on a GC/MS as the L-form does. It is important for the lab to quantitate how much of each stereoisomer are present, which can be done by means of percentages. Some laboratories will allow up to 20% of the D isomer to be present before reporting positive test results for illegal methamphetamine. This blog post explains the concepts of chirality and sterochemistry as they apply to GC/MS testing of suspected methamphetamine.
- If a sample contains a high concentration of pseudoephedrine or ephedrine, it is possible for an “artifact peak” to appear on a GC/MS chromatogram. If not correctly identified as an artifact peak, the sample may be misidentified as methamphetamine. This peak is created by a reaction at the injection point under certain experimental conditions. At higher temperatures (see Hornbeck et al, “Detection of GC/MS Artifact Peak as Methamphetamine,” Journal of Analytical Toxicology (1993) which determined that at 300 degrees an artifact peak existed, while at 185 degrees it did not) pseudoephedrine and ephedrine will react with 4-carboxyhexafluorobutyrl, pentafluoropropionyl, heptafluorobutyryl, and a few other substances to create an artifact peak that is similar to that of methamphetamine. This artifact peak can be eliminated by adding sodium periodate to the urine sample before GC extraction.
- Malfunctions in the equipment can occur. The injection point septum is a part of the machine that has the potential to wear. This port only lasts 100-200 injections. The injection port temperature, which is selected by the analyst, may be high or low causing a shortened septum life span, decreased sensitivity, poor separation of liquid material, or decomposition of the sample.
- Is the machine is properly maintained and calibrated? Were the samples prepared properly by the analyst? Are any reagents out-of-date? Were negative controls, positive controls, and blanks properly used? Was the analyst following the protocol properly?
- Did the analyst perform an individualized interpretation or did s/he rely on the computer generated comparison to the library of standards?
- Interpretation of only the chromatogram (chart generated by the gas chromatograph) or a spectrum (chart generated from the mass spectrometer) is not sufficient. Both the chromatogram and spectrum must be interpreted together and a final identification must be supported by both.
- Non-specific
- Used for molecules with high molecular weights.
- Similar to GC, but where the GC is filled with gas, the HPLC column is filled with liquid solvent or solvent mixture.
- The State Crime Laboratory uses this technique for quantitation of methamphetamine in drug analysis cases.
- HPLC can be used for qualitative identification: figuring out what is in a sample. HPLC is a separation technique that uses a stationary and mobile phase, a pump, and a detector to separate the compounds in a mixture. The result of the process is a computer generated graph known as a liquid chromatogram.
- First, a small portion of the sample (approximately 5 to 20 µL) is injected into the stationary phase or column. The stationary phase is a small tube, also known as a column that is 3-5 microns in diameter and packed with microscopic particles. Next, the liquid mobile phase is pumped by the exterior pump through the machine. The mobile phase is similar to the gas flowing through a GC. The liquid mobile phase flows through the stationary phase similarly to how water flows through a water filter. The stationary phase separates the molecules in the sample, carrying them down the column at different rates. Eventually, the molecules will travel through the column and reach the detector at the end. The detector will record which molecules are present, and also will record the number of molecules of the exact same chemical makeup are detected. As with a GC, the time it takes the molecule to travel through the column and be measured by the detector is called the molecule’s retention time. Similarly, the greater the number of molecules present in the sample, the higher the intensity of the peaks.
- HPLC can also be used for quantitative measurement: figuring out how much of a compound is in a sample. A calibration curve is used to calculate the concentration of the sample.
- The standards used in both GC and HPLC are measured using certain conditions. The analyst chooses the speed of the gas or liquid that travels through the column. They also choose the temperature of the column for each experiment. The temperature of the column, the gas/liquid flow rates, and other factors affect the results. To recreate the experiment and replicate results, the exact conditions chosen by the analyst must be known. The same flow rate and temperature must be used if a second analyst wants to receive the same results on the earlier tested sample. If an analyst wants to compare their results to a known standard sample, they must have performed the experiment under the same conditions that the standard was performed under. As a whole, HPLC results are not as reproducible as GC results.
- This animation shows how these conditions can change the results in an HPLC chromatogram.
- A single HPLC chromatogram cannot be used to calculate the concentration of the compound of interest. A calibration curve must be used to calculate the concentration of the sample. The calibration curve must also be within the accepted ranges allowed by the experiment (QC requirements). Always check that the QC and calibration curve were in range. A calibration curve with a correlation coefficient or R2 value equal to 1.000 is the most desirable. An R2 value of greater than 0.995 is required by the State Crime Laboratory for accurate reporting.
- An HPLC machine cannot test all ranges of concentrations. For a highly concentrated drug, a sample (here referring to a small portion of the overall evidence) will be taken from the whole and diluted to measure the diluted sample’s concentration. A concentration for the diluted sample will be calculated using the concentration curve mentioned above. The diluted sample’s concentration will be multiplied by the dilution factor to find the calculation of the entire evidentiary sample. The concentrations used in the calibration curve must encompass the concentration of the diluted sample. For example, if the analyst is reporting the concentration of the diluted sample of methamphetamine to be 55 mg/mL, and the standards used to create the calibration curve range from 0.5 mg/mL to 50 mg/mL, then the concentration is out of range and cannot be used. The analyst must either further dilute a second sample to be within the range of the calibration curve or create another calibration curve. The curve’s limits may not be extrapolated to determine a sample’s concentration. Only the concentrations within the range of the concentration curve are valid.
- Link to the State Crime Lab’s procedure
- Highly specific.
- Many substances will create an unequivocal IR spectrum that is easily identifiable by analysts.
- An IR spectrum by itself does not provide an exact chemical structure of a compound, but will provide information about functional groups that are present in the molecule.
- Presence or absence of certain functional groups will guide an analyst as to the possible identity of a compound.
- Infrared light is light that is not visible to the human eye. FTIR measures the amount of infrared light that is absorbed by a sample. Molecules (multiple atoms held together by bonds) are constantly moving. The bonds that hold the atoms together will bend, rock, stretch, compress, or twist. These actions collectively are called vibrations. Different functional groups, mentioned above, will absorb infrared light differently and will cause the atoms to vibrate more or less frequently. How quickly the molecule vibrates is called the frequency. More frequent vibrations will create a higher frequency, and fewer vibrations will generate a lower frequency.
- An infrared light beam, or source, is aimed directly at an instrument called an interferometer . The interferometer uses a combination of a beam splitter, a mobile mirror, and a stationary mirror to separate the beam of light into individual wavelengths. First, the beam splitter splits the beam of infrared light at right angles into two smaller beams. One beam will hit the stationary mirror and the other will hit the mobile mirror. The angles of the mirrors allow the two separated beams to reflect, meet again, and reconstitute as one beam. The mirror mechanism separates the light into different wavelengths that are measured at the point of reconstitution. The interferometer records this information and creates a graph called an interferogram. An interferogram records information about every frequency of light from the infrared source. Most importantly, every frequency is measured at once instead of individually as earlier scientific devices required. Once the frequencies of infrared light have been measured by the interferometer, the reconstituted beam continues through the machine to the sample. The beam is applied to the sample where it will either transmit (go through) or reflect (come back towards the source). At this point, specific frequencies of energy will be absorbed by the sample and some will transmit through. The light that does transmit through the source will reach a detector on the other side. Not only does the light that reaches the detector generate information, but the absence of some wavelengths of light, the light absorbed by the sample, also generates information for analysis. The information gathered from the detector is finally sent to a computer where a chart is created specifically for that sample. This computer generated chart is called an IR spectrum .
- Analysts use the IR spectrum and compare the sample’s spectrum to a known reference sample. This can be done either by computer comparison, or by an individual analyst manually comparing the two spectra. Each “peak,” explained below, is representative of a bond between two atoms. Specific well-known bonds that also have definable physical/chemical characteristics are called functional groups. Common functional groups include alcohols (- OH), ketones (=O), esters (- COOCH3), ethers (-COC-), and carboxylic acids (-COOH), to name a few. Each of these functional groups, if present, will create a distinct peak that typically is easily identifiable by a trained analyst. These peaks will be a specific intensity and be found within a specific wavelength range. When analyzing a spectra, it is important to know that the presence of a peak in a certain place does not necessarily indicate that a specific functional group or bond exists in a sample’s chemical structure. However, absence of a peak in the area where a known functional group or bond would present itself does indicate that that functional group or bond does not exist in the sample’s chemical structure. Some peaks will be more or less intense due to the bond’s nearness to other bonds (the molecule’s stereochemistry), the number of the exact same type of bonds present in a molecule (aromatic C-H bonds), and many other more complex reasons. Identification of compounds by IR spectra is an art which takes a lot of practice. Analysts must not only remember the locations of where peaks should be, but must also remember the patterns common to certain types of molecules that could be present in a larger macromolecule. The comparison of the sample’s spectra to the known standards is mandatory, either manually or by a computer, and the match must be exact, and forensically, must be supported by another confirmatory test.
- IR cannot differentiate between isomers of the same drug.
- For an accurate spectrum, the substance must be reasonably pure (generally >90%). Testing of pharmaceuticals using IR may be more successful than the testing of street drugs which are often impure.
- Diamond cell IR, if used, allows smaller amounts of samples to be tested. If diamond cell IR is not used, then the amount of sample required for testing is much higher.
- Peaks from an IR spectrum are read “upside-down.” A peak, obviously poorly named, is actually observed at the lowest point on the graph, or the valley of the observed area. IR measures absorption, the opposite of transmission. Absorption and transmission are inversely related: the more a sample absorbs light, less light is actually transmitted through the sample. The more light that transmits through a sample, less light is absorbed by that sample. The y-axis of a spectra measures the percent transmission of a sample. Samples with the highest percent transmission will generate higher (deeper) peaks, and those with the lowest percent transmission will show a lower (shallower) peak or possibly no peak.
- Relative intensities of the bands are important, any mismatch with reference spectrum negates identification. Significant for the identification of the source of an absorption band are intensity (weak, medium or strong), shape (broad or sharp), and position (cm-1) in the spectrum. Many compounds look similar, but an exact match is necessary to confirm the identity of an unknown.
- Overview of the use of FTIR for analysis of controlled substances.
- FTIR Identification – this blog post by Dr. Fred Whitehurst discusses the limitations of the FTIR for use with forensic samples.
- Organic Chemistry Lecture – by David Van Vranken, Ph.D. of UC Irvine explaining the science behind IR Spectroscopy.
- Video on the science of FTIR
- Video of an FTIR run from start to finish.
- For a fun and rudimentary explanation of light, see Bill Nye The Science Guy’s December 24, 1993 Episode on Light and Optics.
Which Items are Tested?
When a suspected controlled substance contains many individual packages, crime labs decide which and how many samples to test by following a Sampling Plan. The State Crime Lab’s Sampling Procedure contains three types of sampling: Administrative, Threshold, and Hypergeometric Sample Selection.
- Considerations: the State Crime Lab’s procedure does not allow analysts to infer what substances may be present in the untested pills. This means there should not be testimony about the chemical composition of pills that were not chemically analyzed. The analyst may visually inspect the untested pills and compare their appearance to pictures of pills in the Micromedex database. Visual inspection is not sufficiently reliable to identify a substance. State v. Ward , 364 N.C. 133 (2010).
- Threshold Sample Selection is used when it is practicable to test individual analysis of enough units to meet a statutory threshold.
- Homogeneity: Each package and its contents must be visually inspected for homogeneity of size, weight, color, packaging, markings, labeling, indications of tampering and other characteristics before the samples are subjected to a sampling plan. Section 4.6.2. It may not be possible to detect significant weight variations when visually examining a very small amount of a substance.
- Extrapolation of weight: If the analyzed portion does not meet a weight threshold, additional indiscriminately chosen samples can be weighed to meet the threshold. The lab does not require that those additional samples be tested. Section 4.10.5. The lab allows analysts to extrapolate weight if it is impracticable to obtain individual weights. Section 4.10.5.1.1.
- Previously used method: From 1996-2010, the crime lab and many other forensic labs determined the number of samples to be tested by using the √n+1 formula where n is the total number of samples. The analyst would make an inference about the chemical composition of the remaining samples after testing √n+1 samples. There was no scientific basis for using this method to determine how many samples to test. For additional information on this method, see this paper by Fred Whitehurst, JD, Ph.D.
Residue amounts will not be tested by the State Crime Lab unless accompanied by a written request from a prosecuting attorney. If the case consists of items that are all residue amounts, analysis will be performed on items until any controlled substance is identified. See Section 4.5.2.1.
Practical Tips
For practical tips on reviewing lab reports, challenging tests, and working with experts, see the papers written by Diane Savage and Dean Loven .
This report by the Penn Carey Law Quattrone Center provides the first-ever comprehensive analysis of presumptive drug field test usage across law enforcement agencies in the United States. Inexpensive and fast, these tests have become a tool of choice for law enforcement agencies. Unfortunately, they are notoriously imprecise and are known to produce “false positives,” leading to frequent wrongful arrests and wrongful convictions.
- OSAC Registry Approved Standards , NIST Organization of Scientific Area Committees for Forensic Evidence (OSAC) is developing documentary standards for each forensic discipline. Standards under consideration as well as approved standards are available in the OSAC Registry.
- In Glowing Colors: Seeing the Spread of Drug Particles in a Forensic Lab , NIST
The Health In Justice Action Lab of the Northeastern University School of Law has created a toolkit for attorneys defending death by distribution of drugs. This toolkit will be useful to defenders in handling charges of this sort in NC, both for the old murder by distribution and the new death by distribution.
See pp. 133-136 for the National Research Counsel’s assessment of the analysis of controlled substances.
- Possession of Khat , 2/24/2023 Originally posted on North Carolina Criminal Law – A UNC School of Government Blog Readers may have heard of the plant commonly known as khat or qat (or Catha edulis, for the botanically inclined). The plant is indigenous to Africa and is popular in parts of that continent, as well as parts of the Middle East, and is …
- State v. Booth and marijuana identification , 10/26/2022 In case you missed it, the COA released State v. Booth on Oct. 18, 2022, dealing in part with lay opinions by officers identifying marijuana as such based on sight and odor only and without a proper lab test identifying the levels of delta-9 THC. The officer in Booth was permitted to testify that he could …
- Hemp remains legal in NC , 7/1/2022 With Governor Cooper signing it into law yesterday Senate Bill 455 which permanently excludes hemp from the legal definition of marijuana under state law in NC, hemp’s future as a widely-available consumer product in our state seems secure. Under the new legislation, the previous requirement that the hemp be cultivated or possessed by a grower …
- Announcing Expert Services Project , 3/28/2022 We want court appointed attorneys to use experts, and we want using experts to be easier for attorneys. Access to qualified expert services is essential to the provision of indigent defense. To better equip the North Carolina public defense community with the resources it needs to achieve fair and just outcomes for clients, Indigent Defense …
- Challenging an officer’s identification of marijuana by sight or smell , 11/16/2020 There have been several posts on this and related topics here and here. This post will attempt to compile all of the resources and walk attorneys through the process of making these challenges. N.C. Gen. Stat. § 90-87(16) provides the statutory definition of marijuana, specifically excluding from its definition industrial hemp. Industrial hemp, as defined in …
- Newly-Developed Drug Test May Be Able to Help Distinguish Hemp from Marijuana , 8/24/2020 In July of 2019, Virginia became the first US state to acquire a new drug test created by a team of forensic chemists in Switzerland. The 4-AP test, or “Cannabis Typification” or “Swiss Test,” purports to be able to help differentiate between hemp– a legal variety of cannabis– and marijuana. However, laboratory documentation says that …
- Summer 2020 Hemp Update , 6/18/2020 Originally posted on June 16, 2020 on the North Carolina Criminal Law blog On Thursday, June 4, 2020, the North Carolina General Assembly passed S.B. 315, referred to as the State Farm Bill, which was subsequently signed into law by the Governor. The bill was pending all last session and stalled, allegedly over a dispute about how …
- What to watch when quarantined , 4/23/2020 Are you done with Tiger King and don’t know what to watch next? Each of the series below is available on Netflix and offers insight into various forensic evidence methods. How to Fix a Drug ScandalThis four-episode series chronicles what happens when two drug analysts in Massachusetts commit misconduct in the lab. The series examines …
- Carts, Wax, and Oh, My: The New World of Marijuana Extracts , 11/12/2019 Originally posted on Nov. 12, 2019 on the North Carolina Criminal Law blog. The advent of cannabis legalization across the country has led to a proliferation of new types of cannabis products. There are skin patches, food and drinks (for humans and pets), vaporizer or “vape” cartridges (or “carts”), and different concentrate or extract products (“dabs”, “wax” or “shatter”, among other names). [Click …
- Some Texas District Attorneys require lab tests for marijuana charges , 7/10/2019 The Texas Legislature recently legalized the cultivation of hemp, complicating enforcement of existing marijuana laws in the state. Hemp and marijuana are the same species of plant, Cannabis sativa, and are distinguishable only through a chemical analysis which shows the THC concentration. The concentration of THC, the psychoactive compound in marijuana, is the legal difference …
Jeff Welty posted a 2-part series on the effect of legal hemp on drug dog sniffs on the SOG’s blog, North Carolina Criminal Law. They are linked here:
Part 1 Part 2
NIST article about testing of drugs that may contain fentanyl or other dangerous synthetic opioids. The article contains video clips showing how powder substances can contaminate various surfaces in laboratories and addresses ways that labs work to eliminate contamination.
- NIST Study Will Help Labs Distinguish Between Hemp and Marijuana , NIST
Discusses the paper below and shows black-light videos that help illustrate the risk of cross contamination in a forensic drug lab.
E. Sisco, M.E. Staymates, A. Burns. An easy to implement approach for laboratories to visualize particle spread during the handling and analysis of drug evidence. Forensic Chemistry. Published online March 11, 2020. DOI: 10.1016/j.forc.2020.100232
Jan. 21, 2020 NC Criminal Law blog post by Phil Dixon with links to resources for defenders litigating the new crimes of death by distribution and aggravated death by distribution in G.S. 14-18.4 cases.
by Joanna Gin and Edward Imwinkelreid. UC Davis Legal Studies Research Paper, available for free download. Like nuclear DNA testing, GC/MS analysis has important limitations. Courts should not assume it is a nearly infallible technique. When GC/MS is used in drug testing, the court must inquire as to the mode of analysis: full scan, selective ion reliance, or selective ion monitoring. When GC/MS is employed to identify ignitable liquids in arson investigations, the court should inquire as to the condition of the sample tested: Has it been subjected to weathering, microbial degradation, or pyrolysis?
- What’s Known and Unknown about Marijuana , NIJ Two podcast episodes describing how suspected marijuana is tested by forensic labs.
- Don’t Plead to Weed Webinar Free webinar offered by Emancipate NC
Live webinar presented by Ed Brown, Ph.D. April 1, 2021, 1:00 pm 60 min of CLE credit anticipated
Materials: PowerPoint Slides
This one-hour live webinar will provide attorneys having little science training with the basics about the various chemical analysis techniques employed by forensic laboratories and other contract labs around the US. Although a few technical details will be presented about each method so that a greater understanding of each technique can be obtained, we will help attendees understand the basic principles that these methods rely upon.
We will cover methods such as presumptive tests like color tests, microcrystalline tests, spectroscopy, and immunoassay testing. Attendees will be provided with a clear foundation about these concepts and methodologies as well as about their utility and limitations for use which will aid in understanding of the forensic evidence in cases involving suspected controlled substances.
Registration: This program is the second of the 2021 IDS Forensic Science Education Series. The webinars will be presented monthly and are free to attend. Attorneys who want CLE credit for attending will be billed $3.50 per credit hour by the State Bar. Use this link to register for all webinars in the series and attend any that are of interest. If you already registered for the series, there’s no need to register again. More information about the webinar series is available here .
Presenter: Edward G. Brown, Ph.D. obtained his Bachelor of Science degree in Chemistry from U.C. Berkeley in 1980 and his Doctoral degree in Organic Chemistry from U.C. Davis in 1988. Two post-doctoral chemistry research fellowships from University of Auckland, New Zealand, and Yale University furthered his academic studies in medicinal chemistry and analytical chemistry techniques through 1990.
Dr. Brown’s productivity during his career as a medicinal and organic chemistry researcher in academics and while working at several pharmaceutical companies and other laboratories has led to his co-inventorship on ten US patents and his co-authorship on over thirty research articles and conference presentations.
Dr. Brown is also a patent agent who specializes in chemistry patent drafting and assists in patent litigation and prosecution issues for patent law firm clients in North Carolina and around the US.
In 1991, Dr. Brown began his consulting work as an expert witness in an LSD case. He has since continued to develop his expert witness practice throughout the intervening years, and to date, has assisted nearly 100 attorneys in law firms from North Carolina, Virginia, Maryland, Pennsylvania, and many other States, as well as in Canada and Great Britain on a large variety of drug cases and DWI/DUI cases, both at the State and Federal levels. He has testified in criminal drug cases and DWI/DUI cases as a Chemistry Expert in both Federal and State courts around the United States and has been deposed as a Chemistry Expert in a patent litigation case.
- Defending Drug Overdose Homicides Training , NACDL Live webinar, Sponsored by NACDL
- Emerging Issues in Laboratory Analysis for the Differentiation between Marijuana and Hemp , NCSTL Presenters: Reta Newman and Michael Gilbert. Offered by National Clearinghouse for Science, Technology, and the Law. Recording and powerpoints are available.
Join Stetson University College of Law for Emerging Issues in Laboratory Analysis for the Differentiation between Marijuana and Hemp, a scientific and legal analysis regarding the differentiation between marijuana and hemp. Ms. Reta Newman, Director for the Pinellas County Forensic Laboratory, and Mr. Michael Gilbert, Assistant Director for the Pinellas County Forensic Laboratory, will give an in-depth look at how forensic labs analyze marijuana and hemp. They will break down the process and where the legal issues arise. In addition, they will discuss the 2018 Farm Bill and look at different state statutes and the changing legal landscape.
Follow up to a previous training posted here . To view this free webinar, linked here , register to create and account and then enroll in the program. Duration of the program: 2 hours.
Due to the high attendance and interaction from Marijuana or Hemp: From Farm Bill to Forensic Analysis, the webinar held on January 15th, 2020, the Forensic Technology Center of Excellence is hosting a follow-up session to give in-depth answers to questions the speakers were unable to address in the first session.
In the first session, experts in agricultural policy, hemp industry analytical testing, and the DEA Special Testing and Research laboratory discussed the state of forensic drug testing following the passage of the Agricultural Improvement Act of 2018 (2018 “Farm Bill.”) The webinar discussed the legal history of hemp and THC as it relates to farm policy, farming and quality testing techniques used in the hemp industry, and the DEA’s enhanced forensic testing program for the identification of suspected marijuana samples.
Learning Objectives:
1) Understand the 2018 Farm Bill, which created a USDA-administered hemp program, requiring the USDA to establish a regulatory framework to monitor compliance and regulate hemp production.
2) Understand the difference between Δ9-THC and Δ9-THCA, along with the testing and quality systems being used within the hemp and marijuana industries.
3) Provide the audience with an overview of the new analysis protocol implemented in DEA laboritories to efficiently identify marijuana submissions.
Renee Johnson; Mike Goodrich; and Dr. Sandra Rodriguez-Cruz
Forensic Sciences at RTI International is offering a free webinar program on Mar. 31, 2020.
The presentation will discuss analytical strategies to answer the question: is the material presented for analysis an illegal product based on its total THC content?
The question “is this sample illegal based on its psychoactive ∆9-tetrahydrocannabinol content” has been common in forensic labs for decades. Traditionally, this has been answered in a binary fashion as “yes” or “no” based on the results from a semi-quantitative analysis using GC/MS technologies. Since the passage of the Agriculture Improvement Act, also known as the Farm Bill, this question has become more complicated due in large part to the legalization of hemp as an industrial crop.
The United States Federal Register defines industrial hemp as any part or derivative (including seeds) of the plant Cannabis sativa L. with a dry weight concentration of “tetrahydrocannabinols” not greater than 0.3% by dry weight of the plant material. Tetrahydrocannabinols specifically refer to salts and isomers of ∆9- tetrahydrocannabinol (THC). Any hemp plant material that exceeds this threshold is defined as marijuana and considered an illegal scheduled drug.
Recently, the USDA has published guidance for the determination of total THC (0.877*[THCA] + [THC]) in hemp and hemp-derived cannabinoid products. It has been stipulated that this testing must occur in DEA registered labs and that HPLC and or GC technologies are to be used for the quantitative determinations. Here, subject matter experts discuss an orthogonal LC/MS and GC/MS analytical strategy for quantification of total THC in hemp and hemp products. They will also discuss sample preparation of various products and analytical challenges using LC and GC methodologies.
Detailed Learning Objectives:
a) Understand the legal definition of hemp and its differentiation from marijuana
b) Learn about HPLC, LC/MS, and GC/MS testing strategies
c) Understand the analytical challenges for the determination of total THC in cannabis, hemp, and cannabinoid products
The Forensic Technology Center of Excellence will offer a webinar on marijuana and hemp analysis. Attendees will hear from experts in agricultural policy, hemp industry analytical testing, and the DEA Special Testing and Research laboratory to obtain a better understanding of the issues that have developed within the field of forensic drug testing since the signing of the Agriculture Improvement Act of 2018 (“2018 Farm Bill”). This webinar will provide a history of US farm policy as it relates to the legalities of hemp and tetrahydrocannabinol (THC). Information on farming and quality testing used by the hemp industry will be presented. The DEA will share their revised and enhanced forensic testing program for the effective and efficient identification of suspected marijuana submissions.
This free webinar will present results based on a NIST-led, multi-agency collaboration focused on establishing drug background levels in forensic laboratories and understanding the implications for data quality, data integrity, and occupational health and safety.
Hemp must be legally differentiated from marijuana. In this free webinar, Dr. Anthony Macherone, Senior Scientist with Agilent Technologies and a Visiting Professor at the Johns Hopkins University School of Medicine, will discuss strategy for screening and quantification of THC in hemp or marijuana. Archived webinar is available for on-demand viewing.
2nd Annual Online Symposium hosted by RTI and ForensicEd on May 13-17, 2019. This program offers information on best practices in forensic toxicology, drug analysis, and trace analysis such as sample preparation, method development, and forensic method validation. Presentations are geared toward forensic practitioners, but several of the sessions should be of interest to attorneys.
The mission of SWGDRUG is to recommend minimum standards for the forensic examination of seized drugs and to seek the international acceptance of such standards. Click here for a PDF of the current approved guidelines for Drug Analysis.
A collaboration between the Orlando Public Defender and the National Center for Forensic Science at UC Florida. The site has links to many helpful training videos that help attorneys understand forensic science evidence.
The National Forensic Science Technology Center created this website to explain in simplified terms the principles of each type of forensic analysis and how the analysis is performed. Topics include DNA, digital evidence, fingerprints, firearms, trace evidence, blood stains, and more.
This US National Library of Medicine website allows users to learn the possible identity of pills based on their appearance, color, shape, and markings.
This National Institute of Health searchable database allows users to search for a compound and learn more about its chemical structure, uses, properties, toxicity, and other information.
This website contains a library of GCMS data for compounds and is organized by molecular weight, base peak and second base peak. This library may be useful for limited purposes.
- James Shellow, Cross-Examination of the Analyst in Drug Prosecutions
- Donnell R. Christian, Forensic Investigation of Clandestine Laboratories
- Terry Mills, III et al., Instrumental Data for Drug Analysis, Vol. 6 and 7
- John E. Leffler, A Short Course in Modern Organic Chemistry
- Eugene W. Berg et al., Physical and Chemical Methods of Separation
- Ralph L. Shriner et al., The Systematic Identification of Organic Compounds (5d ed.)
- Roger Adams et al., Laboratory Experiments in Organic Chemistry (4th ed.)
NCSCL drug chemistry analyst Jennifer West testified about whether fentanyl was an opiate or opioid. Trial court erred in admitting West’s testimony because she lacked training on the issue of whether fentanyl was an opiate or opioid.
- State v. Hills (N.C. Ct. App. 2021)
Evidence that cocaine was the identity of the substance was admissible not withstanding the substance being handled with bare hands and stored in a glove box where cocaine had previously been stored. The court found concerns over cross contamination went to the evidence’s weight, not matters of admissibility and authentication.
The court held that it was not plain error for the analyst to testify that GCMS testing is used to confirm results of presumptive testing but not to testify that GCMS was performed in the case at hand.
The court held that it was not plain error for the analyst to testify to the identity of a controlled substance without explaining what type of chemical analysis she performed. She testified she performed a color test and an instrumental analysis.
In August, the North Carolina Supreme Court weighed in on drug identification once again in State v. Osborne , ___ N.C. ___ (August 16, 2019). Defender Educator Phil Dixon discusses admissibility and sufficiency of evidence in drug cases in this blog post.
This document provides the relevant statutes and summarizes the case law on the issue of how marijuana should be weighed. It addresses issues such as whether water weight and mature stalks should be included. Links to the State Crime Lab’s relevant procedures are provided, as well as contact information of experts who are available to weigh suspected marijuana.
Today, the court of appeals reversed a defendant’s drug convictions because the indictments identified the controlled substances in question using terms that are widely used to describe the drugs, but that are neither the chemical names listed in the controlled substance schedules nor – according to the court – “trade names” for the drugs. Because more and more drug cases involve pharmaceuticals that have many names, it is worth reviewing the case. The case is State v. Sullivan . It arose when police used an informant to buy steroids from the defendant…
A NC Supreme Court decision finding the trial court abused its discretion by allowing the State’s expert to visually identify drugs using an insufficiently established method. The court found the expert’s use of information in Micromedex literature to make drug identification did not meet the first prong of Rule 702 as it was never established as reliable before the trial court. The court indicated that chemical analysis might be required within the bounds of “common sense.”
A California Attorneys for Criminal Justice (CACJ) report by John Kelly. The report is largely based on the research of Dr. Frederic Whitehurst who tested field drug test kits and exposed and documented that they render false positives with legal substances. The report focuses on the Duquenois-Levine and KN Reagent tests used to test for marijuana.
A draft 702 motion to exclude expert testimony from an arresting officer identifying a substance as marijuana.
A draft motion to suppress evidence collected after a stop made based on the sight/smell of what the officer believes to be marijuana. Please note the motion does not contain an accompanying affidavit as required by law.
Sample discovery motion regarding drug chemistry or toxicology evidence.
Drug Analysis in the News
- Study Estimates Roadside Drug Tests Result in 30,000 Wrongful Arrests Every Year , by CJ Ciaramella, Reason , 1/9/2024
- Even After Legalization, Maryland Cops Wanted to Search People Based on the Odor of Pot. Legislators Said No. , by Jacob Sullum, Reason , 5/24/2023
- Roadside Drug Tests Used to Convict People Aren’t Particularly Accurate. Courts Are Beginning to Prevent Their Use. , by Ryan Gabrielson, ProPublica , 4/25/2023
- Renovation at State Crime Lab in Raleigh expected to bring courts more efficient results , by Sarah Krueger, WRAL , 4/19/2023
- Animal Tranquilizer Floods Illicit Drug Markets in Maryland , NIST , 3/16/2023
- Annie Dookhan took the blame for the state drug lab scandal, but she wasn’t the ‘sole bad actor,’ new documents show , by Andrea Estes, Boston Globe , 2/13/2023
- Here’s an Officer Who Might Have Actually ODed From Fentanyl Contact—but Not Because He Just Touched It , by Scott Shackford, Reason , 1/9/2023
- Study Reveals Inaccurate Labeling of Marijuana as Hemp , by Walter Wilson, National Institute of Justice , 10/17/2022
Drug Analysis Experts
- Christopher Michael (Mike) Bailiff , Lewisville, NC
- Edward G. Brown, Ph.D. , Durham, NC
- Barry Funck , Tallahassee, FL
- Lyle Liechty , Indianapolis, IN
- Carol Murren , Shoreline, WA
- Guy Oldaker, Ph.D. , Lewisville, NC
- Bethany P. Pridgen, MFS , Wilmington, NC
- Amy Swaim , Wendell, NC
- Chris Trevey , Greensboro, NC
- Patra Watson , Columbia, SC
- Dr. Frederic Whitehurst , Bethel, NC
- Charles Bruce Williams, III, Ph.D. , Wilmington, NC
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Pharmaceutical Formulation Design - Recent Practices
Drug Analysis
Submitted: 15 July 2019 Reviewed: 21 July 2019 Published: 20 August 2019
DOI: 10.5772/intechopen.88739
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Pharmaceutical Formulation Design - Recent Practices
Edited by Usama Ahmad and Juber Akhtar
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
2,370 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Instrumental methods are widely used for the analysis and stability studies of compounds in bulk and pharmaceutical forms. They vary in their sensitivity, techniques and reagents involved. This chapter will overview those different techniques and the application of the analytical methods. It will also describe how to design and develop simple, sensitive and accurate method for routine quality control of specified compound depending on its molecular structure. Quality control and assurance of the analytical process will be discussed. Furthermore, the chapter will describe a number of factors affecting the chemical and physical stability of Pharmaceutical formulations and how to develop stability-indicating methods to qualify and quantify the drug degradation.
- instrumental
- development
- quality control
Author Information
Shaza w. shantier *.
- Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Khartoum, Sudan
*Address all correspondence to: [email protected]
1. Introduction
Drug discovery and development process can be divided into two major stages: drug discovery which involves isolation of the active constituent, purified, and standardized. The second major stage, drug development, starts with a solitary compound, which at that point progresses through different studies intended to support its endorsement as a new drug [ 1 ]. The new drug will then be formulated as an appropriate pharmaceutical dosage form.
Pharmaceutical product is medicine intended for human or veterinary use in cure, alleviation, prevention or diagnosis of disease. The use of ineffective, harmful or poor-quality drugs will cause health hazards and waste of funds. The problem is aggravated by adverse climatic conditions and weak drug supply system (including storage and transport). These lead to deterioration of drug quality, loss of activity and may be formation of harmful degradation products [ 2 ]. All this made it a must that any pharmaceutical product should be subjected to different analytical procedures in order to ensure its efficacy and safety. Therefore, an effective drug quality assurance and assessment system should be developed and maintained.
2. Pharmaceutical analysis
Broadly speaking, this is the application of a process in order to identify a drug (single or combined) in its bulk or pharmaceutical dosage form. Testing pharmaceutical product involves chemical, physical and sometimes microbiological analysis [ 3 ].
Qualitative methods: these methods usually are used to ascertain the presence or identity of a component and\or impurities (predicted or expected).
Quantitative methods: determine how much of known drugs are present in bulk form or in a formulation.
Correct identification of the drug in bulk form or as a formulated product.
Indicating the percentage of the stated content of a drug present in formulation within acceptable stated limits.
Indicating the stability of the drug in the formulation and hence the shelf life i.e. indicating the presence of a drug in its intact form and or the presence of any impurities (whether as drug precursors, decomposition products due to chemical or photochemical causes).
Application in the dissolution rate studies i.e. at what rate is the drug released from its formulation so that can be absorbed by the body (bioavailability studies).
Ensuring that the identity and purity of pure drug (bulk form) meet official standards or monograph.
Ensuring that the identity and purity of excipients used in formulation meet specifications set by official standards.
Indicating the concentration of the specified impurities in the pure drug substance (limit test application).
3. Drug stability
The stability of a pharmaceutical product is defined as the capability of the product, in a specific container, to retain its efficacy, properties and characteristics throughout its shelf-life [ 4 ]. The recommended shelf-life (expiry date) for a commercial pharmaceutical product is 3–5 years. During this time, the concentration of the drug should not be reduced more than 95% of its value when originally prepared [ 5 ].
Chemical (including photochemical): the product retains its chemical integrity and potency.
Physical: the conformity of the pharmaceutical product (color, appearance, dissolution, etc.) does not change upon storage or handling.
Microbiological: sterilized products should remain sterile (no pyrogenicity).
Therapeutic: the therapeutic effect remains unchanged within the specified dosage regimen.
Toxicological: no significant increase in a predetermined toxicity effect is noted.
Stability types (therapeutic, microbiological, and toxicological) are basically dependent on the chemical and physical properties of the drug.
Knowledge of the chemical stability of a drug is of great concern for selecting suitable storage condition against the effects of light, temperature, humidity, etc. and for anticipation of drugs interaction with each other or with excipients [ 6 , 7 ].
A stable drug is of great concern to the pharmacist (in view of marketing, storage and distribution); to the physician and patient (in view of safety and efficacy); and to the regulator and quality control analyst (in view of quality, strength, purity and identity).
3.1 Chemical reactions that cause drug degradation
Many drugs are derivatives of carboxylic acid or contain functional groups based on this moiety, for example esters, amides, lactones, lactams, imides or carbamates [ 5 ]. Accordingly, various chemical reactions can result in the degradation of the drug. These reactions include hydrolysis, oxidation, photochemical reactions, polymerization, isomerization, racemization and dehydration [ 4 , 5 , 8 ].
3.1.1 Hydrolysis (or solvolysis)
Hydrolysis forms the most common pathway by which drugs become degraded since many drugs contain hydrolysable functional groups. It can be defined as the process by which drug molecules interact with water to yield breakdown products of different chemical constitution. Hydrolysis occurs more readily in liquid state than in the solid state. It may occur in aqueous suspensions of sparingly soluble drugs. In tablets and other solid dosage forms, there may be sufficient water to allow hydrolysis of the drug [ 8 ].
Solvolysis is a term used for the reactions involving the decomposition of the active drugs with their solvent present (not water).
3.1.2 Ester hydrolysis
Hydrolysis of drugs with an ester functional group (e.g. procaine, atropine, etc.) forms one of the most common types of drug instability. It is usually a bimolecular reaction involving acyl-oxygen cleavage. Ester hydrolysis is (H + ) or (OH − ) ion catalyzed and is dependent on the specific compound and the pH of the solution. Atropine hydrolysis is totally pH dependent and this was characterized by the slopes of −1 and +1. In some cases, the hydrolysis of the drug can show a pH-profile with three regions: a hydrogen ion (proton) catalyzed region, (slope = −1), an uncatalyzed region (solvent dependent, slope = 0) and a hydroxyl ion-catalyzed region (slope = +1) ( Figure 1 ) [ 5 ].

Log rate - pH profile for the degradation of atropine at 60°C.
3.1.3 Amide hydrolysis
Amides are generally more stable to hydrolysis than esters. In general the rate of hydroxyl ion-catalyzed reaction of amides is greater than the proton-catalyzed hydrolysis [ 6 ].
Penicillins and cephalosporins are amides in which the amide bond is part of the strained four membered ß-lactam rings. Their decomposition is catalyzed by hydrogen ion, hydroxyl ion and many buffers. Therefore, these compounds are too unstable to be formulated as solutions. Their pH profile is generally similar to the pH-profile shown in Figure 1 .
In addition to acid-base catalyzed hydrolysis, enzyme-catalyzed hydrolysis may take place in drugs of natural origin; for example enzymes catalyzes the hydrolysis of cardiac glycosides in digitalis leaf [ 8 ].
3.1.4 Oxidation
Oxidation involves the removal of an electropositive atom, radical or electron, or the addition of an electronegative atom or radical. When a reaction involves molecular oxygen (O▬O), it is commonly called autoxidation and this forms the most common pathway of oxidative decomposition of pharmaceuticals.
Oxidative degradation by autoxidation may involve chain processes consisting of three concurrent reactions—initiation, propagation and termination. Initiation can be via free radicals formed from organic compounds by the action of light, heat or transition metals such as copper and iron which are present in trace amounts in almost every buffer [ 5 ].
Many drugs are complex molecules and can be subjected to both hydrolysis and oxidation e.g. steroids, anti-inflammatory, polyene antibiotics (amphotericin B) etc.
Figure 2 show the oxidation of phenothiazines to the sulfoxide which involves two single-electron transfer reactions involving a radical cation intermediate. The sulfoxide is subsequently formed by reaction of the cation with water [ 5 ].

Phenothiazine oxidation process.
Oxidation in solution generally follows first or second order kinetics. Some oxidation reactions are redox reactions that involve the loss of electrons without the addition of oxygen e.g. oxidation of ascorbic acid, ferrous sulfate, adrenaline and riboflavin [ 8 ].
In addition to oxidation and hydrolysis, many other degradative reactions had been studied including addition, dehydration, polymerization, isomerization, acylation, transesterification, etc.
3.1.5 Photochemical degradation
These are the reactions that take place by absorption of the visible or ultraviolet light. The reactant molecule absorbs photons of light (energy) and get excited. The excited molecule then produces the photodecomposition product.
In many photochemical reactions, the reactant molecule may not absorb the radiation directly but through a mediator which absorbs the incident radiation and subsequently transfers its energy to the reactant molecule that becomes activated. Such type of mediator is called photosensitizer.
At times, a molecule can act as a protector for the photolabile drug by preferentially absorbing the radiant energy and produce products. These compounds are referred to as screening agents [ 9 ].
3.1.5.1 Light sources for photodegradation studies
The majority of therapeutic substances are white in appearance, which means that they may absorb in the UV region depending on their chemical structure.
UV-C: which ranges between 200 and 290 nm and is termed shortwave or far UV. Sunlight at the earth’s surface is devoid of this band due to its absorption by molecular oxygen and ozone in the upper atmosphere. Artificial radiation sources such as discharge and germicidal lamps and welding arcs form the sources of UV-C which are used for forced drug photodegradation studies (stress-conditions). These also cause serious damage to the skin and cornea [ 11 ].
UV-B band: this covers the region 280–320 nm. It causes sunburn, skin cancer and other biological effects and it is responsible for the direct photoreaction of many chemicals in natural sunlight.
UV-A band: this is the long wavelength region from 320 to 400 nm, also called near-UV because it is near the visible region.
The most commonly sources for photostability studies include: day light, window-glass filtered day light and room light [ 12 ]. All these sources can be generated artificially. The artificial light source should have an output with spectral power distribution (SPD) as near as possible to the sunlight. This can be achieved by the use of arc lamps and fluorescent tubes.
3.1.5.2 Drug molecules labile to photodecomposition
A number of medicinal products have been studied for their photostability. Carbonyl, nitroaromatic and N -oxide functions, aryl halides, alkenes, polyenes and sulfides are certain chemical functions that are expected to introduce photoreactivity [ 13 ].
Photodegradation of a drug is considered of practical significance if the compound absorbs light >300 nm and the photodegradation becomes evident in a short period.
Factors that govern photochemical reaction rate include aerobic (most reactions proceed in presence of oxygen) and anaerobic (N 2 ) conditions, solvents (H 2 O, organic solvents), buffers, temperature, metals, intensity of radiation and spectral distribution of light, drug concentration and volume of the sample [ 14 ].
Thus, in formulations that contain low drug concentrations, the primary photochemical reaction follows first-order kinetics; the kinetics is more complicated at higher concentrations and in the solid state because most of the light is then absorbed near the surface of the product.
The mechanisms of photodegradation are of such complexity as to have been fully elucidated in only a few cases. For example, the phenothiazine chlorpromazine (CLP) is rapidly decomposed under the action of ultraviolet light, the decomposition being accompanied by discoloration of the solutions ( Figure 3 ). Chlorpromazine behaves differently towards ultraviolet irradiation under anaerobic conditions.

The effect of ultraviolet light on chlorpromazine (CLP).
A polymerization process has been proposed which involves the liberation of HCl in its initial stages [ 5 ].
The photodegradation of ketoprofen can involve decarboxylation to form an intermediate which then undergoes reduction, or dimerization of the ketoprofen itself.
4. Analytical methods
4.1 method development.
Prior the development of any method for the analysis of certain compound or formulation, there are many factors must be considered before developing the method and applying it to the intended use. The first step include collecting information about the analyte itself (the analyte structure and its physicochemical properties). The mode of detection should be selected (e.g. UV detection). Sample preparation which may include centrifugation, sonication and filtration. The type of the diluent also plays an integral role in the analysis as it should be transparent and does not interfere in the analysis. The stability of the prepared solution, the mobile phase; stationary phase and mode of elution in case of chromatographic elution. All these factors and much more should be considered, optimized and the developed method is then validated and applied for the analysis.
4.2 Spectrophotometric methods
4.2.1 uv/vis spectrophotometry.
Absorption spectrophotometry is the measurement of an interaction between electromagnetic radiation and the molecules, or atoms, of a chemical substance [ 15 ]. Techniques frequently employed in pharmaceutical analysis include UV, visible, IR and atomic absorption. Spectrophotometric measurement in the visible region was referred to as colorimetry.
The procedure of UV-unmistakable spectrophotometry includes the estimation of the measure of bright (190–380 nm) or noticeable (380–800 nm) radiation consumed by a substance in arrangement. Retention of light in both the UV and unmistakable area of the electromagnetic range happens when the vitality of the light matches the vitality required to instigate an electronic change and it is related with vibration and rotational progress in the atom. There are two systems of utilizing spectroscopic estimations in medication examination, the total and the similar strategies for measure, and the one utilized relies upon which side of the Atlantic Ocean you complete the investigation. In the UK and Europe the Beer-Lambert condition will in general be utilized in what is known as the outright technique for examine. In this strategy the absorbance is estimated tentatively and the Beer-Lambert condition is comprehended for c, the medication fixation. Hence, the British Pharmacopeia and European Pharmacopeia quote A1% 1 cm qualities in medication monographs. In the US Pharmacopeia, the near strategy for test is liked. In this sort of examine a standard arrangement of the medication to be investigated is readied, the absorbance of the example and the standard are estimated under indistinguishable conditions, and the centralization of the example is determined from the relationship:
Where [test] is the centralization of the example and [std] is the convergence of the readied standard. The relative strategy for test has the bit of leeway that it very well may be utilized regardless of whether the medication experiences a substance response during the measure (for example development of a shaded subsidiary to permit estimation in the obvious district of the range), yet experiences the hindrance that a credible example of the medication being referred to must be accessible for examination. When doing medication examines by spectroscopy it is frequently important to set up a scope of groupings of a standard example of the analyte and measure the absorbance of every arrangement. At the point when these information are plotted, a straight line of positive incline ought to be acquired that goes through the inception. Developing diagrams of this sort not just confirms that the Beer-Lambert law applies to the test at the wavelength of estimation yet additionally enables the chart to be utilized for alignment purposes. An answer of obscure fixation is set up in the very same manner as the benchmarks and its absorbance is estimated at a similar wavelength as the principles. This absorbance is then perused off the alignment chart and the fixation is determined. Standard arrangements arranged independently from the example along these lines are known as outer models. An increasingly thorough system includes the utilization of inside models. An inside standard is an exacerbate that is comparative in compound structure and physical properties to the example being investigated. The inner standard ought to be added to the example being referred to before extraction or measure initiates and is then present in the example framework all through the consequent test. In the measure of complex examples, some example pre-treatment is normally required and the recuperation of the example from the extraction procedure may not be 100%. On the off chance that an inner standard is utilized, misfortunes in test will be reflected by comparative misfortunes in the standard and the proportion of test to standard ought to stay consistent. Inner measures are especially utilized in chromatographic examination (particularly gas chromatography and elite fluid chromatography), where fluctuations in instrumental parameters (for example flow rate of versatile stage) influence precision. In certain spectroscopic examinations a comparable way to deal with the utilization of inner benchmarks is utilized. This is the strategy of standard augmentations and includes expansion of expanding volumes of a standard arrangement of the analyte to a fixed volume of the example and development of an alignment diagram. The diagram in a standard expansion examine is of positive incline however converges they-pivot at a positive estimation of absorbance. The measure of medication in the example is found by extrapolation of the alignment chart back to the point where the line crosses the x-pivot (for example at the point when y 0 in the condition of the line). The strategy for standard increments is generally utilized in nuclear spectroscopy (for example assurance of Ca 2+ particles in serum by nuclear emanation spectrophotometry) and, since a few aliquots of test are examined to create the alignment chart, should expand the exactness and accuracy of the measure. The chief advantage of colorimetric and spectrophotometric methods is that they provide a simple means for determining minute quantities of substances [ 16 , 17 ]. Although spectral interference (degradation products, excipients, etc.) can often occur, the selectivity and sensitivity of these methods can be improved by employing an instrumental technique such as derivative spectrophotometry.
4.2.2 Derivative spectrophotometry
In derivative spectrophotometry the absorbance (A) of a sample is differentiated with respect to wavelength (λ) to generate the first, second or higher order derivative [ 18 ].
In the context of derivative spectrophotometry, the normal absorption spectrum is referred to as the fundamental, zero order or 0 D spectrum.
A = f (λ)dA/dλ = f (λ)d 2 A/λ 2 = f ∆(λ), etc.
Zero orderfirst ordersecond derivative
The first derivative ( 1 D) spectrum is a plot of the rate of change of absorbance with wavelength against wavelength, i.e. a plot of the slope of the fundamental spectrum against wavelength. The second derivative ( 2 D) spectrum is a plot of the curvature of the 0 D spectrum against wavelength.
The first order derivative spectrum of an absorption band is characterized by a maximum, a minimum and a crossover point at λ max of the absorption band. This bipolar function is characteristic of all odd-order derivatives.
The second derivative spectrum is characterized by two satellite maxima and an inverted band of which the minimum corresponds to the λ max of the fundamental band.
A derivative spectrum is therefore gives better resolution of overlapping bands than the corresponding fundamental spectrum and may permit the accurate determination of the λ max of the individual bands. Secondly, it discriminates in favor of substances of narrow spectral band width against those with broad bandwidth. And consequently, substances with narrow spectral bandwidth display larger derivative amplitude than those with broad bandwidth [ 15 ].
These advantages of enhanced resolution and band width discrimination found in derivative spectrophotometry permit the selective determination of certain absorbing substances in samples in which non-specific interference may limit the application of simple spectrophotometric methods. Ephedrine hydrochloride in ephedrine hydrochloride elixir is assayed by second derivative spectrophotometry, which eliminates the broad band absorption of the excipient.
Derivative spectrophotometry has found significant application in clinical, forensic and biomedical analysis. It has been widely applied in the analysis of different pharmaceutical dosage forms. It solves the problem of analysis associated with drug combination, stability studies of drug and degradation products, drug impurities and interference of excipient in drugs [ 19 , 20 ]. It also solves the problem of analysis of drugs in biological fluids.
4.2.3 Difference spectrophotometry
Both selectivity and accuracy of spectrophotometric analysis of samples, which contain absorbing interferons, may be greatly improved by the technique of difference spectrophotometry. In difference spectrophotometry assays the measured value is the difference in absorbance (∆A) between two equimolar solutions of the analyte, in different chemical forms which exhibit different spectral characteristics. It is sometimes referred to as differential spectrophotometry.
Reproducible changes are induced in the spectrum of the analyte by the addition of one or more reagents.
The absorbance of the interfering substances is not altered by the addition of such reagents.
The simplest and most commonly used techniques for altering the spectral properties of the analyte is the adjustment of the pH of the solution by means of aqueous solution of acids, alkali or buffers [ 21 ]. The measured value (∆A) in a quantitative difference spectrophotometric assay can be proportional to the concentration of the analyte and so it obeys Beer’s law. A modified equation may be derived.
Where ∆a is the difference absorptivity of the substance at the wavelength of measurement.
The accuracy and selectivity of the method was found to be increased by conversion of normal zero-order or differential UV spectra into higher order [ 21 , 22 ]. Therefore, the application of difference spectrophotometry is expected to have the totality of advantages of both derivative spectrophotometry (first, second, etc.) combined with delta spectrophotometry [ 23 ].
On the other hand, the stability-indicating property, coupled with the selectivity and simplicity of application, of the derivative spectrophotometry (first, second, etc.) and ∆D 1 make these methods more preferable to use for drug analysis than the costly HPLC methods, especially in developing countries.
4.2.4 Colorimetric method
Colorimetric methods, although are generally dependent on functional group in the drug molecule (NH 2 , OH, SH), are sometimes utilized as stability-indicating methods. This can be achieved by selectively transforming a drug, its degradation product or its impurity into a derivative so that the spectrum of the derivative is shifted to the visible region.
There are several parameters, which require careful and critical consideration in colorimetry. Firstly, the color reagent should be selective for the drug molecule itself, discriminating degradation products which might be present. Secondly, the effect of any parameters which can affect the development and stability of the color should be established. Moreover, the time required to establish the chromophore should be carefully monitored and assessed.
4.3 Chromatographic methods (HPLC and TLC)
Chromatography is essentially a group of techniques for the separation of the compounds of mixtures by their continuous distribution between two phases. One of the two phases is the fixed (stationary) phase, which can be solid or a liquid supported on a solid. The other phase is a moving (mobile) phase which can be gas or a liquid that flows continuously around the stationary phase.
According to the nature of the mobile phase, chromatography is subdivided into liquid chromatography (LC) where the mobile phase is a liquid, and gas chromatography (GC) where the mobile phase is a gas [ 15 ].
4.3.1 Liquid chromatography
Adsorption chromatography: liquid solid chromatography (LSC).
Partition chromatography: liquid-liquid chromatography (LLC).
Ion exchange chromatography: an ionic liquid mobile phase and a solid polymeric stationary phase containing replaceable ions.
Size-exclusion chromatography.
Column chromatography and thin-layer chromatography (TLC): solid stationary phase and liquid mobile phase.
Column, paper and thin-layer chromatography: liquid stationary phase and liquid mobile phase.
High performance liquid chromatography (HPLC) belongs to the category of column chromatography and it covers four classes of chromatography: adsorption (LSC), partition (LLC), ion exchange and size-exclusion.
4.3.2 Thin-layer chromatography (TLC)
Thin-layer chromatography has developed into a very sophisticated technique for identification of compounds and for determination of the presence of trace impurities. Separation in TLC occurs by either adsorption or partition. For adsorption, the stationary phase consists of a thin layer of sorbent (e.g. silica) which is activated by heating at 105°C to evaporate water and the mobile phase is devoid of water (usually a mixture of organic solvents).
The term retention time used in TLC is referred to as R f value which is the distance traveled by the compound from the origin (where the compound is spotted on the plate) divided by the distance traveled by the solvent. Although TLC is widely used for qualitative analysis, it does not in general provide quantitative information of high precision and accuracy. Changes in the practice of TLC have resulted in improved performance of separation and quantitative measurement. These developments are referred to as high-performance thin-layer chromatography (HPTLC) [ 24 ].
Identification of the components of a mixture by comparing their Rf values with those of reference standard.
Detection of any impurities (synthetic route, stability during manufacturing process or storage).
Separation of a mixture of compounds and recovery by elution technique.
Following synthetic reactions for their completion.
Forensic application in drug poisoning or addiction.
4.3.3 High performance liquid chromatography (HPLC)
HPLC is the most commonly used technique for the quantification of drugs in formulations. The principal advantages of HPLC compared to column chromatography are improved resolution of the separated substances, faster separation time and the increased accuracy, precision and sensitivity.
HPLC is based on the same separation modes of column chromatography i.e. adsorption and partition. Unmodified silica (silanol group) is the most widely used in adsorption HPLC. Partition HPLC is divided into two categories, normal-phase and reverse-phase, based on the relative polarities of the stationary and mobile phases.
Suitable solvent for the drug.
Molecular structure.
Nature of analysis: whether for quantification analysis or stability-indicating method.
The following diagram ( Figure 4 ) gives a general guide to the selection of a chromatographic method for separation of compounds of molecular weight ˂ 2000; for samples of higher molecular weight the method of choice would be size-exclusion [ 25 , 26 ].

General guide for selection of chromatographic method.
5. Quality assurance and quality control
In the pharmaceutical Industry, quality management is defined as the aspect of management function that determines and implements the quality policy.
A quality system describing the organizational structure, procedures, processes and resources.
A systematic action or actions necessary to ensure adequate confidence that a product (or service) will satisfy given requirement for quality.
The concepts of quality assurance, GMP and quality control are interrelated aspects of quality management. They are inter-related and have fundamental importance to the production and control of pharmaceutical products.
In fact quality assurance covers quality control in exactly the same manner as it covers other functions such as manufacturing and ware-housing. It approves methods and standards and sees to it that good laboratory practices are operative.
Each manufacturing unit must have a quality control department independent from the production and other departments and under the control of a qualified and experienced personnel and has one or several quality control laboratories at his or her disposal.
Quality control is integral part to all modern industrial processes and the pharmaceutical industry is not an exception. Testing a pharmaceutical product involves physical, chemical and sometimes microbiological analysis. It is a critical function of any business offering a product or service to consumers. In the field of pharmaceutical chemistry, quality control is vital to the successful development, manufacturing, and use of drugs meant to save lives. It determines the quality and stability of drug products via pharmaceutical analysis; it includes areas such as method validation, handling raw materials and finished products, documentations, inspections that impact the development of pharmaceutical products that are governed by specified rules.
Pharmaceutical products are developed and produced according to GMP requirements and other associated codes e.g. good laboratory practices (GLP), and good clinical laboratory practices (GcLP), … etc.
Production and control operations are clearly specified in a written form i.e. standard operating procedures (SOP’s) and GMP requirements are adopted.
Control procedures on starting materials, intermediate products and finished products and other in process controls should be carried out according to written and validated procedures.
The finished products should be correctly processed, checked, packed according to defined procedures.
Finished pharmaceutical products should not be sold or supplied unless they are released by an authorized person.
6. Method validation and statistical interpretation of the analytical method
The function of the analyst is to obtain a result as near to the true value as possible by the correct application of the analytical procedure employed. Quantitative analysis is not simply a case of taking sample, carrying out a single determination and then claim that the value obtained is irrefutable. It also requires knowledge of the chemistry involvements and the possibilities of the interferences from other ions, elements and compounds as well as of the statistical distribution of values [ 27 ].
Gross errors: easily recognized as it leads to definite unreliable results: could be due to contaminated reagents, defective instruments, accidental loss of crucial sample etc. It is defined as a serious error so that there is no way to correct the experiment.
Random error (in determinate error): the average of the results are very close to the true value, so there is no evidence of bias i.e. some results could be high and some results could be low. Arises from sources that cannot be corrected i.e. the degree of sensitivity of the balance: fourth decimal, fifth decimal. Types of burettes or pipettes (A, B, C types) etc.
Systematic (determinant errors): this causes all the results to be in error in the same sense (constant error). May be due to (1) faults in analytical procedure or (2) the equipment used. Observed result could be too low or too high i.e. inaccuracy should be constant (all answers are 10% too high or too low). Example: True value for three samples were 25, 20 and 30% assay result was 35, 30 and 40% respectively i.e. 10% too high. It makes the assay precise but inaccurate sometimes the inaccuracy may be proportional to the true answer, giving rise to proportional error such as 10% of the answer i.e. for the above example for 25% result is 27.5%, 22% for 20% and 33% for30%.
Validation of methods for the quantitative analysis of drugs involves determining as a minimum, their selectivity, and limit of detection, limit of quantification, linearity, working range, accuracy and precision [ 28 ].
6.1 Accuracy
Accuracy is a measure of how closely the result of an experiment agrees with the expected result. The difference between the obtained result and the expected result is usually divided by the expected result and reported as a percent relative error [ 29 ].
6.2 Precision
Precision is a measure of how close a set of results are to each other [ 30 ]. It is often measured under repeatable (same analyst, same day, same instruments and same materials) and reproducible conditions. Precision always accompanies accuracy, but a high degree of precision does not imply accuracy.
6.3 Linearity
For any developed analytical method, standard curve is constructed to verify the linear relationship between the concentration and a characteristic parameter for a component such as peak area, peak height or peak ratio in chromatographic analysis or UV-absorption in spectrophotometry.
Most analytical methods are based on processes where the method produces a response that is linear and which increases or decreases linearly with analyte concentration. In other words, it is the ability of the method to elicit test results that directly proportional to the concentration of analyte within a given range.
Statistical application is important in evaluating calibration graphs in instrumental analysis. The equation of a straight line takes the form:
Where a is the intercept of the straight line with the y axis and b is the slope of the line.
The statistical measure of the goodness of the fit of the line through the data is the correlation coefficient “r”. It falls in the range −1 ≤ r ≥+1. Negative r-values indicate negative slope and vice-versa. It is important to note that calculated r-values can be sometimes misleading and a calibration curve must be physically plotted to ensure the shape of the plot. From the calculated regression line data, the concentration of the analyte can be estimated by interpolation. Each value of y is subjected to random error and likely an error in the slope and intercept values can occur. This can be resolved by calculating standard deviations of the slope (S b ) and intercept (S a ). S b and S a are obtained from a calculated statistic value S y/x [ 29 ]. The values of S b and S a are used to calculate the confidence limits for the slope and intercept using a t -value at a desired confidence level, normally 95% level. These limits are important to indicate if there is a significant difference between these values and certain true values, which reflects the effect of random or systemic errors.
6.4 Limit of detection
The limit of detection is the lowest content of analyte that can be distinguished from background noise and measured with reasonable statistical certainty. It can be calculated by the reduced formula:
Where SB = Sy/x (calculated from the regression analysis data), b is slope [ 29 ].
6.5 Limit of quantification
The lower limit of quantification is the amount equal to or greater than the lowest concentration point on the calibration curve that can be measured with an acceptable level of accuracy and precision [ 29 ].
It can be calculated by the equation:
Where SB = Sy/x (calculated from the regression analysis data), b is slope.
6.6 Method comparison
The comparison of two methods should be carried out using a suitable statistical procedure to test if there are significant differences between them. The t -test provides a simple check on accuracy and the F -test on precision. These tests require the knowledge of what is known as the number of degrees of freedom.
7. Conclusion
Pharmaceutical products must be analyzed regularly to ensure their safety and effectiveness. This chapter described the quality assurance and quality control of materials and finished products. The requirements to develop a suitable method and its validation. Also different analytical methods and their application in the field of pharmaceutical analysis was also discussed.
Conflict of interest
The author declares that there is no conflict of interest.
- 1. Blass B. Basic Principles of Drug Discovery and Development. 1st ed. Amsterdam: Academic Press; 2015
- 2. Cairns D. Essentials of Pharmaceutical Chemistry. 3rd ed. UK: Pharmaceutical Press; 2008
- 3. Jeffery GH, Bassett J, Mendham J, Denney RC. Vogel’s Textbook of Quantitative Chemical Analysis. 5th ed. England, UK: Longman Scientific and Technical; 1989
- 4. Aulton ME. The Science of Dosage Forms Design. 2nd ed. Toronto: Churchill Livingstoe; 2002. p. 129
- 5. Florence AT, Attwood D. Physicochemical Properties of Pharmacy, Chapter 4. 4th ed. Chicago, London: Pharmaceutical Press; 2006
- 6. Genaro AR. Remington, The Science and Practice of Pharmacy. 20th ed. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 986
- 7. Ansel HC, Popovich NG, Allen LV. Pharmaceuticals Dosage Forms and Drug Delivery Systems. 6th ed. Philadelphia: Williams and Wilkins; 1995. p. 117
- 8. Lund W. The Pharmaceutical Codex. Principles and Practice of Pharmaceutics. 12th ed. London: Pharmaceutical Press; 1994. pp. 69, 277, 283
- 9. Carstensen JT. Drug Stability Principles and Practices. 2nd ed. New York: Marcel Deckker; 1995. p. 91
- 10. Grossweiner LI. Photophysics. In: Smith KC, editor. The Science of Photobiology. New York: Plenum Press; 1989. p. 12
- 11. Cadet J, Anselmino C, Douki T, Voituriez L. Photochemistry of nucleic acid in cells. Journal of Photochemistry and Photobiology B: Biology. 1992; 15 :277
- 12. Albini A, Fasani E. Drugs Photochemistry and Photostability. Cambridge: The Royal Society of Chemistry; 1998. pp. 29, 54, 132, 171
- 13. Greenhill JV, McLelland MA. Photodecomposition of drugs. Progress in Medicinal Chemistry. 1990; 27 :51-121
- 14. Tonnesen HH. Photostability of Drugs and Drug Formulations. London: Tylor and Francis; 1996. pp. 5-121
- 15. Beckett AH, Stenlake JB. Practical Pharmaceutical Chemistry. 4th ed. UK: Athlone Press; 2004. pp. 260, 296-300
- 16. Shantier SW, Gadkariem EA, Ibrahim KAE. A colorimetric method for the determination of tobramycin. International Journal of Drug Formulation and Research. 2011; 2 (4):260-272
- 17. Shantier SW, Gadkriem EA. Colorimetric methods for determination of cefquinome sulphate. American Journal of Applied Sciences. 2014; 11 (2):202-206
- 18. Anthony CM, Widdop B, Moss MS. Clarck’s Isolation and Identification of Drugs. 2nd ed. UK: The Pharmaceutical Press; 2006
- 19. Shantier SW, Gadkriem EA, Adam MO, Mohamed MA. Development of stability-indicating methods for cefquinome sulphate. International Journal of Biomedical Sciences. 2013; 9 (3):161-167
- 20. Elimam MM, Shantier SW, Gadkariem EA, Mohamed MA. Derivative spectrophotometric methods for the analysis and stability studies of colistin sulphate. Journal of Chemistry. 2015; 2015 :5. Article ID 624316
- 21. Davidson AG, Hassan SV. Assay of benzenoid drugs in tablet and capsule formulations by second-derivative ultraviolet spectrophotometry. Journal of Pharmaceutical Sciences. 1984; 73 :413
- 22. Abounassif MA. pH-Induced difference spectrophotometric assay of bromazepam. Journal de Pharmacie de Belgique. 1989; 44 :329
- 23. Shantier SW, Gadkriem EA. Differential spectrophotometric method for determination of cefquinome sulphate. British Journal of Pharmaceutical Research. 2014; 4 (5):617-625
- 24. Yuri K, Rosario L. HPLC for Pharmaceutical Scientists. Hoboken, New Jersey: John Wiley and Sons; 2007
- 25. Veronika RM. Practical High-Performance Liquid Chromatography. 3rd ed. New York: John Wiley and Sons; 1999. p. 15
- 26. Watson DG. Pharmaceutical Analysis. 2nd ed. UK: Harcourt Publishers Limited; 1999. pp. 80, 90-92, 238, 278
- 27. Harvey D. Modern Analytical Chemistry. USA: The McGraw-Hill Companies; 2000. p. 38
- 28. ICH Harmonized Tripartite Guideline, Stability Testing of New Drug Substances and Products; 2003. pp. 1-15
- 29. Miller JC, Miller JN. Statistics and Chemometrics for Analytical Chemistry. 5th ed. England: Pearson Education; 2005
- 30. Robinson JW. Undergraduate Instrumental Analysis. 5th ed. New York, Basel: Marcel Dekker; 1995. p. 16
© 2019 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Pharmaceutical formulation design.
Edited by Usama Ahmad
Published: 05 February 2020
By Anis Yohana Chaerunisaa, Sriwidodo Sriwidodo and M...
4735 downloads
By Divvela Hema Nagadurga
4672 downloads
By Zermina Rashid
1579 downloads
- Open access
- Published: 31 July 2017
An overview of forensic drug testing methods and their suitability for harm reduction point-of-care services
- Lane Harper 1 ,
- Jeff Powell 2 &
- Em M. Pijl 1
Harm Reduction Journal volume 14 , Article number: 52 ( 2017 ) Cite this article
74k Accesses
206 Citations
104 Altmetric
Metrics details
Given the current opioid crisis around the world, harm reduction agencies are seeking to help people who use drugs to do so more safely. Many harm reduction agencies are exploring techniques to test illicit drugs to identify and, where possible, quantify their constituents allowing their users to make informed decisions. While these technologies have been used for years in Europe (Nightlife Empowerment & Well-being Implementation Project, Drug Checking Service: Good Practice Standards; Trans European Drugs Information (TEDI) Workgroup, Factsheet on Drug Checking in Europe, 2011; European Monitoring Centre for Drugs and Drug Addiction, An Inventory of On-site Pill-Testing Interventions in the EU: Fact Files, 2001), they are only now starting to be utilized in this context in North America. The goal of this paper is to describe the most common methods for testing illicit substances and then, based on this broad, encompassing review, recommend the most appropriate methods for testing at point of care.
Based on our review, the best methods for point-of-care drug testing are handheld infrared spectroscopy, Raman spectroscopy, and ion mobility spectrometry; mass spectrometry is the current gold standard in forensic drug analysis. It would be prudent for agencies or clinics that can obtain the funding to contact the companies who produce these devices to discuss possible usage in a harm reduction setting. Lower tech options, such as spot/color tests and immunoassays, are limited in their use but affordable and easy to use.
Given the current opioid crisis in Canada [ 1 , 2 , 3 ] and around the world [ 4 ], harm reduction agencies are seeking to help people who use drugs to do so more safely. Harm reduction sites and/or clinics are increasing in number and service provision across the world, making it crucial to provide point-of-care workers with the tools and knowledge necessary to provide proper care for people who use drugs. Drug, pill, and substance testing are increasingly being used as a harm reduction strategy throughout the world [ 5 , 6 , 7 , 8 ] to decrease the risk of adverse effects. Indeed, various approaches to drug testing have been around, even in North America, for decades [ 9 , 10 , 11 ]. More recently, in Canada, drug testing is becoming more common at music festivals [ 12 ]. In Canada, the Standing Committee on Health [ 13 ] recommended that the Government of Canada grant exemptions under the Controlled Drugs and Substances Act so that drug testing could occur at designated sites. While there are certainly legal hurdles to overcome when it comes to drug testing [ 6 ], there are three primary advantages to testing drugs before they are consumed: short- and long-term adverse effects (including overdose and fatality) can be avoided by the person using the substance; other institutions (such as hospitals) and public health authorities can be made aware when a lethal or novel substance begins to circulate; and, a global picture of drugs in circulation can be generated [ 5 , 14 , 15 , 16 ]. The goal of this paper is to describe the most common methods of testing chemical substances in both laboratory and point-of-care settings. We will conclude with recommendations for point-of-care testing of illicit substances. In this paper, we use the term “drug testing” to refer to the forensic testing of illicit substances in their intended consumption form. Please note that the legal issues surrounding, and the service models of, drug testing are beyond the scope of this paper.
Introduction to substance testing methods
The following methods have been validated by the Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG). Scientific Working Groups consist of scientific subject-matter experts who collaborate to determine best practices and develop consensus standards. As such, these methods have been proven to be effective in the analysis of unknown (forensic) examination of illicit substances and are therefore also the best methods to use in identifying unknown substances. Not all of these methods are easily accessible in a point-of-care framework, as some require high technical knowledge and/or a laboratory setting. Therefore, any of the following methods may be suitable on a case-by-case basis. This is due to the fact that some clinics may be able to easily access more discriminatory methods, through direct funding or industry partnership, whereas some clinics may have to rely on less precise testing methodologies and equipment due to lack of funding or support.
More discriminatory methods carry a much larger price tag to invest in the proper equipment. This may require community partnerships or a serious cost-benefit analysis or both. To keep the information precise and to attempt to interpret some of the associated technical details, the methods have been broken down into subheadings. Each method has three subheadings: “How does it work?” (a brief discussion of the theory behind the method), “What substances can be detected and how accurately?”, and “How easy is it to use?” The methods have also been broadly assigned into two larger categories: most discriminatory, or methods that will accurately identify a substance/mixture and that also have the potential to quantify the amount of substance, and least discriminatory, or methods that presumptively identify a substance and/or mixture without quantification. At the end of the paper, there will be a recommendation section that will focus strictly on the best methods/devices considering only point-of-care situations. The methods are summarized in Table 1 .
Most discriminatory
Mass spectrometry
How does it work?
Mass spectrometry (MS) is the most discriminatory of the drug testing techniques. Mass spectrometry measures the precise molecular mass of ions as determined by their mass to charge ratio ( m / z ) and is the current gold standard in forensic drug analysis [ 17 ]. In general, mass spectrometry requires separation, ionization, and finally detection. Separation can be accomplished through gas chromatography (GC), liquid chromatography (LC), or capillary electrophoresis (CE). There are various ionization methods. The most commonly used in analysis of illicit substances are electron ionization (EI), atmospheric pressure chemical ionization (APCI), electrospray ionization (ESI), matrix-assisted laser desorption ionization (MALDI), atmospheric pressure photoionization (APPI), fast atom bombardment (FAB), and more recently direct analysis in real time (DART). Ionization methods can be grouped into hard or soft techniques.
Hard techniques like EI, FAB, and APCI cause molecules to fragment generating complex mass spectra. Fragmentation is useful in analysis because molecules have known fragmentation patterns. A spectral database allows for a computer to quickly match spectra and determine the molecular species. Hard techniques are limited to detecting small molecules. Most illicit drugs are small molecules with the exception of drugs of a biological nature being consumed in their raw form.
Soft ionization techniques such as MALDI and ESI minimize fragmentation and allow for the molecules being analyzed to remain intact. Soft ionization techniques are useful for large biomolecules such as proteins.
DART is of particular interest as it allows non-destructive testing, is fast, and can quickly quantify when used with an internal standard. A pill can be held in front of the gas stream and within seconds determine the molecular species present. DART does not require separation of each molecular species prior to analysis allowing untrained personnel to collect data [ 18 ].
What substances can be detected and how accurately?
Virtually, any substance can be identified using MS in combination with a separation (chromatographic) technique. Sensitivity of current mass spectrometers allows for detection of analytes at concentration in the attomolar range (10 −18 ) [ 19 ]. MS has increased sensitivity over some other analytical techniques as the analyzer, a mass-charge filter, reduces background interference (i.e., a clearer reading/analyte fingerprint can be produced). It demonstrates excellent specificity due to characteristic fragmentation patterns, high resolution, and unique filtering abilities available especially in tandem or higher order mass spectrometry [ 20 ].
MS provides information about molecular mass and isotopic abundance of elements and temporally resolved chemical data, allowing for highly accurate identification. Newer devices are easier to utilize and much smaller than older versions. Interfacing with computers allows for refined database searches, making the drug identification process easier.
A major drawback of MS is that the tested sample taken from the supply is destroyed by the testing process (DART being an exception). Only a very small sample size (milligrams) is required. There are also continuing costs due to consumable materials required, and some of these consumables are poisonous/hazardous. Complex mixtures must be separated with a chromatographic technique (either gas or liquid chromatography) to correctly identify each constituent (unless using DART).
How easy is it to use?
The expertise required to utilize this technology is intermediate to expert (for definitions of terms in context with this paper please, see Table 1 ). Individuals should have some theoretical knowledge of how the technology and specific instrument work and specialized training from an expert. The cost of a mass spectrometer can vary from US$5000 to US$1,000,000. While an older used mass spectrometer may be less expensive upfront, it is not necessarily suitable for point-of-care drug testing. There are also considerable ongoing operational costs, such as chromatography (separation) reagents, gas consumables (nitrogen, helium, etc.), sample preparation items, and routine maintenance and service. Some labs offer MS services with costs between US$5 and US$100 per sample.
Ion mobility spectrometry
Ion mobility spectrometry (IMS) separates and identifies ions based on their speed through a carrier gas. Ion mobility is dependent on three molecular characteristics: the charge, reduced mass, and the collision cross section of the ion. IMS requires ionization before samples are passed into the instrument. This can be accomplished by ESI, MALDI, APPI, and coronal discharge or by using radioactive sources such as nickel-63.
There are many designs for ion mobility spectrometers including drift tube, ion trap, traveling wave, high-field asymmetric waveform, and differential mobility types. Drift tube IMS determines the ion mobility based on the amount of time it takes for ions to reach the detector. Many modern instruments use a drift tube for analysis.
Of interest is the field asymmetric subtype of the high-field asymmetric waveform IMS. A field asymmetric ion mobility spectrometer (FAIMS) uses a high (strong) electric field to control the movement of the ions through a physical filter. A pulsing electric field can then be applied to select for ions with specific ion mobility. Only ions with the specifically selected mobility will be able to maintain a stable trajectory through the filter. The others will crash into the side walls and not reach the detector.
Any small molecule of illicit substance can be detected very quickly and accurately. FAIMS sensitivity is based on multiple characteristics of both the ion of interest and the physical environment. IMS can detect one molecule in a billion (ppb) and is very selective. IMS selectivity can be further enhanced when using FAIMS. FAIMS is able to operate in environments with high levels of interference with minimal adjustment to operating conditions [ 21 ]. IMS is non-destructive and only requires a very small sample if a quantitative method calls for destructive testing. Determination is very quick and can be accomplished in a few seconds even for a complex sample.
IMS instruments do not require a trained operator. They can be used to quickly analyze a sample. Identification does require a database of known molecules to compare the sample against. The process of building a database would require a trained chemist using another technique or a standard. Once built, a database could be referenced from any instrument without additional technical help [ 22 ]. Quantification is possible when using internal standards or prebuilt methods. IMS is regularly used by law enforcement agencies at airports to detect narcotics and explosives. Minimal maintenance, ease of use by non-technical personnel, low cost, fast and accurate determination, minimal cost of consumables, and robust methodologies make IMS one the best choices for drug identification.
Infrared spectrometry
Infrared (IR) spectroscopy is another highly discriminatory method and is based on the measurement of the amount of IR radiation which is absorbed or emitted by a sample as a function of wavelength. A spectrum is obtained by passing infrared radiation through a sample and determining the amount of the incident radiation (radiation that actually hits the molecule rather than passing through) that is absorbed at each IR frequency [ 23 ]. Interpretation of the spectra allows for determination of molecular functional groups. The IR spectra of a pure molecular compound provides a distinctive fingerprint which can be easily differentiated from the IR absorption pattern of other compounds, including compounds with the same chemical formula, but a different arrangement of atoms in the molecule (known as isomers) [ 23 ]. An advantage of IR techniques is that virtually, all compounds have IR active vibrational modes and can therefore be investigated both qualitatively and quantitatively. However, quantitative analysis can pose a problem with unknown samples and mixtures. The spectroscopic expertise required to forensically analyze and quantify a substance may be difficult or impossible to find in harm reduction clinics. Most papers that describe relatively simple quantification methods are carried out in pharmaceutical research with controlled standards, methodologies, and standards. While quantification of unknown substances is technically possible, it really comes down to a case-by-case basis and is generally a laborious process undertaken by advanced to expert level technicians and chemists in forensic laboratories. It is highly unlikely that quantification would be viable using this technology in this kind of setting. Recent advances in IR technology have allowed for the development of portable IR devices.
When reference spectra are available, most compounds can be unambiguously identified based on their IR spectra. Drugs can be identified through a searchable database (such as http://webbook.nist.gov /). IR cannot distinguish enantiomers (similar to MS) [ 24 ]. According to the SWGDRUG [ 24 ], IR can produce structural information that will provide sufficient selectivity that generates the highest discriminating capability. IR can discriminate between diastereomers (such as pseudoephedrine and ephedrine) and free base/acid and salt forms. Free base/acid and salt forms refer to differences in physical properties that can alter the application of the substance. Free base is usually more volatile and normally has a lower boiling point, allowing the substance to be smoked. The salt form is usually more stable and tends to be crystalline and dissolvable in water, allowing for ingestion, insufflation (inhaling through the nose), or injection. A common example is crack cocaine (free base) and cocaine (salt); they are in fact the same drug (cocaine), and the actual effect on the body is the same, but due to different absorption and dosages based on method of use, it is possible to observe a spectrum of differing responses to each of the drugs. One of the notable benefits of IR spectroscopy is that it does not destroy the sample provided—an important consideration when working with drugs and the people who use them. As well, it requires only a very small sample size in the range of milligrams or less. Additionally, samples can be studied in virtually any physical state (primarily solid or liquid). Interference is very common and causes difficulty in identification.
The level of expertise required to use this technology varies depending on the device. There are portable IR devices on the market that have been optimized for basic to intermediate knowledge base, such as by outreach workers. These devices can analyze the obtained spectrum and search internal databases to display the identified substance or substances in a mixture (to a certain concentration, based on the specifications of a given device). This is considered presumptive or qualitative testing, in that it may only give an accurate breakdown of the constituents of a substance or mixture and sometimes offer a semi-quantitative analysis (i.e., rank-ordered most to least in a mixture). For quantification (as percent mass by total mixture weight), Sorak et al. have shown that some portable IR devices may be used for low error quantitative analysis [ 25 ]; although in order to interpret the obtained spectrum in the these devices in a quantitative manner, advanced to expert level knowledge is required as the devices do not perform this task for the user. Many other IR devices also require at least an intermediate level understanding of the procedures and some require advanced to expert knowledge to correctly analyze and quantify the substances (including operation of the equipment and database searching). Costs of IR devices can be anywhere from the low thousands to US$60,000 and above.
Raman spectroscopy
Raman spectroscopy is an optical technique based on the inelastic scattering of radiation after it interacts with matter. The interaction of incident radiation with the molecules of the substance gives spectral vibrational information [ 26 ]. The technique involves shining a laser on a sample and detecting the scattered light. A small amount of the scattered light is shifted in energy from the laser frequency due to electromagnetic and molecular interactions in the sample [ 26 ]. Plotting the intensity of the shifted light versus frequency gives a Raman spectrum of the sample. An exciting breakthrough in this technology is the development of handheld, portable Raman spectrometers. Many of these devices, most notably the TruNarc device by Thermo Fisher Scientific, have been optimized for drugs of abuse detection with simple “point and shoot” action. These devices also search databases in real time at a device level and give a clear readout of what substance(s) were detected.
Virtually, any drug can be identified with Raman spectroscopy. It can be used to determine active pharmaceutical ingredients (APIs) as well as molecules with the same chemical formula but different molecular arrangement and polymorphs. This is important as many of the novel psychoactive substances that have been emerging are isomers, derivatives, and analogues of many of the classical drugs of abuse. Being able to differentiate between small differences in physical or chemical structure aids greatly in unambiguous identification. Portable Raman spectroscopy has even been reported to be able to detect the date-rape drug rohypnol (flunitrazepam) in spiked beverages [ 27 ].
Raman spectroscopy may have difficulty in identifying substances that exhibit strong fluorescence. These substances tend to be plant-based narcotics such as heroin. However, with proper sample preparation, it is possible to analyze even these substances. The TruNarc Raman spectroscopy device has been shown to have a very high level of agreement with laboratory results (MS) for cocaine, heroin, and methamphetamine; inconclusive results are generally related to illicit substances that are present at extremely low percentages of the total mixture. Some studies have indicated that cocaine can be detected at concentrations as low as 5% when the cocaine was cut with sorbitol [ 28 ]. Others have detected amphetamine residues (milli- to micrograms) on paper currency using Raman spectroscopy [ 29 ]. It must be stressed that the particular technology discussed (TruNarc by Thermo Fisher Scientific) does not offer quantitative data in its “point and shoot” identification action, although it does offer highly accurate and extremely easy-to-use qualitative testing. The Raman technique as a whole is able to identify and quantify (depending on the device) a wide range of illicit drugs, even in the presence of contaminants and adulterants [ 26 ]. Given that there are many substances used to “cut” illicit drugs, this feature is an important one.
RS is rapid and non-destructive, does not require chemical reagents, can detect separate substances in mixtures, is not subject to interference from water or moisture, and importantly, can detect substances through transparent packaging (such as plastic bags and glass containers). Little or no sample preparation is required, although some sample preparation is required for substances that exhibit high fluorescence (including some cutting agents). RS is ideal for both organic and inorganic species and can be used for both qualitative and quantitative analysis. Due to the similarity to IR (detecting forms of molecular movement to identify), Raman has similar issues with quantitative analysis. While quantitative analysis can absolutely be done with Raman spectroscopy, it can be a much more difficult process that may not be possible in a harm reduction setting. Due to the difficulty of quickly and easily performing quantitative analysis on many unknown samples, an important consideration for outreach is that portable handheld devices specifically designed to detect drugs of abuse are available. Qualitative results can be obtained in a fraction of seconds to several minutes.
The cost of a RS unit can vary widely (in the low thousands of dollars to US$50,000 and above). Like all of the previous devices, care must absolutely be taken in selecting the appropriate tool. Advanced knowledge is required for devices that are not optimized for drug testing.
The level of expertise required to use this technology varies depending on the device, similar to IR. Some Raman spectrometers have been optimized for “point and shoot” action, giving a clear interpretation/reread of the substance(s) analyzed, and thus require merely basic to intermediate expertise for presumptive analysis. The requirements for quantitative analysis for portable “point and shoot” Raman spectrometers are similar to IR. Sorak et al. have also shown that some portable Raman spectrometers can offer quantitative analysis to a high degree of precision [ 25 ], although it must be stressed that this comes with the exact same considerations as the portable IR, as stated above. Other bench top or lab specific devices are most often not as simple and may require some database searching and interpretation of results. This can push the level of expertise required to intermediate, advanced, or expert, depending on the chosen device.
X-ray diffractometry
In X-ray diffractometry (X-ray D), the drug sample is bombarded with high-energy X-ray radiation and crystalline atoms in the substance cause incident X-ray beams to diffract in various directions [ 30 ]. This allows for the determination of the spatial structure of molecules by measurement of how X-ray radiation is scattered by the molecular crystal lattice structure. By measuring the angles and intensities of the diffracted X-rays, it is possible to produce a three-dimensional picture of the density of electrons in the crystal, and, from this, it is possible to determine the positions of the atoms in the crystal as well as their chemical bonds and other structural information [ 30 ].
Any crystalline or partially crystalline substance (i.e., substances that are solid and usually either evidently crystalline or powder or pill, such as methamphetamine, ketamine, and cocaine) including those in mixtures and compounds with currently unidentified structure can be identified [ 31 , 32 ]. This method is generally restricted to solid substances. X-ray D is used to identify precise chemical forms but not to quantify them. It can be used to identify diluents or adulterants [ 31 ]. This method is sensitive to both polymorphs and contaminants (common in illicit drugs). X-ray diffractometry determines structural information of the substance, so the substance can be identified with a very high degree of accuracy. This method is specific because substances have unique diffraction lines or an “X-ray fingerprint.” It is also sensitive in that drug concentrations and any additional agents used in cutting can be discerned through the obtained data. Studies have shown that this method can be used to identify a specific drug at only 5% of the total pharmaceutical formulation [ 33 ].
One benefit of X-ray D is that it requires no sample preparation and does not destroy the substance being tested. As well, only a very small sample size is needed (milligrams to micrograms) [ 31 ]. While it is the most reliable structural determination method and can determine the structure of currently unknown molecules, it is not suitable outside of a laboratory environment.
X-rays are highly radioactive and very damaging to organic cells/DNA. Thus, this method requires a high level of training and safety procedures and is restricted to laboratory environments. The skill level involved in operation is advanced to expert.
Least discriminatory
Microcrystalline tests.
These chemical tests result in the formation of unique microcrystals of a given analyte when a specific reagent is applied. The unique crystal formation is compared to a reference standard/control using a common light microscope. Microcrystals are compared based on shape, size, color, and spatial arrangement [ 34 ].
Several commonly abused substances can be identified, including cocaine, heroin, methadone, GHB (gamma hydroxybutyrate ) , ketamine, phencyclidine, amphetamines, and methamphetamine [ 34 ]. With test reagents chosen to induce development of specific microcrystals with the analyte and a reference/control standard available, these tests can be highly specific as the crystals formed are a direct consequence of choice of reagent and analyte and are unique under these circumstances. This is provided that other substances do not react in a similar way, if at all, with the reagent, and provided that impurities, dilutents, and adulterants do not prevent or mask the formation of characteristic microcrystals for the drug tested. In these cases, a microcrystalline test can be considered highly characteristic but non-specific enough for a confirmatory test. Thus, this method is best suited to pure and/or separated samples. Sensitivity is high as samples require only micrograms of substance.
The benefit of microcrystalline tests is their relatively low cost. Minute amounts of reagents are required. Instrumentation is simple; however, this method does not quantify how much of a substance is present. Unfortunately, the sample that is tested is destroyed in the process, which may be less than ideal for people who are bringing the samples for identification.
The expertise required is intermediate to advanced and requires adept interpretation of results.
Thin-layer chromatography
Thin-layer chromatography (TLC) is a technique in which a sample is placed onto a planar stationary phase then a liquid mobile phase resulting in capillary action. The analyte is either adsorbed to the stationary phase or is in the mobile phase, and the time spent on the stationary phase or time spent in the mobile phase determines its retention time. Components of the sample travel at differing rates depending on the component’s size and affinity for the mobile phase [ 35 ]. The result is a plate of spots (separated components of the mixture) that have moved various distances on the stationary phase.
TLC can detect barbiturates, benzodiazepines, GHB, heroin, morphine, opium, oxycodone, and other opiates, amphetamines, cocaine, methamphetamine, MDMA (methylenedioxymethamphetamine or Ecstasy), ketamine, LSD, marijuana, mescaline, synthetic cannabinoids, and cathinones (commonly referred to as “bath salts”). Using TLC, it may be difficult to separate and identify novel psychoactive substances [ 36 ]. TLC performs fairly poorly at separating complex mixtures. Sensitivity is in the micro-nanogram range. Specificity can range from intermediate to high depending on the mixture, and measured retention factors can be used to make a preliminary identification of a substance but are not specific to a single compound [ 35 ]. In order to increase specificity in cases of similar retention factors, it must be used in conjunction with another technique such as Raman spectroscopy or colorimetric testing or in the case of UV active species, UV.
TLC is a relatively low-cost way to test substances and demonstrates good sensitivity and speed of separation. It can be used as a presumptive test with a fairly high degree of accuracy depending on sample purity. While TLC can identify some known substances in provided samples, it does not indicate (quantify) how much of a substance is present in the sample. TLC is best used in conjunction with a more discriminating technique such as Raman spectroscopy, MS, or IR.
TLC is relatively simple to use and interpret and is thus suitable for basic to advanced skill level. This means that someone with basic skill may be able to perform a test following instructions but have trouble interpreting the results, whereas someone with intermediate to advanced skill level would have greater ability to interpret a test and could supervise basic skill level users.
Spot/color tests
Spot/color tests offer presumptive testing based on chemical reactions between analytes and indicators. There are many possible indicator tests such as cobalt thiocyanate, Dille-Koppanyi, Duquenois-Levine, Mandelin, Marquis, nitric acid, para-dimethylaminobenzaldehyde, ferric chloride, Froehde, Mecke, Zwikker, and Simon’s (nitroprusside) [ 37 ]. The indicator chemically reacts with the analyte and causes a reaction that creates a certain color staining depending on the analyte tested. Spots are then compared visually with reference charts, the current standard being the Munsell color charts. There is a method that bypasses the human eye and its subjectivity by using a simple smartphone app to identify colors with high precision and accompanying software that matches the results in a searchable database [ 38 ]. This allows for a more precise quantitation of the color and therefore higher accuracy identification.
What substances can be detected, and how accurately?
Colorimetric tests exist for most drugs of abuse, including cocaine, various pharmaceutical opioids, amphetamines, LSD (lysergic acid diethylamide), cathinones (bath salts), heroin, and fentanyl. There may be other novel psychoactive substances that do not (yet) have any associated colorimetric tests. Each specific named test will have information on what analytes it can be used with. Unfortunately, the test also destroys the sample provided. That said, color tests do not require much sample: if it can be seen, it can be tested.
Colorimetric tests can be quite sensitive, with limits of detection in the microgram range depending on the spot test utilized and the analyte [ 37 ]. Multiple tests with multiple reagents can be used if a mixture of drugs is suspected, though each test requires in the low milligram range of substance and destroys the substance in testing. With the proper standards, these tests can be quite specific, although multiple analyses may be required for high specificity. Some knowledge about what the substance is supposed to be and about general appearance of certain substances can increase specificity. Colorimetric tests are considered presumptive, in that they can only identify presence or non-presence of a particular substance based on the test administered. A single test/reagent will only test for the presence or absence of a drug or class of drugs. A typical test is not sufficient for a suspected mixture or even an unsuspected mixture if there is any reason at all to have suspicion of the substance. An example battery test protocol for considerations of how to test a suspected mixture is included below.
Actual color results may vary depending on the concentration, whether the drug is in salt or free base form, additional diluents, or contaminants; positive result may indicate a specific drug or class of drugs present, but not always specific for a single drug or class. Colorimetric tests rely on simple chemical reactions and produce visible results that can be interpreted with the naked eye.
Reagents and laboratory materials needed are inexpensive and readily available and can be performed with minimal training. Because each individual perceives color uniquely and because lighting conditions are not always optimal in non-lab settings, accuracy can be greatly enhanced with the use of smartphone apps to report color test results quantitatively [ 38 ]. Overall skill level required is basic to intermediate. A basic user can run the simple test and obtain results, whereas an intermediate user would run a standard protocol. An example of an intermediate protocol would be to run a battery of tests based on how much sample can be obtained without objection from the user. The tests should be based on an educated guess system, narrowing down possibilities through analysis and questions. Potential questions would be as follows: What did the user think it was or was told it was? What are recent novel substances that have been appearing in the clinic or on the street lately? What is the most dangerous substances worth testing for (smallest window of dosage)? Is there any knowledge of common mixtures, such as opioid mixtures?
The tests should be interpreted within a maximal 10-min window. The tests can be analyzed via smartphone or at least under good lighting if using the naked eye in order to most accurately determine color. The tests can then be matched against a database if a computer or the internet is available. From a system such as this, a presumptive test can then become a much more powerful tool.
Immunoassay
Immunoassay involves the binding of an antibody that is selective for the drug or drug group of interest (antigen) and a label that will be part of the antibody-antigen complex that can be detected using some means (such as fluorescence). Antigen-antibody binding is based on a typical immune system response in which antibodies in biological tissue bind to antigens in order to neutralize or remove them. This technique is rarely used in drug analysis because these methods were originally designed for analysis in biological materials (primarily metabolites in urine). Thus, traditionally, immunoassay provides important patient information for clinicians but does not provide a determination of the type or amount of a drug prior to its ingestion/injection. ELISA can, however, be used to perform other types of biochemical assays in the detection of an analyte in a liquid sample. Very little scholarly information is easily accessible about which specific drugs ELISA can detect outside of biological samples (post ingestion/metabolization).
Various opioids and cocaine can be detected rapidly and somewhat effectively using immunoassay technology. There are problems with specificity regarding immunoassays, and there have been many instances of false positives due to similarity in drug structures or metabolites. Sensitivity is quite high with detection in the microgram range as antibody-antigen interactions occur on a molecular level [ 39 ].
Immunoassay is fast and relatively inexpensive and in most instances, does not require high-level scientific knowledge to perform and interpret. Running such tests can require intermediate skill level. However, there is very little information available that has been scientifically published or available for public access on the usage of immunoassays for whole drug analysis. Immunoassay is most often employed to detect drug usage after the fact, such as in urine drug screens.
Urine dipstick test
This method has recently come under attention as a relatively cheap, easy-to-use presumptive test for fentanyl [ 40 ]. A sample of the drug sample is dissolved in water, and if the drug contains fentanyl in a concentration above the cut-off levels, an indicator on the strip will appear. The methodology works via chromatographic immunoassay, and in the presence of an appropriate analyte, a strip on the indicator stick appears/changes color.
To date, fentanyl is the only drug for which this method of drug checking has been reported being used [ 25 ], and there is little published data about this methodology. There is no scientific data on sensitivity, although the strips have been developed to detect fentanyl in urine and are therefore specific to testing for fentanyl and/or fentanyl metabolites.
The provided sample is destroyed in the testing process. Urine dipsticks are very easy to use, quick to check, specific for fentanyl, proven in urine test situations, and recently been proven efficacious in testing unknown drug mixtures for the presence of fentanyl. However, dipsticks were designed for drug detection in urine, and therefore, due to low specific weight in other mediums, it may be possible that false positives occur.
Another potential concern with this method is that many retailers will only sell to health professionals, and thus, these items may be difficult to procure for harm reduction agencies unless they are affiliated with a health clinic. Some medical device companies may object to such a test being used in a harm reduction setting, even in the presence of qualified health professionals for liability reasons.
Ultraviolet spectroscopy
This method is based on the absorption of light energy in the ultraviolet (UV) wavelength range. Light in this range can raise the energy levels of the electrons within a molecule from ground state to higher energy levels. Each transition to a higher energy level requires a given amount of energy, provided by light of a particular wavelength. Using a particular wavelength of light, a characteristic UV absorption spectrum can be obtained based on the electronic structure of the whole molecule as this structure will determine what wavelength(s) are absorbed versus which pass through a sample. UV-vis (ultraviolet visible) spectrophotometers measure the intensity of light passing through a sample and compare it to the intensity of light before it passes through the sample and capture this information to create a characteristic spectrum.
Drugs with similar structures may provide the same UV spectra. UV-vis has been used to identify MDMA, ketamine hydrochloride, cocaine hydrochloride, diazepam, phenobarbital, and barbital concentrations in the microgram range, as well as specifically identify six different compounds and for the first time, accurately discriminate some mixtures [ 41 ]. Other substances may be identifiable although literature is sparse on confirmatory usage for a broad spectrum of illegal drugs. UV spectrometry can be used on solid samples and therefore can be non-destructive in nature, although some samples may need preparation that can make them unsuitable for use afterwards. UV can be used quantitatively (amounts) and qualitatively (identification) and yields rough structural information providing modest selectivity to allow for some discriminating capability [ 24 ].
UV can be combined with chromatographic techniques for greater selectivity and specificity. It is not suitable for detection of several drugs in a mixture. Samples must be diluted or the technique can yield saturated spectra. Compounds lacking suitable chromophore provide no signal (for example, GHB has a low wavelength chromophore which makes analysis by UV-vis much more difficult without further sample preparation), although most drugs of abuse have a suitable chromophore due to aromatic ring structures in their chemical structures. Additionally, UV spectrum can vary depending upon the pH of the sample solution, and it is possible for chemical composition to change during the analysis. The level of expertise involved in UV is basic to advanced. The technique may be easily taught to someone with little to know theoretical knowledge of the technique, although interpretation of results would require intermediate to advanced knowledge.
There are many variables to consider when selecting technology for drug checking on the front lines of harm reduction. Harm reduction agencies, if pursuing the addition of drug testing services, will need to consider not only the quantitative capabilities of the tests but whether the agency can afford the human and fiscal resources to support the use of the technology. Thus, the recommendations include a strong bias to cost-benefit and beg the important question of whether some of the less discriminatory interventions are better than no intervention at all. With these considerations in mind, the following recommendations will summarize the methods for drug testing at a point-of-care level.
The techniques that are the strongest candidates based on all considerations are IMS, IR, Raman spectroscopy, and spot/color tests, although these too have some associated drawbacks. Spot/color tests are purely presumptive. In most cases, quantitation is contingent on expert interpretation. In some cases, the therapeutic index is so small and such miniscule quantities can be used as an additive to mixtures that only the highest discriminatory techniques mentioned above are capable of proving unequivocally that the quantity present would fall in therapeutic index (i.e., would produce a high but not be fatal, barring extraneous circumstances).
In our review, the best methods for point-of-care drug testing are handheld IR or Raman spectroscopy. From a cost-to-benefit analysis, these methods (specifically the portable/handheld units) are superior in almost every way to every other method. Manufacturers have simply made these technologies extremely easy to use and effective at identification of unknown analytes. The major downsides of this technology are that quantitation may require advanced expertise and that these units are still fairly expensive. To use these units qualitatively usually requires very little technical expertise or training. Intended for use in the field, these units are small and portable and tend to be fairly rugged, while also being able to have near-lab identification ability [ 25 ]. While many of these devices are only currently in use in law/drug enforcement settings, use in harm reduction settings would be worth exploring.
IMS spectrometers are very robust and require minimal maintenance. They are routinely used in airports worldwide for narcotics detection. Training is easy and quick, and sensitivity and selectivity are very high. Consumables are cheap and have long lives. Sampling is non-destructive and quantification is possible without expert level understanding. Analysis is quick and accurate. IMS is the best option available for clinics with a moderate level of funding. Some gas analyzers allow online updating; rapid sample analysis of liquid, solid, and gas; and discrimination of multiple interfering species in a complex matrix. The capability to update online allows methodologies and new molecular species to be shared instantly among clinics enabling point-of-care testing to remain current.
Other methods worth considering for point-of-care drug testing are MS, TLC, and UV spectroscopy. MS is considered the current gold standard in forensic drug analysis. Since MS units have been in use for a long time, it is actually possible to obtain one for a decent price (low-to-mid thousands) in the used market. However, in order to obtain a newer device optimized for drug testing or for testing extremely low concentrations, it would come with a higher price tag, usually in the hundreds of thousands of dollars. This presents a difficulty of its own because of the wide range of machines available, it would take some considerable research at clinic level to determine the cost-benefit analysis of a new or used machine to ensure acquisition of a machine that is suitable for its intended purpose. Additionally, operation and maintenance of MS machines is still complex, so a clinic would have to assess training, operation, maintenance, and associated ongoing costs which may place such a device beyond the time and/or monetary costs to the clinic compared to the benefits provided.
UV spectroscopy and TLC are more affordable options, but also much less discriminatory. Both of these methods tend to be less technical in operation, maintenance, and interpretation of results, but also do not offer quantification at the same level of the more discriminatory methods. They are also less expensive than all of the more discriminatory techniques. However, when used in conjunction, TLC and UV can be quite powerful in identification of a wide variety of substances (including mixtures) and offer a more rudimentary quantification than the more discriminatory techniques.
A lower technology option is the spot/color tests, which are purely presumptive in nature, although they can be fairly specific at identification of a compound and/or mixture when utilizing a standardized procedure utilizing a battery of tests (as described above). Information about optimal technique can be easily accessed via the internet. Color tests are cost effective, fast to complete, and very easy to perform. The use of a smartphone app can aid in identifying the exact color profile. This can then be used in conjunction with a searchable database to perform the most accurate identifications. The fact that this technology is so cost-effective, easy to perform, and requires a very minute amount of substance makes it really stand out from many of the other presumptive methods [ 16 ]. This type of test is widely used in Europe [ 16 ]. These tests are not perfect and can be performed incorrectly. A proper standardization of technique should be implemented at the clinic level to maximize the accuracy of these tests.
Drug testing methods that are less suited to point-of-care drug testing situations include immunoassay, microcrystalline testing, and X-ray diffractometry. Immunoassays are traditionally designed for usage in biological samples as they work based on antibody-antigen interactions and as such are best suited for testing excreted metabolites (such as in urine). At best, an immunoassay can indicate the presence of drug(s), and at worst, they can give a high proportion of false positives. This may result in people using the substances anyways or serve to give the clinic a poor reputation, and users may soon stop going to the site for drug testing. That said, they are affordable and portable and can detect potentially fatal drugs like fentanyl.
Microcrystalline testing is a highly limited method as the drug needs to be mostly (or completely) pure. This testing has no quantification capabilities at all and requires high skills and knowledge to identify drugs based purely on crystal structure. X-ray diffractometry is a highly discriminating testing method; however, this method basically requires partnership with a specialized lab/institution. X-ray diffractometers are incredibly expensive (mid-to-high tens of thousands), difficult to maintain and operate, and have the added factor of using radioactivity which may present health and safety concerns.
There is a wide variety of techniques that have been validated for drug identification and/or quantification. Each of these techniques has a variety of associated pros and cons that must be considered. With this in mind, this review is not meant to be an in-depth rigorous scientific treatment of each of these methods, but a guide for the practical consideration of usage and recommendations for point-of-care harm reduction purposes. It is sincerely expected that this document will help to narrow down consideration of each of these techniques and that each clinic would then determine a smaller subset of techniques to consider implementing. It would be prudent for clinics that can obtain the funding to contact the companies who produce and design these devices and discuss possible usage in a harm reduction setting as many of the devices are only currently in use in law enforcement and research.
Abbreviations
Active pharmaceutical ingredients
Field asymmetric ion mobility spectrometer
Gas chromatography
Gamma hydroxybutyrate
Liquid chromatography
Lysergic acid diethylamide
Methylenedioxymethamphetamine or Ecstasy
Scientific Working Group for the Analysis of Seized Drugs
Ultraviolet
Ultraviolet visible
Canadian Community Epidemiology Network on Drug Use. Deaths involving fentanyl in Canada, 2009–2014. Ottawa: Canadian Centre on Substance Abuse; 2015.
Google Scholar
Canadian Institute for Health Information. Hospitalizations and emergency department visits due to opioid poisoning in Canada. Ottawa: Author; 2016.
Canadian Institute for Health Information. 13 Canadians hospitalized each day for opioid poisoning. Ottawa; 2017. https://secure.cihi.ca/estore/productFamily.htm?locale=en&pf=PFC3328&media=0 . Accessed 16 Mar 2017.
Mounteney J, Griffiths P, Sedefov R, Noor A, Vicente J, Simon R. The drug situation in Europe: an overview of data available on illicit drugs and new psychoactive substances from European monitoring in 2015. Addiction. 2016;111(1):34–48.
Article PubMed Google Scholar
Trans European Drugs Information (TEDI) Workgroup. Factsheet on drug checking in Europe. In: Executive Agency for Health and Consumers; 2011.
European Monitoring Centre for Drugs and Drug Addiction. An inventory of on-site pill-testing interventions in the EU: fact files. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2001.
Butterfield RJ, Barratt MJ, Ezard N, Day RO. Drug checking to improve monitoring of new psychoactive substances in Australia. Med J Aust. 2016;204(4):144–5.
Tregoning W. Drug checking services. Austalia: Unharm; 2016.
Brown JK, Malone MH. Some U.S. street drug identification programs. J Am Pharm Assoc. 1973;13(12):670–4.
CAS PubMed Google Scholar
Klatt EC, Namiki T, Noguchi TT. Misrepresentation of stimulant street drugs: a decade of experience in an analysis program. J Toxicol Clin Toxicol. 1986;24(5):441–50.
Article CAS PubMed Google Scholar
Marshman JA, Ontario AaDARFo. Street drug analysis and its social and clinical implications. Toronto: University of Toronto; 1974.
Sage C, Michelow W. Drug checking at music festivals: a how-to guide. ANKORS: Nelson, BC; 2016.
Standing Committee on Health. Interim report and recommendations on the opioid crisis in Canada. In: Vol. 42nd parliament, 1st session. Ottawa: House of Commons; 2016.
Nightlife Empowerment & Well-being Implementation Project: Drug checking service: good practice standards.
Drug checking and pill testing—what it can and cannot do and why it matters. https://www.globaldrugsurvey.com . Accessed 28 June 2017.
Brunt TM, Nagy C, Bücheli A, Martins D, Ugarte M, Beduwe C, Ventura Vilamala M. Drug testing in Europe: monitoring results of the Trans European Drug Information (TEDI) project. Drug Test Anal. 2017;9(2):188–98.
Tsai JSC, Lin GL. Drug-testing technologies and applications. In: Wong RC, Tse HY, editors. Drug-testing technologies and applications. Totowa: Humana Press; 2005. p. 29–69.
Cody RB, Laramee JA, Nilles JM, Durst HD. Direct analysis in real time (DART) mass spectrometry. JEOL News. 2005;40(1):8–12.
Forsgard N, Salehpour M, Possnert G. Accelerator mass spectrometry in the attomolar concentration range for 14 C-labeled biologically active compounds in complex matrixes. J Anal At Spectrom. 2010;25(1):74–8.
Article CAS Google Scholar
Manimala YS, Gautam S, Reddy BG. Mass spectrometry: an analytical method. J Pharm Anal. 2016;5(2):118–25.
Verkouteren JR, Staymates JL. Reliability of ion mobility spectrometry for qualitative analysis of complex, multicomponent illicit drug samples. Forensic Sci Int. 2011;206(1–3):190–6.
Sisco E, Verkouteren J, Staymates J, Lawrence J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry. Forensic Chemistry. 2017;4:108–15.
Article Google Scholar
Bunaciu AA, Aboul-Enein HY, Fleschin S. Application of Fourier transform infrared spectrophotometry in pharmaceutical drugs analysis. Appl Spectrosc Rev. 2010;45(3):206–19.
Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG): Supplemental document SD-2 for part IVB, quality assurance/validation of analytical methods. 2006.
Sorak D, Herberholz L, Iwascek S, Altinpinar S, Pfeifer F, Siesler HW. New developments and applications of handheld Raman, mid-infrared, and near-infrared spectrometers. Appl Spectrosc Rev. 2012;47(2):83–115.
de Oliveira Penido CAF, Pacheco MTT, Lednev IK, Silveira L. Raman spectroscopy in forensic analysis: identification of cocaine and other illegal drugs of abuse. J Raman Spectrosc. 2016;47(1):28–38.
Ali EMA, Edwards HGM. The detection of flunitrazepam in beverages using portable Raman spectroscopy. Drug Test Anal. 2017;9(2):256–9.
Hodges CM, Hendra PJ, Willis HA, Farley T. Fourier transform Raman spectroscopy of illicit drugs. J Raman Spectrosc. 1989;20(11):745–9.
Frederick KA, Pertaub R, Kam NWS. Identification of individual drug crystals on paper currency using Raman microspectroscopy. Spectrosc Lett. 2004;37(3):301–10.
Engh RA. X-ray crystallography: basic principles. In: Encyclopedic reference of genomics and proteomics in molecular medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 2026–9.
Chapter Google Scholar
Rendle DF. X-ray diffraction in forensic science. Rigaku J. 2003;19(2):11–22.
Trzybiński D, Niedziałkowski P, Ossowski T, Trynda A, Sikorski A. Single-crystal X-ray diffraction analysis of designer drugs: hydrochlorides of metaphedrone and pentedrone. Forensic Sci Int. 2013;232(1):e28–32.
Phadnis NV, Cavatur RK, Suryanarayanan R. Identification of drugs in pharmaceutical dosage forms by X-ray powder diffractometry. J Pharm Biomed Anal. 1997;15(7):929–43.
Elie MP, Elie LE. Microcrystalline tests in forensic drug analysis. In: Meyers RA, editor. Encyclopedia of analytical chemistry, vol. 1. Hoboken: Wiley; 2009. p. 471–81.
Cargill K, Kammrath BW. The identification of controlled substances by TLC-SERS. In: 66th Annual Scientific Meeting of the American Academy of Forensic Sciences. Seattle: Forensic Sciences Foundation; 2014.
Kanai K, Takekawa K, Kumamoto T, Ishikawa T, Ohmori T. Simultaneous analysis of six phenethylamine-type designer drugs by TLC, LC-MS, and GC-MS. Forensic Toxicol. 2008;26(1):6–12.
O’Neal CL, Crouch DJ, Fatah AA. Validation of twelve chemical spot tests for the detection of drugs of abuse. Forensic Sci Int. 2000;109(3):189–201.
Elkins KM, Weghorst AC, Quinn AA, Acharya S. Colour quantitation for chemical spot tests for a controlled substances presumptive test database. Drug Test Anal. 2017;9(2):306–10.
Dasgupta A. Clinical chemistry: analysis of drugs of abuse. In: Meyers RA, editor. Encyclopedia of analytical chemistry, vol. 2. Hoboken: Wiley; 2009. p. 1242.
Lysyshyn M, Dohoo C, Forsting S, Kerr T, McNeil R. Evaluation of a fentanyl drug checking program for clients of a supervised injection site, Vancouver, Canada. In: HR17 harm reduction conference. Montreal; 2017.
Li Q, Qiu T, Hao H, Zhou H, Wang T, Zhang Y, Li X, Huang G, Cheng J. Rapid and on-site analysis of illegal drugs on the nano-microscale using a deep ultraviolet–visible reflected optical fiber sensor. Analyst. 2012;137(7):1596–603.
Download references
Acknowledgements
Availability of data and materials, author information, authors and affiliations.
University of Lethbridge, 4401 University Drive, Lethbridge, AB, T1K 3M4, Canada
Lane Harper & Em M. Pijl
Carleton University, 1125 Colonel By Dr, Ottawa, ON, K1S 5B6, Canada
Jeff Powell
You can also search for this author in PubMed Google Scholar
Contributions
LH analyzed the extant literature, creating the basis for the paper. JP offered technical analysis and editorial support. EP worked with LH to make the text suitable to a non-technical audience. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Em M. Pijl .
Ethics declarations
Authors’ information.
LH holds a Bachelors of Engineering, majoring in Biomedical Engineering and minoring in Biotechnology obtained from the University of Guelph in 2016. He is currently enrolled in a second degree program and participating in research in Biochemistry at the University of Lethbridge. Lane is also interested in the politics of sensible drug policies and associated programs, including, but not limited to, the implementation of harm reduction best practices in Canada.
JP received a Bachelors of Science in Chemistry from Carleton University. He currently works on automation and sensing technology.
EP holds degrees in nursing and is an assistant professor in the Faculty of Health Sciences at the University of Lethbridge. EP has a clinical background in outreach nursing and harm reduction and conducts research and evaluation studies with local harm reduction agencies.
Ethics approval and consent to participate
Consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Harper, L., Powell, J. & Pijl, E.M. An overview of forensic drug testing methods and their suitability for harm reduction point-of-care services. Harm Reduct J 14 , 52 (2017). https://doi.org/10.1186/s12954-017-0179-5
Download citation
Received : 26 May 2017
Accepted : 24 July 2017
Published : 31 July 2017
DOI : https://doi.org/10.1186/s12954-017-0179-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Harm reduction
- Substance abuse
- Street drugs
- Drug overdose
- Drug effects
- Drug-related side effects and adverse reactions
- Drug evaluation
Harm Reduction Journal
ISSN: 1477-7517
- Submission enquiries: [email protected]
Eight Commonly Used Techniques for Drug Analysis
- Author Name: Helen Smith
Drug analysis refers to the detection and analysis of ingredients and contents in drugs. Drug analysis and testing involves the study of composition, physical and chemical properties, purity, and the determination of the content of active pharmaceutical ingredients and their preparations, to ensure that the medications are safe, rational, and effective.
Drug analysis and testing are very important for drug development, which generally encompasses disintegration test, dissolution test, tablet hardness test , tablet fragility test, residual solvents test, dosage units uniformity test, bioavailability/bioequivalence detection, microbial limits test, particulate matter test, elemental impurities analysis , and extractables & leachables test. To accomplish these analytical testing tasks, some drug analysis methods are employed. The most commonly used drug analysis methods are as follows:
- Gravimetric analysis
Gravimetric analysis is the basic method of chemical analysis in drug analysis and testing. It refers to weighing a certain weight of the sample and using an appropriate method to separate the tested component from the other components in the sample and convert it into a certain weighing form and determine the content of the component. According to the different sample preparation methods, gravimetric analysis is usually divided into physical gravimetry, thermogravimetry, precipitative gravimetric analysis, and electrodeposition. The advantage of gravimetric analysis lies in the high accuracy of the analysis results.
- Acid-base titration
The acid-base titration method is widely used in the analysis and testing of pharmaceuticals. Many industrial products such as caustic soda, soda ash, ammonium sulfate and ammonium bicarbonate generally use acid-base titration to determine the content of their main components. In addition, acid-base titration is also commonly used in the analysis of raw materials, intermediate products and finished products in the food industry.
- PH measurement
The pH indicator is an electronic potentiometer with high input impedance. The pH measurement method is included in the pharmacopeias of various countries. Unless otherwise specified, the pH value of the aqueous solution should be measured with an acidity meter that has a glass electrode as the indicator electrode and has a saturated calomel electrode as the reference electrode.
- Spectroscopy technology
The main principle of spectroscopy technology is that the drugs detected can be radiated through different frequencies. Vibration and rotation will occur when the frequency in a certain range is accepted by some substances. Through the recording of information such as wavelength, its spectrum will be obtained. Based on the spectrum, the actual structural form of the drug and the elements of the drug can be identified and analyzed, which has the advantages of faster detection speed, higher recognition, and high efficiency.
- Chemiluminescence technology
In drug analysis and detection, chemiluminescence is a relatively common technical method, which is mainly based on the principle that the concentration of the relevant detection substance in the chemical detection system and the chemiluminescence intensity of the system are linear and quantitative under specific conditions. The instrument detects the chemiluminescence intensity of the entire system, and the way to determine the actual content detected is a trace analysis method.
- Chromatography
Chromatography is a means of separation and analysis. The main chromatographic detection techniques include gas chromatography, liquid chromatography, and thin-layer chromatography. These techniques play an important role in the detection of food and drugs.
Thin-layer chromatography uses thin-layer analysis of solutions to ensure that it achieves the purpose of qualitative analysis and rapid separation of substances. This detection technology has high sensitivity and good efficiency in practice, and the entire detection test can be completed with a small amount of detection samples.
- Electrophoresis
Electrophoresis is one of the important methods for biologics analysis. It has the advantages of high sensitivity, good reproducibility, and wide detection range. Electrophoresis means that a charged sample (protein, nucleotide, etc. ) is placed in an inert support medium (such as paper, cellulose acetate, agarose gel, polyacrylamide gel, etc. ) and with the effect of an electric field, would move to the corresponding electrode direction at their respective speeds, thus different components are separated into narrow zones. The last step is to use appropriate detection methods to record their electrophoretic maps or calculate their content.
- DNA amplification method
The DNA amplification technology belongs to the broader category of PCR technology, which can amplify the fragments of the DNA sample in the tube up to a million times, enabling the researchers to directly observe by naked eyes. DNA amplification technology is featured by sensitivity, specificity, convenience, and efficiency.
To ensure the quality of medicines, analytical testing should be carried out in strict accordance with the quality standards of medicines before deciding whether medicines can be marketed and used. Formulationbio has a cGMP-compliant laboratory that is equipped with state-of-the-art analytical instruments and can handle complex drug analytical challenges. Meanwhile, it can also provide insightful suggestions for pharmaceuticals to produce safe medicines.
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
https://www.nist.gov/programs-projects/drug-analysis-opioids-and-emerging-threats
Drug Analysis - Opioids and Emerging Threats
The goal of this focus area is to enhance drug detection capabilities through the development of standard methods for the collection, analysis, and interpretation of drug evidence. Many of these efforts focus on synthetic opioids and other novel psychoactive substances (NPSs) because of the unique analytical challenges they present. The projects cover a broad range of needs of end-users in both the field (first responders, law enforcement officers, crime scene technicians, etc.) and in the laboratory. Projects in these areas focus on package interdiction, in-field detection and screening, and laboratory forensic drug analysis.
Description
Package interdiction.
Many synthetic opioids and novel psychoactive substances enter the country through international mail. Research in this area focuses on measurement challenges associated with detection and analysis of these packages at the point of seizure in international mail facilities. Ongoing efforts in this area include:
Measuring Trace Drug Background in Package Interdiction Environments
This project aims to measure the trace drug background in operational environments for package interdiction scenarios. Through wipe collections, samples in operational environments are taken and used to obtain the quantitative and qualitative drug make-up. This information is then used to better address a range of questions from data quality to personnel health and safety risks to considerations for implementing detection technologies.
Measuring and Understanding the Nature of Trace Drug Residue on Packages
This project aims to establish the likelihood and circumstances under which trace drug residues are present on packages containing illicit substances. In collaboration with Department of Homeland Security counterparts, packages suspected of containing illicit substances are sampled and then analyzed to obtain quantitative values for a range of drugs of interest as well as qualitative preliminary identification of a large range of drugs. The data is then compared to results obtained from analysis of the bulk material, to understand what correlations exist. The project also aims to identify where on packages trace residues would be most likely encountered.
Measuring the Persistence of Trace Drug Residues
Achieving successful trace detection of drug residues requires that drug particulate is released from a source and settles on the surface of interest and that the residue is then able to persist in the environment until it is collected and analyzed. While other projects aim to address the question of whether trace particulate is being released and settling on surfaces, this project aims to address the question of environmental persistence. To measure persistence, trace amounts of drugs are exposed to a range of simulated environmental conditions for extended periods of time. Samples are then analyzed using mass spectrometric techniques to both quantify the amount of drug present and to understand the degradation processes. The project is investigating both novel and traditional drugs of abuse.
In-Field Detection and Screening
Detection and analysis of illicit drugs in the field continues to present a number of analytical challenges due to the high potency of these compounds coupled with changing chemical structures and the potential of complex mixtures. Research in this area focuses on investigating, developing, and understanding the role of existing and novel detection technologies for in-field screening. Efforts in this area include:
Understanding the Capabilities of New and Existing Technologies
Continuing efforts to combat the opioid crisis require technologies that can detect the presence of fentanyl and fentanyl-related substances in a range of applications. This project aims to develop methods for understanding the strengths and limitations of trace detection techniques used in synthetic opioid analysis. This project has studied a range of different technologies to understand how identification of multiple fentanyl analogs, the presence of cutting agents, and the presence of background interferences can affect results. One example, highlighted below, shows how cutting agents can have either deleterious or beneficial effects on fentanyl detection.

Relevant Publications:
- Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory-based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry ( https://doi.org/10.1016/j.forc.2017.04.001 )
- Method for evaluating ion mobility spectrometers for trace detection of fentanyl and fentanyl-related substances ( https://doi.org/10.1039/C9AY02174D )
- Evaluation of a lateral flow immunoassay for the detection of the synthetic opioid fentanyl ( https://doi.org/10.1016/j.forsciint.2019.04.019 )
- Separation and detection of trace fentanyl from complex mixtures using gradient elution moving boundary electrophoresis ( https://doi.org/10.1021/acs.analchem.9b03083 )
Understanding the Characteristics of Drug Evidence
Many of the technologies used for in-field screening rely on trace drug residues to be present on a surface, which are collected and analyzed. However, little research exists on the prevalence and nature of these residues. This project aims to better understand the characteristics of trace drug residues on drug evidence. In collaboration with multiple practicing forensic laboratories, seized items from interdictions are sampled and subsequently analyzed to understand the qualitative and quantitative nature of the residue on drug evidence. Using ground truth information from analysis of the bulk material, the correlation of the drug evidence residue and contents can be understood. The analytical approach for this project is shown below.

Relevant Publications
- What’s in the bag? Analysis of exterior drug packaging by TD-DART-MS to predict the contents ( https://doi.org/10.1016/j.forsciint.2019.109939 )
Laboratory Forensic Drug Analysis
Laboratory analysis of drug evidence is a critical component of all forensic analyses. Drug chemists are faced with a unique set of challenges and are required to balance safety and exposure probabilities with case backlog issues and a rapidly changing drug landscape. Projects focus on the challenges faced by drug chemists in analytical measurement and operations. Efforts in this area include:
Understanding Drug Background Levels and Their Contributing Processes
As drugs become increasingly potent and analytical instrumentation becomes more sensitive, the need to understand and monitor drug background levels becomes imperative. Background monitoring is commonplace in many other industries but has been largely overlooked in forensic laboratories. This project represents a multi-agency collaboration focused on establishing drug background levels in forensic laboratories and in understanding the implications for data quality, data integrity, and occupational health and safety.
Efforts in this project include conducting drug background studies in forensic laboratories to address questions arising from: processes that contribute to drug background; ensuring data quality; developing ways to reduce background; and strategies for self-monitoring. The foundational study for this work measured over 700 background wipes from 20 laboratories across the country, thereby providing insight into the level of background found on different surfaces and sections within the laboratory. A summary of this study is shown below.

Other efforts include visualizing and quantifying the role that different processes have on background contribution – processes such as net weights, crushing pills, and opening evidence, an example of which is shown below. Another important component of this work is understanding the potential occupational health and safety impact on the analyst.

- A snapshot of drug background levels on surfaces in a forensic laboratory ( https://doi.org/10.1016/j.forc.2018.09.001 )
- A Multi-Laboratory Investigation of Drug Background Levels ( https://doi.org/10.1016/j.forc.2019.100184 )
- An easy to implement approach for laboratories to visualize particle spread during the handling and analysis of drug evidence ( https://doi.org/10.1016/j.forc.2020.100232 )
- Net Weights: Visualizing and Quantifying their Contribution to Drug Background Levels in Forensic Laboratories ( https://doi.org/10.1016/j.forc.2020.100259 )
- Webinar: Understanding the impact of drug background levels in forensic laboratories ( https://nij.ojp.gov/events/understanding-impact-drug-background-levels-… )
Development of a Novel Workflow for Forensic Drug Analysis
This project aims to develop a re-envisioned workflow for traditional drug analysis that provides higher fidelity information in a more rapid manner while also increasing the safety of the forensic chemist. Working with the Maryland State Police Forensic Sciences Divisions, the new workflow aims to eliminate colorimetric tests and GC-FID analyses with DART-MS analysis while also employing the use of targeted and batched GC-MS analyses. The utilization of DART-MS search software and GC-MS deconvolution software along with retention time locking and retention indices aims to increase the fidelity of the case results. Under this project other process, such as taking net weights, are also being evaluated and safer methods are being developed.
Tools for DART-MS Analysis of Forensic Samples
This project aims to provide the community with increased tools and resources for the implementation of DART-MS technology through the development of updated and freely available spectral libraries and search tools. This project will also allow for the development and testing of automated curation methods which may allow for more rapid updating of the library to be completed. The initial focus is on providing a spectral library of novel psychoactive substances and cutting agents. Concurrently, a new search tool is being developed that more heavily utilizes fragmentation data to provide users with increased confidence in presumptive compound identification.
Rapid Dissemination of Emerging Drug Samples to Local, State, and Federal Forensic Laboratories
With the continually changing emerging drug landscape, a major need for forensic drug chemists is improved access to standards in a timely and cost-effective manner. When new substances are encountered in casework, drug chemists either cannot identify the substance or they must wait months for a commercially available drug standard. This not only delays the issuance of laboratory reports for prosecution but also prevents relevant information from being given to public health officials and drug task forces to prevent overdoses. This project aims to address one of the main bottlenecks in identification of novel drugs in forensic samples by developing a platform to rapidly provide characterized samples of emerging drugs to forensic laboratories at no cost. The single use samples will allow for laboratories to measure these emerging drugs on their instrumentation, compare their data to curated spectra, and identify their presence in casework to better inform government and public health officials.
Ambient Ionization Mass Spectrometry Tools for Toxin Detection
Detection of toxins is a need for forensic chemists when poisonings or suspected poisonings occur. These toxins can include seed-based toxins (such as scopolamine), toxic industrial chemicals, or other compounds such as rodenticides (anti-coagulating agents). This project aims to investigate potential approaches for rapid detection of toxins using the chemical signatures obtained by either atmospheric solid analysis probe mass spectrometry (ASAP-MS) or direct analysis in real time mass spectrometry (DART-MS) in combination with chemometric analyses.
Detection of Brodifacoum and other Rodenticides in Drug Mixtures using Thermal Desorption Direct Analysis in Real Time Mass Spectrometry (TD ‐ DART ‐ MS) ( https://doi.org/10.1111/1556-4029.13978 )
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: current analytical trends in drug testing in clinical and forensic toxicology.

- 1 Health Sciences Research Center, Faculty of Health Sciences, University of Beira Interior, Covilhã, Portugal
- 2 Instituto Nacional de Medicina Legal e Ciências Forenses (INMLCF), Lisbon, Portugal
- 3 John Jay College of Criminal Justice, New York, NY, United States
- 4 Institute of Forensic Sciences “Luis Concheiro”, University of Santiago de Compostela, Santiago de Compostela, Spain
Editorial on the Research Topic Current Analytical Trends in Drug Testing in Clinical and Forensic Toxicology
The articles included in this collection cover novel analytical approaches, including chromatographic and spectrometric methods, and sample preparation techniques for the investigation and analysis of several classes of compounds. These compounds include novel psychoactive substances (NPS) as well as other drugs and substances within the scope of clinical and forensic toxicology, and other fields, such as doping control.
Current trends in bioanalysis require the constant development of novel analytical tools, which includes efficient sample collection procedures and adequate sample preparation protocols in order to maximize compound detection, even at trace levels. Taking into account that the number of substances possibly present in a sample are increasing, efficient multi-analyte methods are usually necessary. The detection of NPS, including synthetic cathinones and synthetic cannabinoids, is becoming more and more important as several reports of acute intoxications and deaths are often being issued. Therefore, developing new analytical methods and strategies help scientists efficiently face those challenges, allowing laboratories to be one-step ahead.
In this topic collection, four publications focus on the investigation of different critical aspects of NPS. These studies provide new tools for the identification of new NPS derivatives and metabolites ( Frison et al. ; Lopes et al. ), investigate the stability of synthetic cathinones in biological samples and storage solvents ( Ciallella et al. ) or explore the detectability of synthetic cannabinoids in hair samples ( Shi et al. ). Frison et al. described the analytical characterization, following two non-fatal intoxication cases, of 3-methylmethcathinone (3-MMC) and 3-methoxyphencyclidine (3-MeO-PCP) in seized products, and the investigation of 3-MeO-PCP and metabolites in biological samples. Three different analytical approaches were employed to identify 3-MMC and 3-MeO-PCP in seized materials, including gas chromatography-mass spectrometry (GC-MS) with electron impact ionization, liquid chromatography-high-resolution accurate-mass Orbitrap mass spectrometry (LC-HRAM-Orbitrap-MS), and solid deposition gas chromatography-Fourier transform infrared spectroscopy (sd-GC-FTIR). The role of the two latter techniques in attaining full structural characterization of the psychoactive drugs and related metabolites, in both non-biological and biological samples, was highlighted. The novelty of Frison et al. work lies in this aspect of the employment of LC-HRAM-Orbitrap-MS and sd-GC-FTIR instrumentation to identify and characterize new psychoactive substances in the absence of reference standards in different types of samples. Lopes et al. also employed high resolution mass spectrometry (HRMS) for the identification of new metabolites of synthetic cathinones. In particular, they identified the phase I and II metabolites of 4'-methyl-N,N-dimethylcathinone (4-MDMC), 4'-methyl-N,N-diethylcathinone (4-MDEC), 4'-chloro-α-pyrrolidinovalerophenone (4Cl-PVP) and 4'-chloroethylcathinone (4-CEC). The metabolites herein identified are expected to play an important role not only because they act as potential selective biomarkers of the intake of the studied synthetic cathinones, but also because their potential adverse effects may be better understood. In addition, those causative agents may be linked to toxicities, thereby helping understanding and treating non-fatal intoxications. This study highlights the critical role of high resolution mass spectrometry in the investigation of the toxicity of NPS. Ciallella et al. studied the stability of four Schedule I synthetic cathinones, namely mephedrone, naphyrone, MDPV, and α-PVP. Indeed, stability is a critical parameter for toxicology laboratories. Understanding the variability in the analyte concentrations due to stability issues has an impact in the subsequent interpretation of concentration data derived from biological sample analysis. In this research, Ciallella et al. were able to analyze these cathinone derivatives employing solid phase extraction of blood and urine samples, and analyzing the compounds by GC-MS. The results of this study provided a comprehensive overview of the stability of these compounds in biological matrices over an extended period, including the evaluation of an alternative preservative and the inclusion of solvent-based working solutions. Shi et al. developed and validated a novel target analytical method for the determination of the synthetic cannabinoid 5F-MDMB-PICA and five metabolites in hair samples by liquid chromatography-tandem mass spectrometry (LC-MSMS). This new synthetic cannabinoid has been used in the form of “spice-like” herbal incenses or in electronic cigarette oil, and this study provides critical data for the interpretation of hair testing for this type of substances. The sensitivity of LC-MSMS allowed the authors of this study to achieve limits of detection at low pg/mg level in hair.
This topic collection also covers new challenges and strategies of analytical methods. Jurásek et al. investigated the potential of X-ray powder diffraction (XRPD) for rapid and simple identification of drugs of abuse in seized material. In this work, the authors proved that XRPD could be used to unambiguously identify 7 selected psychoactive substances (including 5 NPS) in different street sample mixtures, and proposed this technique as a complement to Infrared and Raman spectroscopies, the most common techniques used for this purpose, when unequivocal drug identification with these techniques is hindered by drug or additives native fluorescence or matrix complexity. Joye, Widmer et al. used matrix-assisted laser desorption/ionization (MALDI) high-resolution mass spectrometric (HRMS) technologies, which have been used to analyze the samples seized in the black market. The authors highlight the potential of MALDI-HRMS as high-throughput analytical strategy in toxicology laboratories, which significantly accelerates the detection and quantification of several drugs of abuse. The developed approach showed qualitative and quantitative results comparable to those obtained using LC-MS and GC-MS, reducing the analytical procedure by six times. With the development of bioinformatics tools and shared online libraries, new drugs of abuse that appear in the markets are easily identified and determined. In a second manuscript, Joye, Rocher et al. also used liquid chromatography hyphenated with Orbitrap high-resolution mass spectrometry with parallel reaction monitoring (PRM) for the quantification of the major classes of psychoactive substances present in the context of driving under the influence of drugs (DUID), such as cannabinoids, cocaine and its metabolites, amphetamines, opiates and opioids, and the major benzodiazepines and z-drugs, achieving the required sensitivity for DUID cases using a sample amount as low as 0.1 mL of whole blood. In addition to high resolution, Orbitrap-based PRM acquires all the selected precursor ions, avoiding a priori knowledge of the fragments of interest for method development, which represents an advantage over classical multiple reaction monitoring (MRM). Habib et al. reviewed the strategies for chemical analysis of drugs of abuse and explosives, using mass spectrometry-based approaches. Several new ionization sources were revisited and the mechanisms of ion formation following their use were addressed for illicit drugs and explosives. The authors concluded presenting the main challenges that the future holds regarding the analysis of non-volatile compounds in what concerns ionization procedures.
Greener sample preparation techniques, like hollow fiber liquid-phase microextraction, are also presented in this collection, namely by de Oliveira Silveira et al. , to determine the main markers of Ayahuasca consumption in urine specimens. This alternative and eco-friendly sample preparation approach was fully validated, showing excellent limits of detection and quantification (1-5 ng/mL), reproducibility, reduced matrix effect interferences, and outstanding recoveries (above 80%).
Finally, new approaches to determine drug use via wastewater analysis were reviewed by Zilles Hahn et al. The authors addressed new insights about wastewater-based epidemiology (WBE) as a useful tool to detect in real time illicit drug use by a population. Also, the most important biomarkers of drugs of abuse consumption in wastewater and the fundamentals of polar organic chemical integrative sampling (POCIS) in WBE were discussed and compared with other strategies.
In summary, this collection covers Research Topics representative of the recent trends and advances in drug testing and new compound identification in biological specimens, with focus on the development of novel analytical approaches, new chromatographic and spectrometric techniques, and sample preparation procedures, including miniaturized and environmentally friendly methodologies.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Keywords: drugs of abuse, chromatographic techniques, drug testing, clinical and forensic toxicology, trends in bioanalysis, novel analytical approaches, new psychoactive substance
Citation: Gallardo E, Barroso M, Concheiro-Guisan M and de-Castro-Ríos A (2021) Editorial: Current Analytical Trends in Drug Testing in Clinical and Forensic Toxicology. Front. Chem. 9:673397. doi: 10.3389/fchem.2021.673397
Received: 27 February 2021; Accepted: 14 April 2021; Published: 07 May 2021.
Edited and reviewed by: Huangxian Ju , Nanjing University, China
Copyright © 2021 Gallardo, Barroso, Concheiro-Guisan and de-Castro-Ríos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eugenia Gallardo, egallardo@fcsaude.ubi.pt ; Mário Barroso, mario.j.barroso@inmlcf.mj.pt ; Marta Concheiro-Guisan, mconcheiro-guisan@jjay.cuny.edu ; Ana de-Castro-Ríos, ana.decastro@usc.es
This article is part of the Research Topic
Current Analytical Trends in Drug Testing in Clinical and Forensic Toxicology

3 Drug Analysis
Introduction.
Identify unknown drugs in powder and pill form using presumptive and confirmatory techniques.
Drug Identification
Analysis of controlled and uncontrolled substances is often fairly routine. After a visual assessment and measuring the mass of the exhibit, we will typically perform presumptive testing then use a different technique to confirm the identity of an unknown. While some labs or specialists may need to quantitatively characterize the various components of an unknown drug, we’ll focus on simple identification since that is the most routine task required.
Explanations and examples of presumptive and confirmatory testing are provided below. Regardless of the specific methods used in our analytical scheme, both analyses must yield results that agree with one another in order to confirm the identity of an unknown substance. There are some possible exceptions to this sequence of events, though. One example of an exception is non-controlled pharmaceutical preparations, which is also explained below.
Presumptive Testing
Presumptive testing is a quick, cheap, useful tool that can help inform future analysis and often serves as 1/2 of the concurrent positives required in most drug identifications. A common method to perform this testing is by using one or more reagents that are expected to change color when exposed to certain functional groups. These color test reagents are usually quite simple and vary in reactivity – they may only change one specific color in response to one specific type of drug or they may exhibit a range of colors in response to a wide variety of drugs.
The Marquis reagent is an example of the latter. A simple formulation of sulfuric acid and formaldehyde technically made for detection of alkaloids, the Marquis reagent may turn shades of orange, brown, red, green, or yellow in the presence of a variety of amphetamines, opiates, and other drugs. While lacking in specificity, the results can often be used in conjunction with other tests or with confirmatory results to reinforce identification of these substances.
Controls should always be used with color tests. A known positive should be used to generate the expected reaction and ensure no false negatives (positive control) . A known negative, or blank, should be used to ensure a reaction does not occur when the target is not present and ensure no false positives (negative control) .
Confirmatory Testing
Confirmatory testing should then typically be performed on presumptive positives. Because confirmatory testing requires expensive instrumentation and personnel, it is helpful to have some direction to save time and money (also – we need two positives most of the time anyways, so confirmatory testing may not be useful without another positive). The gold standard for confirmatory drug testing is usually gas chromatography-mass spectrometry (GC-MS), or, as an alternative, Fourier transform infrared spectroscopy (FTIR).
GC-MS analysis will generate a chromatogram (signal vs. time) and a mass spectrum (signal vs. mass-to-charge ratio, m/z ). The chromatogram will show us the retention time (RT) – how long each compound takes to elute , or travel through the column. The mass spectrum will show us the masses of compound fragments found at each retention time. Together, this information makes GC-MS a very powerful discriminatory tool.
Sample Preparation
Sample preparation can vary depending on the physical form of the drug. For normal GC-MS testing, we need our drug to be dissolved in a volatile organic solvent. So, if the drug is an acidic salt, we need to do a basic liquid-liquid extraction (bicarbonate solution + chloroform, e.g.) to analyze the free-base version in the organic layer via GC-MS. Some labs will perform this extraction on most powders that come in – it saves time when we don’t know what it is, and drugs that are already soluble in the organic layer will still be there. Otherwise, presumptive tests or FTIR analysis can help inform the preparation process. Liquids, plants, and other forms of drugs have other sample prep considerations but we’ll only be looking at powders and pills in this class.
Prior to analysis of unknowns, a control sample must be analyzed using the same instrument method you intend to use on your unknown. This control sample is a mixture containing standards of some or all of the drugs for which that assay is designed and an internal standard (IS) . A blank should also be run to ensure there are no false positives resulting from instrumental/method issues or analyte carryover from a previous run.
The internal standard is a compound that can also be added to our unknown mixture and is ideally structurally similar to our analytes. Internal standards can serve a couple of purposes. First, they are used to confirm the instrument and method are working appropriately for that run – this way, if no other compounds appear in our unknown data, we know the drugs we’re testing for are not present. Second, an internal standard can be used to obtain relative retention times (RT of drugs relative to that of IS to account for acceptable variations).
For a GC-MS analysis to be confirmatory of a drug identification, there are some parameters that must be met:
- If this is not the case, the entire run is generally void
- RT of unknown must be within a given tolerance of the control RT
- Number of peaks required can vary from drug to drug
- Relative intensity of each m/z should also be within a given tolerance compared to control
- No major unidentifiable m/z peaks
Legally Manufactured Pills
Sometimes, legally manufactured, non-controlled drugs are recovered by authorities. Whether or not they were legally possessed, we still often need to identify them. If there are tablets with a pharmaceutical identifier on them, we can often consult reference materials for a presumptive identification and then confirm with FTIR or GC-MS. If you have access to an attenuated total reflectance (ATR) FTIR, you can confirm by placing a tablet directly on the ATR crystal, though grinding into a powder will usually yield a more intense signal. Capsules that can be broken open always need to be emptied prior to confirmatory testing.
If the FTIR results do not support the ID from the pharmaceutical identifiers, GC-MS analysis should generally be performed to confirm the FTIR results.
Materials and Methods
Supplies (part 1).
- internet access
- camera (phone is acceptable)
- FTIR or ATR-FTIR
Procedure (Part 1)
Pills – visual identification.
RxList maintains a free pill lookup resource that allows you to enter markings, shape, and color of pills. If it is a legally manufactured pill, it’s pretty reliable for returning accurate results. The DEA maintains its own Logo Index with over 30000 pills. There is also now software labs can buy to enter photos of pills and automate the identification process.
- Take a close-up photo of any pill evidence you’ve been given
- Enter what information you have into the identifier at https://www.rxlist.com/pill-identification-tool/article.htm
Pills – Analytical Confirmation
To confirm the identity of the pills, we can use FTIR and compare to known results for the same pills.
- Obtain a blank spectrum
- Then, ground the tablet and analyze again to compare
- For capsules, break open and analyze the contents
- Compare to reference spectra
- Take note of parameters and obtain CSVs of your data for your report
- If results do not confirm your ID from the pharmaceutical identifiers, you’ll perform an extraction later to analyze via GC-MS
Supplies (Part 2)
- Components for Marquis Reagent
- Storage bottle
- graduated cylinder
- Testing tray
- Disposable pipette
Procedure (Part 2)
Make your own marquis reagent.
Marquis Reagent is made with concentrated (95–98%) sulfuric acid and 40% formaldehyde. You’ll use it to perform colorimetric tests on a variety of samples provided.
- Add 1 mL of formaldehyde to your storage bottle
- Carefully add 20 mL sulfuric acid to the formaldehyde
- You may adjust the total volume with the same ratio if needed (5 mL + 100 mL, e.g.)
Use Your Marquis Reagent
- Place a small amount of each sample into individual wells of your sample tray
- Label each sample with a marker and note your labels in your notebook
- Carefully add a few drops of Marquis Reagent to each well
- Color changes should happen immediately
- Consult a color reference chart like that provided in class to try to presumptively identify each unknown
Supplies (Part 3)
- Control sample with standard mix (TA provided)
- GC sample vials
- Pills from Part 1, if additional analysis needed
- Internal standard solution (details indicated on board)
- Suitable acids may include: hydrochloric (concentrated or diluted) and acetic (concentrated or diluted)
- Suitable bases may include: sodium hydroxide, sodium bicarbonate, and ammonium hydroxide
- Suitable organic solvents may include: acetone, ethyl ether, chloroform, heptane, hexane, methanol, methylene chloride, isopropanol
- Test tubes or other vessels suitable for liquid-liquid extraction
Procedure (Part 3)
In order to analyze your unknown drugs via GC-MS, you’ll first need to get them into a volatile organic solvent. The type of sample (physical form, acid/base properties, etc.) and your instrumental methodology will determine how to best go about doing this. There are a variety of methods including dry solvent extractions, solvent washing, reconstitution, etc., but we will perform only simple acid/base extractions in this exercise.
General Acid/Base and Solubility Reminders
- We need the unionized form of our drugs, because unionized drugs tend to easily dissolve non-polar organic solvent.
- pKa values can tell us the pH at which 50% of the drug will be ionized, but not whether it behaves as an acid or a base.
- Acidic functional groups most commonly found in drugs are carboxylic acids and phenols .
- Basic functional groups most commonly found in drugs are amines (nitrogen lone pair must be available for interaction with protons).
- Acidic drugs will be ionized at higher pH (and thus more aqueous soluble) and in their free acid form at lower pH (and thus more non-polar soluble).
- Basic drugs will be ionized at lower pH (and thus more aqueous soluble) and in their free base form at higher pH (and thus more non-polar soluble).
- The organic solvent you intend to inject should be immiscible with your acid/base solution.
- Refer to the densities of your solvents to determine if your organic layer will be on top or bottom.
- Place ~1 mg of the unknown in the test tube
- Note: drugs within a given class (opiates, amphetamines, etc.) usually tend to exhibit similar acid/base properties
- Alternately – you can do both acid and base extractions in separate vessels and combine the organic layers (if same solvent used), though this could potentially result in over-dilution in some cases
- Add ~1 mL of the appropriate acid/base solution
- Add ~1 mL of organic solvent
- Mix gently and allow layers to separate
- Transfer the the organic layer to the GC vial
- Dilute as instructed if needed
- Determine how much internal standard solution you need to add for the desired final concentration indicated on the board and add it
GC-MS Analysis
Your control mix will be pre-prepared and the contents detailed on the board. Your instrumental method will be pre-prepared as well, but you should make sure to note the details of the GC and MS settings provided by your TAs for your methods section.
- Run the control mix
- Review RTs and fragments
- may be omitted at TA discretion
- Run unknown samples
- Unless instructed otherwise
- Look over RTs and fragments for unknowns
- Obtain CSVs of chromatograms and spectra to plot for your report
Supplies (Part 4)
- Unknown samples
FTIR of unknowns
We may also be interested in using FTIR to confirm the identity of unknown drugs.
- Obtain blank spectrum
- Analyze unknowns
- Include photos of all samples
- Be sure pharmaceutical identifiers are as clear as possible
- Use CSVs or other raw data to plot your data in Excel or similar
- Include reference spectra and spectra for your unknown samples with explanation of stretches
Forensic Chemistry Laboratory Manual by University of North Texas is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book
share this!
April 4, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
New method reveals secrets of protein interactions with potential for drug discovery
by University of Oulu
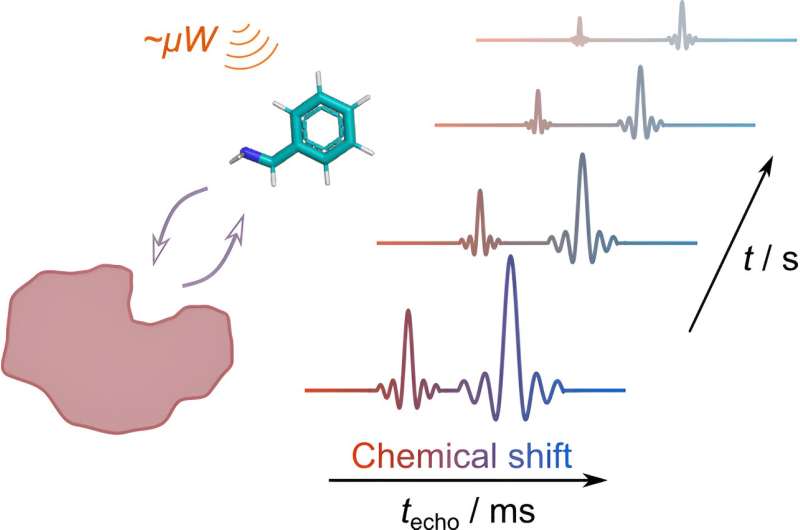
Scientists from the University of Oulu (Finland) and Texas A&M University (U.S.), have developed a new method to study how proteins interact with small ligand molecules, paving the way, for example, for faster and more efficient drug discovery.
This interaction, known as protein–ligand interaction, is crucial for many biological processes, but studying it has traditionally been slow and insensitive. The new method , described in the Journal of the American Chemical Society , combines two advanced techniques to overcome these limitations.
A method has the potential to revolutionize our understanding of protein interactions as part of cells' continuous communication. These interactions and the disruptions that may occur can play a significant role for example in the development of autoimmune diseases and neurodegenerative diseases such as Alzheimer's Disease. For instance, dysfunctional interactions can also lead to aggressive cell growth and cancer.
"The method we have developed could significantly speed up the development of new drugs and help us to understand the mechanisms of many diseases much better," says Dr. Otto Mankinen from the NMR Research Unit, University of Oulu.

Fast and detailed analysis
The first technique, Dissolution Dynamic Nuclear Polarization (d-DNP) hyperpolarization, acts like a signal amplifier, significantly enhancing the signal of the ligand molecule under investigation. Especially when studying low amounts of substances and low abundance nuclei such as carbon-13, hyperpolarization is a crucial tool to make signal observable.
The second technique, Ultrafast NMR, allows the use of hyperpolarization in measurement of multidimensional NMR data. Conventionally measured multidimensional NMR measurements require multiple repetitions to collect full data.
In the ultrafast approach, one of the dimensions is encoded along the sample volume in layers, with a method called spatial encoding. After the encoding, the information is read with the principles of Magnetic Resonance Imaging (MRI). In this case the NMR spectrum was spatially encoded and then the attenuation of signal in time was monitored for multiple spectral peaks.
By combining these techniques, researchers can now obtain detailed information about protein–ligand binding in a single experiment for multiple ligand signals. Conventional approach is limited to a single signal per measurement. This opens doors for more efficient drug discovery by allowing scientists to better understand how potential drug molecules interact with their protein targets.
Journal information: Journal of the American Chemical Society
Provided by University of Oulu
Explore further
Feedback to editors

What do scientists hope to learn from total solar eclipse in US?
6 hours ago

Rare Javan rhino calf spotted in Indonesia
7 hours ago
Scientists investigate information propagation in interacting bosonic systems
13 hours ago

DESI first-year data delivers unprecedented measurements of expanding universe
Apr 6, 2024

Saturday Citations: AI and the prisoner's dilemma; stellar cannibalism; evidence that EVs reduce atmospheric CO₂

Huge star explosion to appear in sky in once-in-a-lifetime event
Innovative sensing platform unlocks ultrahigh sensitivity in conventional sensors
Nonvolatile quantum memory: Discovery points path to flash-like memory for storing qubits

Can language models read the genome? This one decoded mRNA to make better vaccines
A simple, inexpensive way to make carbon atoms bind together
Relevant physicsforums posts, zirconium versus zirconium carbide for use with galinstan.
Mar 29, 2024
Electrolysis: Dark blue oxide from steel?
Mar 28, 2024
Identification of HOMO/LUMO in radicals
Mar 27, 2024
Quantum hybridized orbitals
New insight into the chemistry of solvents.
Mar 23, 2024
Is G10 material dangerous?
More from Chemistry
Related Stories

An upgrade for magnetic resonance methods with a 1,000-fold amplifier
Aug 9, 2022
Magnetic resonance makes the invisible visible
May 17, 2022

Novel ultrafast nuclear magnetic resonance method for investigating molecular exchange
Sep 17, 2020

New synapse type discovered through spatial proteomics

New carbon nanotube transistor enhances sensitivity and resolution of molecule glasses
Novel technology allows comprehensive analysis of membrane protein extracellular interactions
Feb 22, 2024
Recommended for you

Scientists harness chemical dynamics for complex problem solving
Apr 5, 2024

Chemical reactions can scramble quantum information as well as black holes

Research team creates a chemistry map for human cells

Rapid, simultaneous detection of multiple bacteria achieved with handheld sensor
Click chemistry: Research team creates 150 new compounds
Apr 4, 2024
Operando spectroscopy provides a window on water oxidation
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Telling the story of the opioid crisis: A narrative analysis of the TV series Dopesick
Roles Data curation, Investigation, Writing – original draft, Writing – review & editing
Affiliations Faculty of Medicine, Universitat de Vic–Universitat Central de Catalunya, Vic, Spain, Observatory of Humanities in Medicine, Hospital d’Olot i Comarcal de la Garrotxa Foundation, Olot, Spain
Roles Conceptualization, Supervision, Writing – review & editing
Affiliation Faculty of Medicine, Universitat de Vic–Universitat Central de Catalunya, Vic, Spain
Roles Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Faculty of Medicine, Universitat de Vic–Universitat Central de Catalunya, Vic, Spain, Research group on Methodology, Methods, Models and Outcomes of Health and Social Sciences (M3O), Faculty of Health Sciences and Welfare, Center for Health and Social Care Research (CESS), Universitat de Vic–Universitat Central de Catalunya, Vic, Spain, Institute for Research and Innovation in Life Sciences and Health in Central Catalonia (IRIS-CC), Vic, Spain
- Joel Piqué-Buisan,
- Josep-E Baños,
- Irene Cambra-Badii

- Published: April 4, 2024
- https://doi.org/10.1371/journal.pone.0301681
- Reader Comments
Dopesick (2021) is the first TV series whose plot deals exclusively with the opioid crisis in the United States. The current study uses narrative analysis and framing theory to explore this series, discussing its portrayal of the people and themes involved in the opioid crisis. Our analysis found that although Dopesick attempts to portray multiple dimensions of the opioid crisis, its narrative oversimplifies the story in attributing the cause of the problem almost exclusively to Purdue Pharma and its director Richard Sackler, while downplaying other factors that contributed to the opioid crisis. Thus, the narrative in this TV series tends to offer simple explanations to a complex problem for which simple solutions are likely to be inadequate.
Citation: Piqué-Buisan J, Baños J-E, Cambra-Badii I (2024) Telling the story of the opioid crisis: A narrative analysis of the TV series Dopesick . PLoS ONE 19(4): e0301681. https://doi.org/10.1371/journal.pone.0301681
Editor: Sorin Adam Matei, Purdue University, UNITED STATES
Received: June 5, 2023; Accepted: March 20, 2024; Published: April 4, 2024
Copyright: © 2024 Piqué-Buisan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The opioid crisis is one of the most severe public health crises in recent U.S. history [ 1 , 2 ]; it was declared a public health emergency in 2017 [ 3 ]. The latest World Drug Report [ 4 ] highlights the importance of opioids in illegal drug use. In 2020 alone, overdoses of opioids resulted in 68,630 deaths in the U.S., accounting for 74.8% of all drug overdose deaths [ 5 ].
Aiming to improve pain management and alleviate pain-associated suffering, physicians started to prescribe opioids more often in the 1990s [ 6 – 8 ]. However, strong opioid analgesics were sometimes used to treat patients who did not actually need them [ 6 ]. Overprescribing is but one factor among many that contributed to the opioid crisis, which developed through a complex network of agents, including patients, doctors, drug companies, and even the healthcare system and government drug regulatory offices [ 1 , 2 , 9 – 11 ].
The complexity of the opioid crisis and the multiple interests and viewpoints involved result in “opioid storytelling” from a wide variety of different perspectives [ 12 ]. Journalistic approaches portrayed the opioid crisis in books such as Pain killer : an empire of deceit and the origin of America’s opioid epidemic [ 13 ], Dopesick : dealers , doctors and the drug company that addicted America [ 14 ], and Empire of pain : the secret history of the Sackler dynasty [ 15 ].
Likewise, in recent years several audiovisual documentaries have also focused on the opioid crisis, and fiction series, especially medical dramas, have also included opioid addiction in some episodes [ 16 – 23 ]. However, portrayals of the opioid crisis in fiction series have been limited to a single dimension such opioid use disorder and the difficulties of recovery, pharmaceutical companies’ responsibilities, or increased criminality associated with drug addiction.
Dopesick [ 24 ] is the first drama miniseries whose plot deals exclusively with the opioid crisis and includes multiple dimensions in its portrayal. Based on the non-fiction book Dopesick : dealers , doctors , and the drug company that addicted America [ 14 ], the TV series portrays the opioid crisis in the U.S., focusing on the production, marketing, sales, and consumption of OxyContin ® .
Previous studies have looked at how the media and social media depict the opioid crisis [ 25 – 29 ]; a consistent conclusion is that there is an emphasis on the use of personal, emotionally touching, and often stereotyped stories at the expense of in-depth thematic coverage. However, only a few studies with a narrow scope published in academic and opinion journals have explored its depiction in recent TV shows [ 30 , 31 ].
The current study aimed to use a qualitative approach to explore and analyze the narrative in the TV series Dopesick , focusing on how the opioid crisis is portrayed. Rather than carry out a classical content analysis, we decided to perform a theme-based inquiry following the model proposed by Riessman [ 32 ]. We focused our analysis on the contents of the story, in other words, on the elements selected for portrayal and their salience. We sought to determine which elements, characters, and narrative arcs are predominant in the plot.
Therefore, our first two research questions are descriptive and investigate the way Dopesick tells the opioid crisis on its narrative:
- RQ1: Who is involved in the opioid crisis as it is portrayed in Dopesick ?
- RQ2: Which narrative themes are prominent in Dopesick , and how are they presented?
Furthermore, we sought to analyze the fidelity of the narrative according to Fisher’s narrative paradigm [ 33 ]. Fisher argued that narrative coherence and fidelity provide insights into why some stories can be accepted and others cannot [ 34 ]. Whereas coherence refers to how well the story fits together in terms of details, characters, and events, fidelity refers to the “truth qualities” of the story [ 33 ]. Considering that the opioid crisis is a real public health crisis, we chose to evaluate the fidelity of Dopesick ’s narrative by comparing the information presented in the series with reports on the opioid crisis in the scientific literature through a third research question:
- RQ 3: To what extent does Dopesick’s narrative agree with the information in the scientific literature?
Theoretical framework and methodological approach
All approaches to narrative analysis explore stories through their narrative arcs, plots, and motives [ 32 , 35 ]. Especially in TV series, characters, institutions, and storylines are important and can be used specifically to persuade or entertain an audience [ 36 ]. The way TV series portray complex, real problems in their fictional narratives is of particular interest. Media can tell audiences not only which issues to consider, but also how to think about them [ 37 ].
Framing theory offers a comprehensive framework for organizing and managing information in everyday life [ 38 ]. Basically, frames are persistent, shared organizing principles that work symbolically to structure the social world [ 39 ]. Gitlin [ 40 ] characterized framing as the principles involved in the selection, emphasis, and presentation of narrative elements that reflect implicit theories regarding the existence and significance of events. Framing selects aspects of perceived reality that serve to “ define problems —determine what a causal agent is doing with what costs and benefits, usually measured in terms of common cultural values; diagnose causes —identify the forces creating the problem; make moral judgments —evaluate causal agents and their effects; and suggest remedies —offer and justify treatments for the problems and predict their likely effects” [ 41 ].
Framing theory underscores the importance of two key elements: selection and salience [ 41 ]. This theory posits that any given issue can be examined from multiple perspectives, each emphasizing distinct implications for various values or factors; and this process involves the deliberate selection of specific facets of reality and elevating their prominence within a communication text. Gamson and Modigliani [ 42 ] formulated a notion of frames as “a central organizing idea or story line that provides meaning to an unfolding strip of events. The frame suggests what the controversy is about, the essence of the issue”.
Although framing theory originated in attempts to explain how individuals interpret and understand reality in the fields of communication and sociology [ 38 , 41 – 44 ], this approach has also been employed in health communication research. Framing has been useful in analyzing how health information is presented in the media and how different ways of presenting health information can affect audiences [ 45 – 47 ]. Studies focusing on “media frames” are those that analyze how frames are presented, while studies focusing on “audience frames” analyze the impact the frames have on the audience, typically in the short term [ 37 ].
The methodology within this framework has yet to be fully standardized [ 47 ]. Health communication studies have applied media framing through diverse methodologies: for example, Wang and Parris [ 48 ] combined framing theory with narrative analysis to examine the risks associated with the depiction of teenage suicide in the TV series 13 reasons why . Framing theory was combined with quantitative content analysis by Kim and Willis to analyze the American news media’s portrayal of individual and societal responsibility in obesity [ 49 ] and by Van den Bulck et al. [ 50 ] to analyze the use of alcohol in the prime-time American youth TV series The OC . However, these analyses lack a unified methodology, and the bridge between framing theory and content analysis, especially qualitative approaches, is not always clear.
Since the presentation of a narrative invariably involves adopting a perspective or frame, we aimed to align the three research questions presented above with framing theory by considering a fourth research question:
- RQ 4: How is the opioid crisis framed in Dopesick ?
Materials and methods
The present study analyzes the narrative told through the eight episodes of Dopesick . Each episode is about one-hour long and contains an average of 57 scenes ( Table 1 ). Each scene is framed in a certain time and location; changes in either of these coordinates signal a change to a different scene. According to this definition, we analyzed 458 scenes.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0301681.t001
Coding procedure and analysis
Following the approaches used in recent narrative analyses of TV series [ 48 , 51 ], we sought to “take apart the logic of the stories to determine their meanings” [ 33 ]. Using scenes as the unit of analysis, two researchers analyzed the content independently, while a third one supervised the whole process. The two independent analyses were compared, and discrepancies or disagreements were settled through discussion with the third researcher. The results were merged into a single dataset.
To address the question of who was involved in the opioid crisis according to Dopesick (RQ 1), we followed these steps. Each of the two researchers independently watched the entire series to identify characters, record actions, delineate plots, reconstruct timelines and identify salient elements, noting the year of the event depicted and the characters involved in each scene. To determine the year of the event depicted in the scene, we used extradiegetic notes in the episodes (i.e., the display of the year as a number in the transition to the scene). In the few cases where no extradiegetic signal was present, we relied on cues of scene continuity provided by characters’ clothing and the settings where the scene took place to determine the year in which the event occurred. Then, we grouped the characters by their affiliations (e.g., with Purdue Pharma or U.S. Attorney’s office) or main role in the narrative (e.g., physician or addicted patient). It was especially challenging to categorize physicians for analysis because of their pervasive presence across all categories; in addition to acting as clinicians prescribing pain medication to patients, some medical professionals were associated with Purdue Pharma and its sales, were witnesses in the trials, or became addicted themselves. Thus, although David Haddox and Russel Portenoy were both medical physicians, they were grouped according to their affiliation with the pharmaceutical company, rather than as clinical physicians, because they both promote OxyContin ® at scientific meetings and in the media, and Haddox was hired by the Purdue Pharma. In analyzing the character Samuel Finnix, we considered the scenes related to his role as a physician separately from those related to his addiction and recovery.
To determine the prominent themes in Dopesick and explore how they are developed (RQ 2), we took detailed notes about the key narrative elements such as character development, character interactions, and recurring themes. We developed a system to categorize and code the narrative elements as themes. Themes were not predefined; rather, they emerged from the analysis as the data accumulated. Again, two researchers reviewed each episode independently and abstracted data. To keep subjectivity in this classification to a minimum, two coding rounds were needed. To classify themes, we considered the characters present in the scene, taking the overarching theme into account.
We decided on three main narrative themes: 1) the government’s investigation, mainly by the U.S. Attorneys’ office and to a lesser extent by the DEA, including the grand jury proceedings; 2) unveiling OxyContin’s ® accountability network: the responsibilities of different agents, from the Sackler family to healthcare providers, including Purdue Pharma’s sales agents and the U.S. Food and Drug Administration (FDA) through its approval and labeling; and 3) the impact of the opioid crisis on society explained through the lives of individuals who become substance-dependent after being prescribed OxyContin ® , including their background stories (e.g., everyday life and work before) and the narratives of their addictions. Then, we counted the number of scenes involving each of these themes to determine their salience.
For each of these narrative themes, we have included a subcategory called “background stories” for scenes that include one or more of the main characters of the storyline articulating the theme without dealing directly with the thematic concept. For example, in Episode 1, scenes showing Betsy Mallum’s life at work are not directly related to her addiction, but they form part of her storyline because they enable viewers to track the development of her character and story.
To analyze the extent to which Dopesick ’s narrative aligns with reports on the opioid crisis in the scientific literature (RQ 3), we compared information about the opioid crisis in the U.S. presented in the series with information from other sources, especially articles in scientific journals obtained from Pubmed scientific database. As in the content analysis, each of the two researchers independently assessed narrative fidelity in Dopesick and then compared results to mitigate subjective discrepancies.
To our knowledge, no published studies have examined the opioid epidemic through the perspective of framing. Thus, to analyze how the opioid crisis is framed in Dopesick (RQ 4), we decided to apply an inductive method to analyze the narrative themes identified in RQ 2 to determine which frame(s) underlie(s) the narrative.
Exploring the roles of the characters in Dopesick
Table 2 reports the frequency of appearance Dopesick’s main characters, some of whom are based on real people like the Sackler family, owners of Purdue Pharma. The groups of characters appearing in the most scenes are members of the U.S. attorneys’ offices (n = 198; 43.2%) scenes, followed by employees of Purdue Pharma (n = 164; 35.8%), patients who use OxyContin ® and end up becoming addicts (n = 136; 29.7%), the Sackler family (n = 119; 25.9%), health professionals (n = 63; 13.7%), DEA agents (n = 57; 12.5%), and FDA officials (n = 87; 1.9%).
https://doi.org/10.1371/journal.pone.0301681.t002
Narrative themes in Dopesick
Nearly all the events presented in Dopesick’s narrative take place between 1996 and 2007. Rather than presenting events in a strictly chronological order, the narrative of the series jumps around in time to focus on different aspects of the story. Thus, each episode includes scenes from different years, and to help viewers place the scene in a particular year, the series normally uses extradiegetic announcements. S1 Annex relates the events in each episode in chronological order.
Of the 458 scenes, 94 (20.5%) are set in 1996, the year in which OxyContin ® was launched in the United States, 87 (19.0%) in 2002, the year in which the assistant U.S. attorneys for Virginia (19.0%) opened their investigation, and 54 (11.8%) in 1999, the year in which the DEA opened their investigation.
Of the three main narrative themes, the one that is developed in the greatest number of episodes is the stories of individuals who become substance-dependent after being prescribed OxyContin ® (n = 190; 41.5%), followed by the government’s investigation (n = 154; 33.6%) and Oxycontin’s ® accountability network (n = 114; 24.9%). Table 3 details the number of scenes that deal with different aspects of each of the three main narrative themes in each episode.
https://doi.org/10.1371/journal.pone.0301681.t003
OxyContin ® and the impact on society and substance-dependent individuals’ lives.
The most frequently recurring theme in Dopesick is the impact of OxyContin ® on the lives of individuals who became substance-dependent and on their families. Although these stories are heterogeneous, they share some elements. Samuel Finnix, Betsy Mallum, and Logan Parker all started using OxyContin ® in 1996 when they were prescribed the drug. Only young Elizabeth Ann McClung first uses OxyContin ® recreationally, snorting a pill at a party in 1997. Samuel Finnix is the only one who starts treatment with a dose of 20 mg rather than 10 mg, as a consequence of Purdue Pharma’s “individualize the dose” marketing campaign. Betsy Mallum’s dose is increased as a result of Purdue Pharma’s “breakthrough pain” marketing campaign (5 scenes). Both Finnix’s and Mallum’s addictions spiral after self-medication. The series shows how patients became dependent on OxyContin ® and suffer withdrawal syndrome (13 scenes), which consists of the same symptoms and signs in all cases: drowsiness, hallucinations, sweating, itching, and tics.
Dependent individuals will go to great lengths to obtain the drug. The series shows how patients obtain OxyContin ® legally in hospitals and private medical clinics (11 scenes) as well as in pain clinics of questionable medical ethics (4 scenes). It also shows how substance abusers obtain the drug illegally. Walt, a local drug dealer, sells pills to both Betsy Mallum and Samuel Finnix (5 scenes), and one of the attendees at a community meeting to help addicts recover sells OxyContin ® in the restroom (1 scene). Samuel Finnix looks for medication in different states, steals medication belonging to his patients, asks a Purdue sales representative for samples, and hides medication in his house and in his office (6 scenes).
Desperate actions to obtain drugs include prostitution, which is depicted in specific scenes from the first episode, where Elizabeth Ann McClung offers to have sex in exchange for money in a parking lot (1 scene). A doctor in a pain clinic suggests that Betsy Mallum can pay for her consultation with sex (1 scene); although she refuses his advances, she does agree to have sex with a pawnshop employee in exchange for more money when she sells her mother’s family jewels (1 scene).
The series shows how many individuals who were prescribed OxyContin ® eventually switched to cheaper, more easily obtainable opioids when their access to OxyContin ® was restricted. Betsy Mallum’s first tries heroin in 2000 and goes on to die of an overdose in 2002 (2 scenes). Betsy uses heroin in derelict areas with high concentrations of drug users.
Dopesick also shows various approaches to overcoming OxyContin ® addiction. Betsy Mallum undergoes three different types of treatments: she attends a church-based support group for addicts (2 scenes), is admitted to a rehabilitation clinic where she is tied to a bed (1 scene), and she is subjected to exorcism-like ceremonies at the community church (2 scenes). After Samuel Finnix’s addiction leads to malpractice and eventually to his medical license being revoked after he botches a routine minor surgery (4 scenes), he is forced to enter a rehabilitation clinic for 90 days, where he undergoes group therapy (9 scenes). After he is discharged, Finnix undergoes treatment with methadone at Dr. Art Van Zee’s clinic (8 scenes) and psychotherapy with Sister Beth Davies (2 scenes). Later, the methadone treatment is replaced with Suboxone ® (3 scenes). Finnix also arranges for Logan Park and Elizabeth Ann McClung to undergo methadone treatment with Dr. Art Van Zee and personally transports them to the clinic (9 scenes).
Families are unevenly represented in the narratives of addictions. Only Betsy Mallum’s family is portrayed in any depth (34 scenes). Betsy’s family is representative of the town’s inhabitants: they are poor, hard-working, religious, and have difficulties accepting their daughter’s homosexuality. When Betsy becomes addicted, she initially refuses their help. She argues regularly with her parents and steals all the family jewelry to pawn for drug money, bringing additional hardship on the family. Episodes 7 and 8 show Betsy’s mother participating in demonstrations at a museum to denounce the Sacklers, meeting with Dr. Art Van Zee and Sister Beth Davies, and attending the Abingdon Court trial. Another character, Maryanne Skolnick, based on a real person, also represents family involvement, but her role is limited to the process of seeking justice after her daughter’s death (6 scenes).
The government’s investigation.
This theme comprises the stories of the characters working at the U.S. Attorney’s office and at the DEA. The U.S. Attorney’s office’s investigation shows the manipulation of the promotional video "I got my life back" (13 scenes); Purdue Pharma’s subsidization of networks purporting to be independent that recommended the use of OxyContin ® , such as the American Society of Pain, the Academy of Pain Medicine, and patients’ associations such as the National Foundation for the Treatment of Pain and the American Chronic Pain Association (8 scenes); and suspected corruption in Purdue Pharma’s hiring of former government officials (FDA’s Curtis Wright, 6 scenes; and the U.S. Attorney for Maine, Jay McCloskey, 6 scenes).
Regarding grand jury proceedings, two preliminary court hearings that take place in 2003 are represented in Episode 3. In the first, U.S. Attorneys Rick Mountcastle and Randy Ramseyer introduce the promotional video “I got my life back” as evidence that Purdue Pharma “manipulated basic facts about the drug” by claiming that it was not addictive, alleging that the participants were deceived when they were recorded for the video. At the second hearing, the U.S. Attorneys provide evidence that the American Pain Society, which claimed to be an independent medical group, received significant funding from Purdue Pharma.
In Episode 8, court proceedings have a more prominent place in the narrative, where a trial set in 2004 and 2005 accounts for 15 scenes. These scenes include witnesses’ testimony about how Purdue Pharma promoted OxyContin ® , the effects of its use and withdrawal from the drug, and the ease of access to the drug. One trial takes place in 2007 in Abingdon, Virginia federal courthouse (7 scenes). The U.S. attorneys bring charges against Purdue Pharma executives, and viewers see testimony from people whose relatives died from OxyContin ® , often addressing the executives directly. The judge accepts the $600 million settlement, but upholds the plea bargain by which the executives would only be convicted of misdemeanors and sentenced to three years’ probation.
The DEA investigation, in the TV series led by the fictional character Bridget Meyer, began in 1999. In 20 scenes, she alerts her superiors about the increases in overdoses and crime associated with the consumption and diversion of OxyContin ® and confronts Purdue Pharma and the FDA through press conferences to inform the general public about these problems. The tension between the DEA and the FDA over the OxyContin ® label occupies a large part of this narrative axis (12 scenes). When promoted to become the Deputy Director of the DEA Diversion Area, Meyer goes to the FDA to request restrictions on the distribution of OxyContin ® (5 scenes); she also meets with Purdue Pharma executives in the DEA’s offices in 1999 and 2001 (2 scenes). Faced with the FDA’s and Purdue Pharma’s refusals to change the labeling of the drug, Meyer’s DEA team undertakes a forensic investigation to provide evidence that many overdoses occurred among people who were prescribed OxyContin ® and used it exactly as indicated on the label (6 scenes). However, neither Purdue nor the FDA accept the evidence.
OxyContin’s ® accountability network.
The third major narrative theme of Dopesick traces the responsibility of different actors in the opioid crisis. From the very first episode, the FDA’s original labeling of OxyContin ® is presented as a major problem, epitomized in Bridget Meyer’s statement “That damn label caused it all” . Curtis Wright, a former U.S. government official who played an important role in approving OxyContin ® during his tenure at the FDA, is singled out as a major culprit (10 scenes). Just two years after the drug’s market release, Wright was recruited to serve as Purdue Pharma’s Executive Director of Medical Affairs. In Episode 2, when the U.S. prosecutors question Wright’s role in the FDA’s accepting that OxyContin ® ’s addiction rate was < 1% in approving the drug’s label in the light of his career move to Purdue Pharma, FDA officials respond that many people leave government employment for more lucrative jobs in private industry, and that this is not illegal and cannot be considered corruption because it is just how the system works. In Episode 7, entitled “Black Box Warning”, viewers see how the FDA finally agrees to change the OxyContin ® label by adding a black box warning. However, the wording of the warning is crucial. Because it merely states that “addiction is reported to be rare”, the new label continues to allow OxyContin ® to be prescribed for moderate pain; moreover, it also states that the drug can be used even "for an extended period of time". Thus, Richard Sackler and Purdue Pharma executives actually consider the change in the label to be a boon rather than the detriment they had feared.
Purdue Pharma’s organizational structure is portrayed as strongly hierarchical. The focus remains on Richard Sackler, who meets with his family to discuss financial issues at his house or in the Sackler Wing of the Metropolitan Museum of Art in New York City (11 scenes). Meetings with top executives (Michael Friedman, Howard Udell, and Paul Goldenheim) are held in Purdue Pharma’s offices (24 scenes). Top management’s decisions are relayed directly to sales executives and implemented in training sessions for sales representatives (18 scenes). Viewers see how the sales representatives promote OxyContin ® to doctors and how they are motivated to sell more through a system of awards and financial compensation (20 scenes). Although the series lays responsibility on Richard Sackler by emphasizing his leadership and his speeches (e.g., through the narrative of pushing for OxyContin’s ® introduction in Germany, 5 scenes), it is Purdue Pharma’s executives rather than the Sackler family who take the blame, as is shown in the Episode 8 through both fictitious (8 scenes) and documentary images (19 scenes).
Physicians play an important role in the company’s strategy through their prescribing and promoting the drug. The series portrays the intricate web of connections between sales representatives and healthcare professionals, involving gifts and bonuses associated with prescribing OxyContin ® . Dopesick assigns a minor role to one physician based on a real person, Dr. Alan Spanos (3 scenes), who unscrupulously promotes OxyContin® for financial gain. By contrast, one of the major roles in the series, the fictional character Samuel Finnix, is a dedicated physician in a small town in Appalachia who is duped by a Purdue Pharma sales representative into prescribing OxyContin ® to treat his patients. When Finnix himself is prescribed OxyContin ® after an automobile accident, he develops a substance-abuse disorder; after a tumultuous journey to recovery, Finnix is redeemed by dedicating his life to helping others overcome their addictions.
Another physician based on a real person is Art Van Zee, an activist fighting against the opioid epidemic in Appalachia. In Dopesick , Van Zee is portrayed as a physician who cares for his community, addressing the issue of addiction not only within Purdue Pharma but also by treating individuals with substance use disorders (8 scenes). In this capacity, he helps Samuel Finnix to recover from his addiction to OxyContin ® through treatment with methadone and Suboxone ® , a fixed dose combination of buprenorphine and naloxone.
Analysis of narrative fidelity in Dopesick
Regarding narrative fidelity, the temporal and spatial setting appears to be accurate and faithful. According to the scientific literature, the number of prescriptions for OxyContin ® increased from 670,000 in 1997 to 6.2 million in 2002 [ 52 ], thus attesting to the explosive growth depicted in this time period in the Dopesick narrative. Moreover, Appalachia, where the impact of the crisis is shown in the series, was indeed the geographical region with the highest concentration of OxyContin ® abuse in that period [ 53 ], and central Appalachia was an early focal point in the opioid epidemic [ 54 ]. In this largely rural area, many people working in physically demanding industries such as coal mining, agriculture, and logging were vulnerable to prescription opioids’ promise of pain relief [ 55 , 56 ].
The composite portrait of substance abusers in the series is in line with the scientific literature, which indicates that people of all ages, sexes, and socioeconomic backgrounds abuse opioids, especially in rural settings [ 9 ]. The characters mostly adhere to an initial stereotype of people from Appalachia, coming from rural areas, with a low level of education, great gender inequalities, and a high level of crime. Some scholars have speculated that these social stereotypes have been created by economic and political forces to justify the exploitation of Appalachian peoples through industrialization and natural resource extraction [ 57 – 59 ]. The fact that all the substance abusers in the series are Caucasians could be thought to reinforce the interpretation of the opioid crisis as a “white disability” [ 12 ], but this portrayal truthfully reflects the demographic composition of the Appalachian population. Likewise, the path to addiction depicted in the series, where three of the four cases begin with doctor-prescribed opioid use, is in line with figures from the scientific literature, which indicates that 80% of opioid abusers were prescribed opioids before becoming addicted [ 60 ]. Dopesick’s narrative does not place much importance on the social determinants and contexts of opioid use; this portrayal is not totally discordant with the scientific evidence on the role of economic conditions in driving drug misuse and overdoses, as different studies have reported discrepant results [ 61 – 63 ]. However, the inadequate access to detox treatments and interdisciplinary approaches shown in Betsy Mallum’s story are in line with reports in the scientific literature [ 64 – 66 ]. Moreover, Betsy’s dependence on Oxycontin ® leading to heroin addiction reflects a widely reported occurrence [ 6 , 67 ], and although Betsy eventually dies of an overdose, the incidence in of deaths from heroin overdoses in the series is much lower than in reality [ 68 ].
By contrast, Dopesick ’s opioid storytelling repeatedly emphasizes the inappropriate marketing of OxyContin ® . By focusing on this aspect, the narrative blames the opioid crisis on Purdue Pharma and its executives, singling out Richard Sackler. The series shows how their responsibility extends far beyond Sackler’s role as the originator and driving force behind the OxyContin ® sales system, underlining the financial links between Purdue Pharma and scientific and patients’ societies and highlighting the role of some pain experts and scientific societies as influencers in a clear demonstration of conflict of interest. This portrayal is in line with reality. Prominent pain societies in North America did indeed support the expanded utilization of opioids for treating chronic pain, and they published consensus statements advocating for the supervised and careful use of opioid therapy in patients with chronic pain [ 69 ]. This model underlies the government and DEA’s focus on criminalizing drug use [ 70 ] which is addressed only limitedly in the series.
The series also shows the roles of medical doctors and scientists (real characters Russell Portenoy and David Haddox) in defending the use of opioids as well as their major direct or indirect conflicts of interest through pain societies financed by Purdue Pharma; this portrayal is grounded in different court records [ 53 , 71 – 75 ].
Importantly, however, Purdue Pharma was not an isolated case or solely responsible for the opioid crisis as might be surmised from the information presented in the series. First, it is not the only company that has been found legally responsible in the opioid crisis. In 2022, Johnson and Johnson and the three biggest U.S. drug distributors—Cardinal Health, McKesson, and AmerisourceBergen—ended America’s biggest multi-state legal settlement with a $26bn payout [ 76 ].
Moreover, the complex network of responsibilities also includes the FDA and the government. The role of the FDA can be considered one of the most problematic issues portrayed in the series. This portrayal is in line with Makhinson et al.’s [ 72 ] conclusion that the influence of experts and scientific societies was second only to that of the pharmaceutical companies and government regulators. Manchikanti et al. [ 73 ] consider that the FDA’s uncritical approval of OxyContin’s ® label made a substantial, albeit unintentional, contribution to the opioid crisis. Nevertheless, Dopesick fails to define the FDA’s responsibility.
Finally, the series fails to mention some facts that would likely interfere with the storyline. For instance, the correlation between the number of opioids prescribed and the extent of non-medical use of opioids or opioid addiction is not straightforward: Singer et al. [ 77 ] reported that although the number of prescriptions decreased after 2012, overdose deaths increased. Moreover, no mention is made of other products like fentanyl, Percocet ® , Percodan ® , or tramadol that are also associated with the opioid crisis [ 7 , 73 , 78 – 80 ].
Framing Dopesick
From the start, Dopesick makes viewers aware that the consequences of the way that OxyContin ® was marketed include misuse, prostitution, and death. The narrative makes it clear that substance abusers are not to blame for their woes, despite Richard Sackler’s attempts to shift the blame onto them. The four characters who come to be addicted to OxyContin ® are portrayed as good people who are unknowing victims acting in good faith: they suffer through withdrawal syndrome, try to rehabilitate themselves, seek solutions to their problems, and sometimes succeed in redeeming themselves.
Dopesick’s narrative is diametrically opposed to the discredited but still prevalent “moral model of addiction” that characterizes addiction as a manifestation of willpower weakness, suggesting that substance abusers experience an uncontrollable urge to use psychoactive substances and eventually lose the ability to manage their usage despite adverse repercussions like loss of employment, disengagement from or conflicts within personal relationships, difficulty maintaining housing, and health problems [ 69 ]. The trajectories of the substance abusers and the challenges they face with their families in Dopesick are portrayed in a way that elicits empathy. Moreover, the portrayals of programs based on the moral model (e.g., Betsy’s church) also reflect the consensus in the scientific community.
Our theoretical framework about framing shows that some identities and choices are privileged in the narrative, and others are negated or stigmatized [ 81 ]. Dopesick devotes several scenes to showing patients becoming substance abusers, but none showing patients benefitting from the treatment, except in the “biased” materials Purdue Pharma and the pain societies they control show to push the drug on society. Thus, the question What do we do with people who are in pain ? is still open [ 82 ].
Dopesick ’s narrative only partially tackles physicians’ responsibility in prescribing OxyContin ® . Viewers learn different aspects of this responsibility in courtroom scenes and scenes related to the U.S. Attorney’s investigation, but the healthcare professionals portrayed in these scenes are minor characters and their ethical responsibility is largely unexplored. The exception is Samuel Finnix, whose story is central to the plot. Dr. Finnix is depicted as a competent, dedicated professional who cares deeply for his patients. Finnix is misled by the industry, eventually going from prescribing OxyContin ® to becoming a substance abuser himself. After hitting bottom, Finnix seeks redemption by concentrating all his efforts on helping the victims of the opioid crisis. This portrayal provokes empathy, and the drug company’s deception exonerates the physician from blame.
In summary, the prominent element of this narrative is its characterization of the processes resulting in the development of dependence on OxyContin® and the individuals who suffer from it. Some characters’ identities and choices are shown in a favorable light while those of others are condemned [ 81 ]: there are obvious “good guys” (e.g., the prosecutors Rick Mountcastle and Randy Ramseyer, both based on real people, and Samuel Finnix and Betsy Mallum, who are fictional inventions) and “bad guys” (most notably, Richard Sackler). Returning to Entman’s definition [ 41 ], this frame defines a problem (the opioid crisis in the United States) by identifying a primary causal agent (Purdue Pharma, and particularly Richard Sackler), diagnoses a cause (greed), makes moral judgments (blaming Richard Sackler and his accomplices: sales executives, the FDA, and physicians prescribing opioids and exonerating substance-dependent individuals as victims of inadequately informed medical prescription), and suggests remedies (withdrawing OxyContin ® from the market). To relate this narrative, Dopesick uses the classic protagonist-antagonist format [ 83 ]; however, rather than pitting individual protagonists and antagonists against one another, prosecutors and people harmed by the crisis face off against Purdue Pharma, characterized, as in the title of the book series is based on, as the drug company that addicted America .
Dopesick ’s narrative about the opioid crisis
Based on real events reported in Beth Macy’s bestselling book Dopesick , the eponymous TV series uses a complex narrative involving different groups of characters including businessmen, prosecutors, doctors, and patients to portray the opioid crisis in the United States as a multifactorial problem. This complexity is also reflected in the narrative approach. Each episode comprises scenes from different years to construct the narrative, showing that the causes and consequences are not linear and underlining the interconnectivity of characters and actions and the complexity of the problem. Although many viewers know the outcomes of the characters based on real individual’s stories before watching the series, the outcome of the fictional characters remains a mystery and generates suspense. This suspense is reinforced by the predominant role of the prosecutors’ investigation, which allows viewers to know how the real-life story was developing behind the scenes.
Despite the shifting timeframes within episodes, the bulk of the series takes place in Appalachia between 1996 and 2007. This period comprises the time from the FDA’s approval of OxyContin ® in 1995 to Purdue Pharma’s pleading guilty to criminally misbranding the drug and misrepresenting its risks of addiction.
It is interesting to reflect on the disparity between the frequency of appearance of characters and of the narrative themes. In the analysis of the groups of characters, the U.S. attorneys appear most frequently, followed by characters associated with Purdue Pharma, and lastly, OxyContin ® substance-dependent individuals and their families.
In contrast, in the analysis of Dopesick ’s narrative themes, the order is reversed. Although the investigations by the U.S. Attorney’s office and the DEA are central to the plot, Dopesick is not primarily a legal drama. The theme that appears in the most scenes is the impact of the crisis on substance-dependent individuals and their families, followed by the Government’s investigation, and finally the network of responsibilities related to OxyContin ® . The preeminence of the impact on substance-dependent individuals, their families, and society as a whole, underlines the suffering caused by the opioid epidemic.
The analysis of Dopesick’s narrative reveals that the geographical, temporal, and multidimensional approach taken by the series towards the opioid crisis primarily emphasizes the responsibilities of Richard Sackler and Purdue Pharma. It provides limited consideration of the duties of the FDA, while downplaying the responsibilities of the government and even the DEA. It is striking that the characters working for the FDA and those working for the DEA (except Bridget Meyer) are not developed further in the TV series. The FDA’s position and actions related to the authorization of the OxyContin ® label and of the phrases and graphs used by Purdue Pharma’s sales representatives border on corruption. Finally, Dopesick fails to deal with other elements and characters involved in the opioid crisis: there is no mention of the government’s failure to act by creating laws to stop the crisis, the collapse of the healthcare system, other pharmaceutical companies or opioid distributors, or the press. In summary, the TV series Dopesick frames the opioid crisis in a way that identifies Purdue Pharma (and in particular, Richard Sackler) as the primary causal agent, morally condemning the company and its chief executive and suggesting that withdrawing OxyContin® would be a step toward resolving the crisis.
Methodological, theoretical, and practical implications
Given the complexity of the series Dopesick , we were obliged to employ a complex inductive approach requiring multiple revisions to harmonize criteria and avoid subjectivity. This process elucidated the narrative structure in a manner that we had not initially envisioned. Our approach allowed us to compare and contrast the narrative themes we identified together with the portrayals of the characters in the series to help us understand the themes included in the narrative and their salience.
Applying Fisher’s narrative paradigm to determine Dopesick’s fidelity vis-à-vis the scientific literature enabled us to analyze another important dimension of the series and provided useful data leading to new insights. We considered it unnecessary to use this paradigm to analyze narrative coherence because our analysis of scenes and characters in exploring narrative themes yielded ample results. The lack of empirical measures and standardized analytical methods for the theoretical constructs in Fisher’s narrative analysis would have made this approach challenging [ 33 ]. Therefore, we looked to framing theory to provide an additional theoretical framework to guide our approach.
Framing narratives serves the purpose of simplifying intricate matters, making them easier for audiences to grasp by highlighting particular aspects of the content to match audiences’ pre-established mental frameworks [ 43 ]. Complex issues like the opioid crisis in the U.S. could demand intricate and instructive narratives that may diverge from TV series’ purpose of entertaining viewers.
Studies in the field of narrative research, with a particular focus on health communication, have consistently demonstrated the significance of analyzing media narratives such as TV series [ 84 ], especially in today’s digital age, where TV series have become a dominant form of entertainment and storytelling [ 85 ]. Delving into these narratives provides a more profound insight into intricate societal matters and underscores the crucial role narratives play in fostering knowledge, attitudes, and health-related behaviors [ 84 ].
While it is not our intention to propose contributions regarding framing conceptualization, we would like to address some theoretical implications of this study. The lack of a cohesive theory and methodology for framing in communication research, what Entman [ 41 ] referred to as a “fractured paradigm”, has resulted in both the overuse and misuse of framing [ 86 ], thus making it challenging to clearly differentiate framing from other concepts in communication research [ 87 ]. However, as pointed out by Ardèvol-Abreu [ 88 ], not everyone considers the varied approaches to framing a drawback. D’Angelo [ 89 ] suggests that the diverse array of approaches is necessary to comprehend a phenomenon of great complexity like the media, and Reese [ 90 ] posits that the significance of framing theory does not reside in its potential as a unified research paradigm, but rather in its ability to bridge the gap between qualitative and quantitative, empirical and interpretive, psychological and sociological, as well as academic and professional research.
Framing can be employed both methodologically and theoretically [ 91 ]; however, previous research has used only one or the other approach. Methodological approaches have yet to be standardized [ 47 ]. Theoretical approaches have used framing as a theoretical background to interpret the content of news media or fictional narratives. Much of this research has focused on framing news in the media [ 90 , 92 ]; however, Scheufele [ 43 ] clarifies that study frames apply not only to news media but also to journalistic stories across different media, such as print and television. For example, Whiteman et al. [ 93 ] analyzed the coverage of scientific articles in news media that showed a possible scientific explanation for breast cancer, focusing on scientific accuracy. Our study also delves into the scientific accuracy of the events depicted in the TV series, comparing them with the existing literature on the opioid crisis.
One of the most original aspects of our research is the exploration of the articulation between narrative analysis and framing. Narrative analysis delineates qualitative narrative threads and analyzes specific themes, characters, and stories according to an objective and well-justified methodology. Framing also encompasses these prominent themes, but it goes further in attempting to reach a broader understanding of reality. Few studies have explored this articulation in depth. Although Listyani et al. [ 94 ] used framing to analyze the scripts of Japanese and American cartoon movies, Ye et al. [ 95 ] studied the medical frame on the portrayals of illnesses and diseases in two medical dramas, and Wang and Parris [ 48 ] used framing theory in the literature review in their analysis of the TV series 13 reasons why , all these papers used framing theory only as a theoretical background. In their study analyzing the causes and solutions of obesity as portrayed in newspapers and TV news, Kim and Willis [ 49 ] took this approach one step further by analyzing the framing of the representation of responsibility in these media. Our research forges ahead on the path these authors laid, focusing on the way Dopesick emphasizes different viewpoints to establish a frame of reference (i.e., reference framing) [ 86 ] to explain the opioid crisis. This approach makes it clear that the dominant perspective in Dopesick ascribes blame to the pharmaceutical company and regulatory organisms, while fostering empathy toward opioid users and their families and championing individuals and organizations who strive to bring the culprits to justice and alleviate the victims suffering.
Bulck et al. [ 50 ] combined quantitative content analysis and qualitative framing analysis, providing enhanced insight into thematic elements in their research into the framing of alcohol in a TV series targeting teenagers. Unlike these authors, who were able to rely on codes from prior investigations, we considered it necessary to develop new codes suitable for the opioid crisis. To this end, we used narrative analysis to examine each scene objectively to substantiate the frequency of characters and narrative themes, and we complemented this approach with framing analysis for theoretical and methodological validation.
Furthermore, framing goes beyond narrative themes, inviting us to analyze the manner in which the story is told, its significance, how protagonists and antagonists are constructed, and the narrative aspects of the story. It prompts us to consider from which perspective the story is being narrated and how “that” reality is being constructed.
Regarding methodology, choosing a single TV series for analysis allows for a more in-depth and focused exploration of its narrative elements. By concentrating on one show, we can delve deeply into its characters, plot development, and themes, gaining a comprehensive understanding of its narrative. We can also debate whether the unit of analysis for a narrative analysis should be the scene or the overarching story. We opted for the scene to gain a nuanced perspective on narrative themes, considering both their depth of portrayal and their placement within the overall plot. Moreover, the challenge in conducting such analyses always lies in achieving objectivity and ensuring replicability in subsequent studies, a matter that is more readily defined when segmenting by scenes rather than overarching plots.
From a practical point of view, our analysis can help people understand Dopesick’s potential to raise awareness about social perceptions of the people affected by the opioid crisis and about social condemnation of the Sackler family and Purdue Pharma [ 69 , 82 ]. Dopesick might also influence how healthcare professionals, law enforcement agencies, and legal experts perceive and treat patients struggling with substance dependence [ 96 , 97 ], favoring viewing these individuals within the context of their complete addiction narratives rather than solely through the lens of criminality [ 98 ]. It can encourage healthcare providers to adopt a more holistic and compassionate approach, recognizing that opioid misuse or abuse is a complex issue often rooted in various personal, social, and medical factors. These issues remain especially important considering the ongoing crisis involving fentanyl and tramadol [ 9 , 98 ].
Limitations of the study and future directions
Some limitations of our study require comment. While Dopesick offers valuable insights into the opioid crisis, it focuses on specific characters and situations and largely ignores or deemphasizes other elements and characters that played an important role in the crisis. This was to be expected, because the opioid crisis was a complex phenomenon that cannot be fully encapsulated by any single TV series or movie. Time constraints, narrative structure, and commercial considerations inevitably shape the portrayal of complex events in TV series. Given the finite number of episodes and runtime, the series had to prioritize certain aspects of the crisis over others, potentially oversimplifying or omitting critical dimensions in striving to engage audiences and maintain viewer interest. However, all approaches to portraying complex situations are limited in different ways, and examining the frames and perspectives in which the events are narrated can shed new light on the situation. Dopesick serves as a compelling entry point to raise awareness about the opioid crisis, and the series provides much useful material for discussion and understanding. Nevertheless, it cannot provide a comprehensive understanding of the multifaceted factors contributing to the crisis.
Thus, the series and our analysis cannot hope to provide a comprehensive view of the opioid crisis, but it may help us understand how the narrative deals with (or fails to deal with) elements besides Purdue Pharma that were involved in the crisis, such as the healthcare system, other drug companies, or the press. Certainly, future studies can be conducted on other narratives about the opioid crisis in the U.S.; it would be interesting to see whether they select and highlight the same themes or if different themes emerge. These studies can examine fictional narratives, documentaries, or journalistic portrayals in news media, as well as comparing storytelling in different approaches. It could also be interesting to for new studies to use Fisher’s concepts of fidelity (and even coherence) to compare Dopesick with other TV series (e.g., Netflix’ Painkiller ); such studies could also examine viewers’ experiences, perhaps employing focus groups with audiences from different backgrounds.
Our study focuses on media frames rather than audience frames [ 37 ], because we were interested in studying how the reality of the opioid crisis is depicted in the TV series Dopesick , rather than how the audience accepted or rejected this narrative. Specifically, our emphasis is on examining how the narrative of the opioid crisis is constructed within the TV series; future research might analyze the series’ impact on the audience. It is important to explore whether these narratives have an impact on public opinion or people’s attitudes toward these health-related topics, since health narratives can help people gain a deeper understanding, develop emotional connections, and ultimately enhance well-being while promoting greater empathy towards others [ 81 ]. Future studies might gauge the short- and long-term effects of this TV series on viewers’ knowledge and attitudes.
New studies should also consider the actual value of Dopesick for understanding the most important elements that contributed to the crisis. The series promises to be useful in teaching various disciplines (e.g., health sciences, law, and sociology), and studies collecting empirical data about the effectiveness of activities based on Dopesick in increasing students’ knowledge and understanding would be useful and would enhance their educational value.
Conclusions
Dopesick is the first TV series centered on the opioid crisis in the U.S. Although the series shows the crisis from different perspectives and reveals multiple dimensions in its storytelling, it nevertheless downplays the roles of many agents and focuses on the Sackler family in general and Richard Sackler in particular as the cause of the problem. There is a general tendency to favor simple explanations and simple solutions to complex problems, such as the narrative of overprescribing opioids sparking a public health crisis in the United States [ 77 ], and Dopesick fails to avoid this pitfall. Rather than provide a nuanced analysis of a complex crisis, the series puts the blame almost entirely on OxyContin ® and Purdue Pharma.
Narratives provide a potent avenue for understanding, communicating, and gaining insights from personal experiences of illness and recovery [ 81 ]. In this sense, Dopesick may foster empathy towards substance-dependent individuals. On the other hand, it has the potential to introduce the topic of the misuse of prescription opioids, as it reinforces its connection with addiction and stigmatized beliefs regarding irrationality and lack of control [ 69 , 99 ].
Dopesick provides viewers with an initial framework for sharing and discussing inappropriate opioid use in general, beyond Purdue Pharma. Indeed, the series is innovative and useful, bringing an important topic to open television and making it possible for the public to learn about and discuss science, pharmaceutical companies, regulatory agencies, health institutions, and even stereotypes of Appalachians.
Supporting information
S1 annex. chronological scheme of the events shown in the series dopesick according to the episodes and the main characters..
https://doi.org/10.1371/journal.pone.0301681.s001
S1 Data set.
https://doi.org/10.1371/journal.pone.0301681.s002
- View Article
- PubMed/NCBI
- Google Scholar
- 4. UN Office on Drugs and Crime. World Drug Report 2023. 2023.
- 13. Meier B. Pain killer: an empire of deceit and the origin of America’s opioid epidemic. New York: Random House; 2018.
- 14. Macy B. Dopesick: Dealers, doctors, and the drug company that addicted America. London: Head of Zeus Ltd; 2018.
- 17. Gibney A. The crime of the century [TV Series]. HBO Documentary Films; Jigsaw Productions; Storied Media Group; 2021.
- 18. Holden Jones A, Fuqua A, Noyce P, Obst O, Boorstein D, Harthan T. The Resident [TV Series]. 20th Television; Disney-ABC Domestic Television;
- 32. Riessman CK. Narrative methods for the human sciences. Thousand Oaks: SAGE Publications; 2008.
- 34. Cohen EL. The multidisciplinary, interdisciplinary, and transdisciplinary nature of health communication scholarship. The Routledge Handbook of Health Communication. New York: Routledge; 2021. pp. 3–16.
- 35. Riessman CK. Analysis of personal narratives. In: Holstein J, Gubrium J, editors. Inside interviewing: New lenses, new concerns. Thousand Oaks: SAGE Publications; 2003. pp. 331–346.
- 38. Goffman E. Frame analysis: An essay on the organization of experience. Cambridge: Harvard University Press; 1974.
- 39. Reese S, Gandy O, Grant A. Framing public life. Mahwah: Erlbaum; 2001.
- 40. Gitlin T. The whole world is watching: Mass media in the making and unmaking of the New Left. Berkeley: University of California Press; 1980.
- 44. Bateson GA. A theory of play and fantasy. Steps to an ecology of mind. New York: Chandler; 1955.
- 66. Hurst C, Fitz Gibbon H, Nurse A. Social Inequality. Forms, Causes, and Consequences. New York: Routledge; 2016.
- 77. Singer JA, Sullum JZ, Schatman ME. Today’s nonmedical opioid users are not yesterday’s patients; implications of data indicating stable rates of nonmedical use and pain reliever use disorder. Journal of pain research. New Zealand; 2019. pp. 617–620. doi: https://doi.org/10.2147/JPR.S199750
- 81. Harter LM, Yamasaki J, Kerr AM. Narrative Features, Forms, and Functions: Telling Stories to Foster Well-Being, Humanize Healthcare, and Catalyze Change. The Routledge handbook of health communication. New York: Routledge; 2021. pp. 47–60.
- 83. Bordwell D. Narrative in the fiction film. Toronto: Madison; 1985.
- 84. Thompson T, Harrington NG. The Routledge Handbook of Health Communication, Third Edition. The Routledge Handbook of Health Communication, Third Edition. Routledge; 2021. https://doi.org/10.4324/9781003043379
- 86. D’Angelo P. Framing: Media Frames. The International Encyclopedia of Media Effects. Wiley; 2017. pp. 1–10. https://doi.org/10.1002/9781118783764.wbieme0048
- 87. D’Angelo P, Lule J, Neuman WR, Rodriguez L, Dimitrova D V., Carragee KM. Beyond Framing: A Forum for Framing Researchers. Journalism and Mass Communication Quarterly. SAGE Publications Inc.; 2019. pp. 12–30. https://doi.org/10.1177/1077699018825004
- 99. Meldrum ML. The prescription as stigma: opioid pain relievers and the long walk to the pharmacy counter. In: Watkins , Greene JA, editors. Prescribed: Writing, Filling, Using and Abusing the Prescription in Modern America. Baltimore: Johns Hopkins University Press; 2012.
Analysis of social media language using AI models predicts depression severity for white Americans, but not Black Americans
NIH-supported study also found Black people with depression used different language than white people to express their thoughts on Facebook

Researchers were able to predict depression severity for white people, but not for Black people using standard language-based computer models to analyze Facebook posts. Words and phrases associated with depression, such as first-person pronouns and negative emotion words, were around three times more predictive of depression severity for white people than for Black people. The study , published today in the Proceedings of the National Academy of Sciences , is co-authored by researchers at the University of Pennsylvania, Philadelphia, and the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH), which also funded the study.
While previous research has indicated that social media language could provide useful information as part of mental health assessments, the findings from this study point to potential limitations in generalizing this practice by highlighting key demographic differences in language used by people with depression. The results also highlight the importance of including diverse pools of data to ensure accuracy as machine learning models, an application of artificial intelligence (AI) language models, are developed.
“As society explores the use of AI and other technologies to help deliver much-needed mental health care, we must ensure no one is left behind or misrepresented,” said Nora Volkow, M.D., NIDA director. “More diverse datasets are essential to ensure that healthcare disparities are not perpetuated by AI and that these new technologies can help tailor more effective health care interventions.”
The study, which recruited 868 consenting participants who identified themselves as Black or white, demonstrated that models trained on Facebook language used by white participants with self-reported depression showed strong predictive performance when tested on the white participants. However, when the same models were trained on Facebook language from Black participants, they performed poorly when tested on the Black participants, and showed only slightly better performance when tested on white participants.
While depression severity was associated with increased use of first-person singular pronouns (“I,” “me,” “my”) in white participants, this correlation was absent in Black participants. Additionally, white people used more language to describe feelings of belongingness (“weirdo,” “creep”), self-criticism (“mess,” “wreck”), being an anxious-outsider (“terrified,” “misunderstood”), self-deprecation (“worthless,” “crap”), and despair (“begging,” “hollow”) as depression severity increased, but there was no such correlation for Black people. For decades, clinicians have been aware of demographic differences in how people express depressive symptoms, and this study now demonstrates how this can play out in social media.
Language-based models hold promise as personalized, scalable, and affordable tools to screen for mental health disorders. For example, excessive self-referential language, such as the use of first-person pronouns, and negative emotions, such as self-deprecating language, are often regarded as clinical indicators of depression. However, there has been a notable absence of racial and ethnic consideration in assessing mental disorders through language, an exclusion that leads to inaccurate computer models. Despite evidence showing that demographic factors influence the language people use, previous studies have not systematically explored how race and ethnicity influence the relationship between depression and language expression.
Researchers set up this study to help bridge this gap. They analyzed past Facebook posts from Black and white people who self-reported depression severity through the Patient Health Questionnaire (PHQ-9) – a standard self-report tool used by clinicians to screen for possible depression. The participants consented to share their Facebook status updates. Participants were primarily female (76%) and ranged from 18 to 72 years old. The researchers matched Black and white participants on age and sex so that data from the two groups would be comparable.
The study’s findings challenge assumptions about the link between the use of certain words and depression, particularly among Black participants. Current clinical practices in mental health that have not accounted for racial and ethnic nuances may be less relevant, or even irrelevant, to populations historically excluded from mental health research, the researchers note. They also hypothesize that depression may not manifest in language in the same way for some Black people – for example, tone or speech rate, instead of word selection, may relate more to depression among this population.
“Our research represents a step forward in building more inclusive language models. We must make sure that AI models incorporate everyone's voice to make technology fair for everyone,” said Brenda Curtis, Ph.D., MsPH, chief of the Technology and Translational Research Unit in the Translational Addiction Medicine Branch at NIDA’s Intramural Research Program and one of the study’s senior authors. “Paying attention to the racial nuances in how mental health is expressed lets medical professionals better understand when an individual needs help and provide more personalized interventions.”
Future studies will need to examine differences across other races and demographic features, using various social media platforms, the authors say. They also caveat that social media language is not analogous to everyday language, so future work on language-based models must take this into account.
“It’s important to note that social media language and language-based AI models are not able to diagnose mental health disorders – nor are they replacements for psychologists or therapists – but they do show immense promise to aid in screening and informing personalized interventions,” said the study’s lead author, Sunny Rai, Ph.D., a postdoctoral researcher in Computer and Information Science at the University of Pennsylvania. “Many improvements are needed before we can integrate AI into research or clinical practice, and the use of diverse, representative data is one of the most critical.”
For more information on substance and mental health treatment programs in your area, call the free and confidential National Helpline 1-800-662-HELP (4357) or visit FindTreatment.gov . Anyone who needs assistance with the first steps in pursuing help can find guidance at FindSupport.gov .
If you or someone you know is in crisis and needs immediate help, call the 988 Suicide & Crisis Lifeline at 988. Learn more about suicide prevention and ways you can help someone who might be at risk for self-harm.
- S Rai, et al. Key Language Markers of Depression on Social Media Depend on Race . The Proceedings of the National Academy of Sciences . DOI: 10.1073/pnas.2319837121 (2024).
About the National Institute on Drug Abuse (NIDA): NIDA is a component of the National Institutes of Health, U.S. Department of Health and Human Services. NIDA supports most of the world’s research on the health aspects of drug use and addiction. The Institute carries out a large variety of programs to inform policy, improve practice, and advance addiction science. For more information about NIDA and its programs, visit www.nida.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
About substance use disorders: Substance use disorders are chronic, treatable conditions from which people can recover. In 2022, nearly 49 million people in the United States had at least one substance use disorder. Substance use disorders are defined in part by continued use of substances despite negative consequences. They are also relapsing conditions, in which periods of abstinence (not using substances) can be followed by a return to use. Stigma can make individuals with substance use disorders less likely to seek treatment. Using preferred language can help accurately report on substance use and addiction. View NIDA’s online guide .
NIH…Turning Discovery Into Health®
Related Articles

Law enforcement seizures of psilocybin mushrooms rose dramatically between 2017-2022

Reduced drug use is a meaningful treatment outcome for people with stimulant use disorders

Residential addiction treatment for adolescents is scarce and expensive
Breaking News

ARGUMENT ANALYSIS
Supreme court appears likely to allow abortion drug to remain available.

The Supreme Court on Tuesday signaled that it was likely to allow mifepristone, one of two drugs used in medication abortions, to remain widely available in the United States. During roughly 90 minutes of oral arguments, a majority of the justices appeared ready to throw out the dispute over the FDA’s expansion of access to the drug in 2016 and 2021 because the challengers in the case – several individual doctors and groups of doctors who are opposed to abortion on religious or moral grounds – do not have a legal right to sue, known as standing.
Tuesday’s argument was the first time that the justices had considered efforts to restrict abortion since their 2022 decision to eliminate the constitutional right to an abortion, in Dobbs v. Jackson Women’s Health Organization . After that ruling, 21 states either banned or significantly limited access to abortion. Medication abortions now account for more than half of all abortions performed in the United States, and a ruling in favor of the challengers in this case would restrict access to abortion even in the states where abortion is otherwise legal.
The case began in November 2022, when several individual doctors and groups made up of doctors opposed to abortion went to federal court in Texas. They challenged both the FDA’s initial approval of mifepristone in 2000 and a series of decisions by the FDA in 2016 and 2021 that expanded access to the drug – for example, allowing it to be used through the 10th week of pregnancy, authorizing health-care providers who are not physicians to prescribe it, and permitting it to be prescribed without an initial in-person visit.
The U.S. Court of Appeals for the 5th Circuit ruled that the challenge to the initial approval of the drug had come too late. But it rolled back the FDA’s 2016 and 2021 decisions that expanded access to the drug, pointing to flaws in the FDA’s decision-making process.
The FDA and Danco, which manufactures mifepristone, came to the Supreme Court last fall, asking the justices to review the 5th Circuit’s ruling. The justices agreed in December to take up the case. Meanwhile, the lower court’s ruling is on hold, so that mifepristone remains widely available until the challenge is resolved.

U.S. Solicitor General Elizabeth Prelogar argues on behalf of the FDA. (William Hennessy)
Most of the debate on Tuesday morning focused on whether the challengers have standing, so that the lawsuit can go forward. Representing the FDA, U.S. Solicitor General Elizabeth Prelogar told the justices that the lawsuit can only go forward if the challengers can identify a doctor who faces imminent harm as a result of the 2016 and 2021 changes. But the challengers’ standing argument, she contended, rests on a “long chain of remote contingencies”: Although they are not required to prescribe mifepristone, one of the individual challengers or the groups’ members would nonetheless have to treat women who had been prescribed mifepristone by someone else and then suffered complications, which are very rare. Particularly when federal laws provide an additional layer of protection for health-care providers who believe that providing certain kinds of treatment, such as care related to abortion, would violate their conscience, Prelogar concluded, the connection to the 2016 and 2021 changes is “too attenuated” for the challengers have a right to sue.
Some of the court’s more conservative justices were skeptical – most notably, Justice Samuel Alito, who pressed Prelogar to explain whether anyone would be able to sue to challenge the 2016 and 2021 changes. Even if the FDA acted unlawfully in making those changes, Alito said incredulously, “the American people have no remedy?”
Chief Justice John Roberts asked both Prelogar and Jessica Ellsworth, representing Danco, to identify a point at which an increase in the risks associated with mifepristone might be enough to confer standing. What percentage of adverse consequences would suffice, Roberts asked, or how many visits to the emergency room?

Jessica Ellsworth representing the drug manufacturer. (William Hennessy)
But several other justices seemed persuaded by Prelogar’s contention that the challengers had not alleged the kind of imminent injury required for standing, particularly when federal conscience exemptions are available for health-care providers who object to providing care relating to abortion. Justice Elena Kagan told Erin Hawley, who argued on behalf of the challengers, that her theory of standing sounded “very probabilistic.” When Hawley told Kagan that it was not, Kagan pressed her to provide an example of a health-care provider who could “meet the court’s regular standing requirements.”
Hawley pointed to a declaration by a doctor who said that she had been required to perform a procedure known as a dilation and curettage, which removes tissue from the uterus, “which she was required to perform due to a life-threatening emergency.”
But when, in response to questions, Hawley noted that the doctor had not objected to performing the procedure, Kagan observed that “most hospitals have mechanisms in place, routines in place to ensure that doctors who are allowed to do this … in advance.” But she had not seen anything in the declaration, Kagan continued, to suggest that the doctor had had to perform the procedure over her objection.
Justice Amy Coney Barrett also voiced skepticism that the conscience exemptions would not provide enough protection for health-care providers opposed to abortion. She told Hawley that she read the declaration that Hawley had cited as alleging that the physician had “performed a D&C on a woman who was suffering serious complications, but the fact that she performed a D&C does not necessarily mean that there was a living embryo or a fetus because you can have a D&C after … a miscarriage.”
“So, if that’s right,” Barrett continued, “these affidavits do read more like the conscience objection is strictly to actually participating in the abortion to end the life of the embryo or fetus. And I don’t read either” of the doctors that Hawley cited “to say that they ever participated in that.”
Justice Brett Kavanaugh, who often provides the key vote on ideologically divisive cases, was relatively quiet on Tuesday, but he too focused on the scope of the conscience exemptions. “Just to confirm,” he asked Prelogar, “under federal law, no doctors can be forced against their consciences to perform or assist in an abortion, correct?”
“Yes,” Prelogar responded. “We think that federal conscience protections provide broad coverage here.”
Justice Ketanji Brown Jackson echoed the idea that the existence of the conscious exemptions undermined the challengers’ claim to standing. Jackson described what she characterized as a “significant mismatch” between the injury that the challengers claim and the 5th Circuit’s remedy. The challengers argue that they are injured by “being forced to participate in a medical procedure that they object to.” The “obvious common-sense remedy,” Jackson posited, in this case, “would be to provide them with an exemption,” but they already have that and are instead “seeking an order preventing anyone from having access to these drugs at all.”
Thomas was dubious about the groups’ claim to standing in their own right, known as organizational standing. The challengers contended (among other things) that the groups had been injured because they had had to divert their own resources in response to the FDA’s actions. But in a question for Hawley, Thomas asked whether that diversion wasn’t “just the cost of litigation?”
Hawley countered that the groups had been “forced to divert resources from speaking and advocating for their pro-life mission generally to explaining the dangers of the harm from abortion drugs.”
Thomas seemed unconvinced, however. “[T]hat would be anyone who is aggressive or vigilant about bringing lawsuits. Just simply by using resources to advocate their position in court, you say now, causes an injury. That seems easy to manufacture.”

Erin Hawley, of Alliance Defending Freedom, arguing for the challengers. (William Hennessy)
The justices spent relatively little time on the merits of the challenge to the FDA’s actions. Alito, who along with Thomas dissented from the court’s decision last year to allow mifepristone to remain widely available while the challenge continued, was the challengers’ most vocal supporter. He asked Ellsworth whether she believed that “the FDA is infallible?” “Has the FDA,” Alito continued, “ever approved a drug and then pulled it after experience showed that it had a lot of really serious adverse consequences?” And he questioned the FDA’s decision to roll back the requirement that prescribers report complications from the drug, known as “adverse events,” that are not fatal. Wouldn’t Danco want data, he queried, about such complications?
Ellsworth responded that the FDA had “decided not to continue that reporting requirement in 2016 based on more than 15 years of a well-established safety profile when that reporting was required. There is no drug on the market today,” she stressed, “that requires the kind of reporting that the Plaintiffs are saying should be reimposed here.”
On the other end of the ideological spectrum, Jackson was more sympathetic. She asked Ellsworth whether “courts have specialized scientific knowledge with respect to pharmaceuticals.” “As a company that has pharmaceuticals,” she continued, “do you have concerns about judges parsing medical and scientific studies?”
Justice Neil Gorsuch had a different concern: the scope of the relief ordered by the 5th Circuit. Gorsuch, who has long been a critic of so-called nationwide, or universal, injunctions, emphasized that courts normally provide “a remedy sufficient to address the plaintiff’s asserted injuries and go no further.” But this case, involving a “handful of individuals” opposed to abortion, he told Hawley, “seems like a prime example of turning what could be a small lawsuit into a nationwide legislative assembly on an FDA rule or any other federal government rule.”
In her rebuttal, Prelogar acknowledged the “profound mismatch” between the injury that the challengers are claiming and the remedy that the 5th Circuit ordered. “[W]hat the Court did to guard against that very remote risk” that a health-care provider will have to provide treatment despite the protections available to her “is enter sweeping nationwide relief that restricts access to mifepristone for every single woman in this country.” She urged the justices to reverse the 5th Circuit’s decision and send the case back to the lower courts “with instructions to dismiss to conclusively end this litigation.”
A decision in the case is expected by summer.
This article was originally published at Howe on the Court .
Posted in Featured , Merits Cases
Cases: Danco Laboratories, L.L.C. v. Alliance for Hippocratic Medicine , Food and Drug Administration v. Alliance for Hippocratic Medicine
Recommended Citation: Amy Howe, Supreme Court appears likely to allow abortion drug to remain available , SCOTUSblog (Mar. 26, 2024, 3:12 PM), https://www.scotusblog.com/2024/03/supreme-court-appears-likely-to-allow-abortion-drug-to-remain-available/
Privacy Overview
Drugs of Abuse: Trends and Advanced Analytical Methods
- First Online: 29 October 2023
Cite this chapter

- Sachil Kumar 6
361 Accesses
Abuse of drugs is correlated with various medical, behavioral, psychological, spiritual, financial, social, family, and legal concerns and profoundly impact the people involved, their families, and society. Drugs of abuse (DOA) testing in biological specimens may provide objective information on the usage or misuse of drugs by the person involved and thus it is considered as one of the main tasks in the different disciplines of forensic toxicology and related areas. Testing is carried out for two primary reasons. The first is to test for or prove an alleged acute drug effect or intoxication/poisoning. The second is monitoring abstinence from DOA, e.g., in workplace drug testing.
This chapter deals with the types and methods of analysis for DOA testing that focus on trends and developments in the last decade regarding relevant analytes and analytical methodology. The authors often rely on alternative matrices for monitoring DOAs.
- Drug of abuse
- Forensic toxicology
- Immunoassay
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Aacc.org (2021) Developing standards for oral fluid as an alternative matrix for toxicology testing. AACC.org . Available https://www.aacc.org/cln/articles/2017/november/developing-standards-for-oral-fluid-as-an-alternative-matrix-for-toxicology-testing . Accessed 1 March 2021
Abdallah IA, Hammell DC, Stinchcomb AL, Hassan HE (2016) A fully validated LC–MS/MS method for simultaneous determination of nicotine and its metabolite cotinine in human serum and its application to a pharmacokinetic study after using nicotine transdermal delivery systems with standard heat application in adult smokers. J Chromatogr B 1020:67–77
Article Google Scholar
Abubakar II, Tillmann T, Banerjee A (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171
Agius R, Nadulski T, Kahl HG, Dufaux B (2012) Significantly increased detection rate of drugs of abuse in urine following the introduction of new German driving licence regranting guidelines. Forensic Sci Int 215(1-3):32–37
Article CAS PubMed Google Scholar
Ammann J, McLaren JM, Gerostamoulos D, Beyer J (2012) Detection and quantification of new designer drugs in human blood: part 1–synthetic cannabinoids. J Anal Toxicol 36(6):372–380
Australian/New Zealand Specialist Advisory Group in Toxicology (2012) MS identification guidelines in forensic toxicology – an Australian approach. TIAFT Bull 42(2):52–55
Google Scholar
Baumgartner MR (2014) Nails: an adequate alternative matrix in forensic toxicology for drug analysis? Bioanalysis 6(17):2189–2191
Baumgartner MR, Guglielmello R, Fanger M, Kraemer T (2012) Analysis of drugs of abuse in hair: evaluation of the immunochemical method VMA-T vs. LC–MS/MS or GC–MS. Forensic Sci Int 215(1-3):56–59
Blencowe T, Pehrsson A, Lillsunde P, Vimpari K, Houwing S, Smink B, Verstraete A (2011) An analytical evaluation of eight on-site oral fluid drug screening devices using laboratory confirmation results from oral fluid. Forensic Sci Int 208(1-3):173–179
Bogusz MJ (1999) Hyphenated liquid chromatographic techniques in forensic toxicology. J Chromatogr B Biomed Sci Appl 733(1-2):65–91
Bogusz MJ, Maier RD, Erkens M, Driessen S (1997) Determination of morphine and its 3-and 6-glucuronides, codeine, codeine-glucuronide and 6-monoacetylmorphine in body fluids by liquid chromatography atmospheric pressure chemical ionization mass spectrometry. J Chromatogr B Biomed Sci Appl 703(1-2):115–127
Bogusz MJ, Maier RD, Krüger KD, Kohls U (1998) Determination of common drugs of abuse in body fluids using one isolation procedure and liquid chromatography-atmospheric-pressure chemical-ionization mass spectrometry. J Anal Toxicol 22(7):549–558
Bogusz MJ, Maier RD, Erkens M, Kohls U (2001) Detection of non-prescription heroin markers in urine with liquid chromatography—atmospheric pressure chemical ionization mass spectrometry. J Anal Toxicol 25(6):431–438
Bosker WM, Huestis MA (2009) Oral fluid testing for drugs of abuse. Clin Chem 55(11):1910–1931
Article CAS PubMed PubMed Central Google Scholar
Caplan YH, Goldberger BA (2001) Alternative specimens for workplace drug testing. J Anal Toxicol 25(5):396–399
CDPHE (Colorado Department of Public Health and Environment) (2016) 2016 medical marijuana registry statistics. https://www.colorado.gov/pacific/cdphe/2016-medical-marijuana-registry-statistics
Chèze M, Duffort G, Deveaux M, Pépin G (2005) Hair analysis by liquid chromatography–tandem mass spectrometry in toxicological investigation of drug-facilitated crimes: report of 128 cases over the period June 2003–May 2004 in metropolitan Paris. Forensic Sci Int 153(1):3–10
Article PubMed Google Scholar
Choo RE, Murphy CM, Jones HE, Huestis MA (2005) Determination of methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, 2-ethyl-5-methyl-3, 3-diphenylpyraline and methadol in meconium by liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B 814(2):369–373
Article CAS Google Scholar
Concheiro M, de Castro A, Quintela O, Cruz A, Lopez-Rivadulla M (2004) Chromatogr B 810:319
Concheiro M, Simoes SMDSS, Quintela O, de Castro A, Dias MJR, Cruz A, López-Rivadulla M (2007) Fast LC–MS/MS method for the determination of amphetamine, methamphetamine, MDA, MDMA, MDEA, MBDB and PMA in urine. Forensic Sci Int 171(1):44–51
Cone EJ (1997) New developments in biological measures of drug prevalence. NIDA Res Monogr 167:108–129
CAS PubMed Google Scholar
Dams R, Murphy CM, Lambert WE, Huestis MA (2003) Urine drug testing for opioids, cocaine, and metabolites by direct injection liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 17(14):1665–1670
Dams R, Choo RE, Lambert WE, Jones H, Huestis MA (2007) Oral fluid as an alternative matrix to monitor opiate and cocaine use in substance-abuse treatment patients. Drug Alcohol Depend 87(2-3):258–267
Dasgupta A (2007) Handbook of drug monitoring methods: therapeutics and drugs of abuse. Springer, New York
Dolan K, Rouen D, Kimber JO (2004) An overview of the use of urine, hair, sweat and saliva to detect drug use. Drug Alcohol Rev 23(2):213–217
Dolder PC, Liechti ME, Rentsch KM (2018) Development and validation of an LC-MS/MS method to quantify lysergic acid diethylamide (LSD), iso-LSD, 2-oxo-3-hydroxy-LSD, and nor-LSD and identify novel metabolites in plasma samples in a controlled clinical trial. J Clin Lab Anal 32(2):e22265
Drummer OH (2004) Postmortem toxicology of drugs of abuse. Forensic Sci Int 142(2-3):101–113
Drummer OH (2006) Drug testing in oral fluid. Clin Biochem Rev 27(3):147
PubMed PubMed Central Google Scholar
Eichhorst JC, Etter ML, Rousseaux N, Lehotay DC (2009) Drugs of abuse testing by tandem mass spectrometry: a rapid, simple method to replace immunoassays. Clin Biochem 42(15):1531–1542
EMCDDA (2016) Information on the high-risk drug use (HRDU) (formerly ‘problem drug use’ (PDU)) key indicator. emcdda.europa.eu . Accessed 27 September 2016
Fucci N, De Giovanni N, De Giorgio F, Liddi R, Chiarotti M (2006) An evaluation of the Cozart® RapiScan system as an on-site screening tool for drugs of abuse in a non-conventional biological matrix: vitreous humor. Forensic Sci Int 156(2-3):102–105
Garg U, Ferguson AM (2012) Alternate specimens for drugs-of-abuse testing: preanalytical and interpretative considerations. In: Barbarajean M, Bissell MG, Kwong TC, Wu AHB (eds) Clinical toxicology testing: a guide for laboratory professionals. CAP Press, Durham, pp 71–80
Garside D (2008) Drugs-of-abuse in nails. In: Drug testing in alternate biological specimens. Humana Press, New York, pp 43–65
Chapter Google Scholar
Gjerde H, Verstraete A (2010) Can the prevalence of high blood drug concentrations in a population be estimated by analyzing oral fluid? A study of tetrahydrocannabinol and amphetamine. Forensic Sci Int 195(1-3):153–159
Gjerde H, Verstraete AG (2011) Estimating equivalent cut-off thresholds for drugs in blood and oral fluid using prevalence regression: a study of tetrahydrocannabinol and amphetamine. Forensic Sci Int 212(1-3):e26–e30
Goggin MM, Janis GC (2019) Salt-assisted liquid-liquid extraction of meconium for analysis of cocaine and amphetamines by liquid chromatography-tandem mass spectrometry. In: LC-MS in drug analysis. Humana Press, New York
Guthrie R, Susi A (1963) A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32(3):338–343
Hadland SE, Levy S (2016) Objective testing: urine and other drug tests. Child Adolesc Psychiatr Clin 25(3):549–565
Hancock JR, D’Agostino PA, Chenier CL (2005) Presented at the 3rd conference on mass spectrometry applied to chemical and biological warfare agents, Noordwijkerhout, The Netherlands, 17–20 April 2005
Holmgren P, Druid H, Holmgren A, Ahlner J (2004) Stability of drugs in stored postmortem femoral blood and vitreous humor. J For Sci 49(4):1–6
Jaffee WB, Trucco E, Levy S, Weiss RD (2007) Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abus Treat 33(1):33–42
Janda I, Weinmann W, Kuehnle T, Lahode M, Alt A (2002) Determination of ethyl glucuronide in human hair by SPE and LC–MS/MS. Forensic Sci Int 128(1-2):59–65
Jang M, Kim J, Han I, Yang W (2015) Simultaneous determination of LSD and 2-oxo-3-hydroxy LSD in hair and urine by LC–MS/MS and its application to forensic cases. J Pharm Biomed Anal 115:138–143
Jantos R, Veldstra JL, Mattern R, Brookhuis KA, Skopp G (2011) Analysis of 3,4-methylenedioxymetamphetamine: whole blood versus dried blood spots. J Anal Toxicol 35(5):269–273
Jeanville PM, Woods JH, Baird TJ III, Estapé ES (2000) Direct determination of ecgonine methyl ester and cocaine in rat plasma, utilizing online sample extraction coupled with rapid chromatography/quadrupole orthogonal acceleration time-of-flight detection. J Pharm Biomed Anal 23(5):897–907
Jensen TL, Wu F, McMillin GA (2019) Detection of in utero exposure to Cannabis in paired umbilical cord tissue and meconium by liquid chromatography-tandem mass spectrometry. Clin Mass Spectrometry 14:115–123
Katagi M, Nishikawa M, Tatsuno M, Miyazawa T, Tsuchihashi H, Suzuki A, Shirota O (1998) Direct analysis of methamphetamine and amphatamine enantiomers in human urine by semi-microcolumn HPLC: electrospray ionization mass spectrometry. Eisei Kagaku 44(2):107–115
Katagi M, Tatsuno M, Miki A, Nishikawa M, Tsuchihashi H (2000) Discrimination of dimethylamphetamine and methamphetamine use: simultaneous determination of dimethylamphetamine-N-oxide and other metabolites in urine by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Anal Toxicol 24(5):354–358
Katagi M, Nishikawa M, Tatsuno M, Miki A, Tsuchihashi H (2001) Column-switching high-performance liquid chromatography–electrospray ionization mass spectrometry for identification of heroin metabolites in human urine. J Chromatogr B Biomed Sci Appl 751(1):177–185
Kumar S, Wahid A, Anoop KV (2012) Physiology and anatomy of hair in drug abusing cases. IJMTFM 2:153–159
Lua IA, Lin SL, Lin HR, Lua AC (2012) Replacing immunoassays for mephedrone, ketamines and six amphetamine-type stimulants with flow injection analysis tandem mass spectrometry. J Anal Toxicol 36(8):575–581
Moeller KE, Lee KC, Kissack JC (2008) Urine drug screening: practical guide for clinicians. In: Mayo clinic proceedings, vol 83. Elsevier, Amsterdam, pp 66–76
Musshoff F, Madea B (2007) Analytical pitfalls in hair testing. Anal Bioanal Chem 388(7):1475–1494
Musshoff F, Kirschbaum KM, Graumann K, Herzfeld C, Sachs H, Madea B (2012) Evaluation of two immunoassay procedures for drug testing in hair samples. Forensic Sci Int 215(1-3):60–63
National Center on Substance Abuse and Child Welfare/Substance Abuse and Mental Health Services Administration (2015) Drug testing practice guidelines. Available https://ncsacw.samhsa.gov/files/IA_Drug_Testing_Bench_Card_508.pdf
Nishikawa M, Nakajima K, Tatsuno M, Kasuya F, Igarashi K, Fukui M, Tsuchihashi H (1994) The analysis of cocaine and its metabolites by liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry (LC/APCI-MS). Forensic Sci Int 66(3):149–158
Patel KN, Patel JK, Patel MP, Rajput GC, Patel HA (2010) Introduction to hyphenated techniques and their applications in pharmacy. Pharm Methods 1(1):2–13
Article PubMed PubMed Central Google Scholar
Paul LD, Musshoff F, Aebi B, Auwärter V, Krämer T, Peters F, Schmitt G (2009) Richtlinie der GTFCh zur Qualitätssicherung bei forensisch-toxikologischen Untersuchungen. Toxichem Krimtech 76(3):142–176
Pelander A, Ristimaa J, Ojanperä I (2010) Vitreous humor as an alternative matrix for comprehensive drug screening in postmortem toxicology by liquid chromatography-time-of-flight mass spectrometry. J Anal Toxicol 34(6):312–318
Peters FT, Schaefer S, Staack RF, Kraemer T, Maurer HH (2003) Screening for and validated quantification of amphetamines and of amphetamine-and piperazine-derived designer drugs in human blood plasma by gas chromatography/mass spectrometry. J Mass Spectrom 38(6):659–676
Pujol ML, Cirimele V, Tritsch PJ, Villain M, Kintz P (2007) Evaluation of the IDS One-Step™ ELISA kits for the detection of illicit drugs in hair. Forensic Sci Int 170(2-3):189–192
Quest Diagnostics (n.d.) Common drugs of abuse. https://www.questdiagnostics.com/home/companies/employer/drug-screening/drugs-tested/
Rapinoja M-L, Kuitunen M-L, Bjork H, Rosendahl K, Vanninen P (2005) Presented at the 3rd conference on mass spectrometry applied to chemical and biological warfare agents, Noordwijkerhout, The Netherlands, 17–20 April 2005
Ristimaa J, Gergov M, Pelander A, Halmesmäki E, Ojanperä I (2010) Broad-spectrum drug screening of meconium by liquid chromatography with tandem mass spectrometry and time-of-flight mass spectrometry. Anal Bioanal Chem 398(2):925–935
SAMHSA (2020) Retrieved 24 June 2020. https://www.samhsa.gov/sites/default/files/workplace/2010GuidelinesAnalytesCutoffs.pdf
Saussereau E, Lacroix C, Gaulier JM, Goulle JP (2012) Online liquid chromatography/tandem mass spectrometry simultaneous determination of opiates, cocainics and amphetamines in dried blood spots. J Chromatogr B 885:1–7
Schänzle G, Li S, Mikus G, Hofmann U (1999) Rapid, highly sensitive method for the determination of morphine and its metabolites in body fluids by liquid chromatography–mass spectrometry. J Chromatogr B Biomed Sci Appl 721(1):55–65
Schubert W, Mattern R (2009) Urteilsbildung in der medizinisch-psychologischen Fahreignungsdiagnostik–Beurteilungskriterien. Kirschbaum, Bonn
Segura J, Ventura R, Jurado C (1998) Derivatization procedures for gas chromatographic–mass spectrometric determination of xenobiotics in biological samples, with special attention to drugs of abuse and doping agents. J Chromatogr B Biomed Sci Appl 713(1):61–90
United Nations (2012) World drug report 2012 (PDF). Retrieved 27 September 2016.
Vindenes V, Yttredal B, Øiestad EL, Waal H, Bernard JP, Mørland JG, Christophersen AS (2011) Oral fluid is a viable alternative for monitoring drug abuse: detection of drugs in oral fluid by liquid chromatography-tandem mass spectrometry and comparison to the results from urine samples from patients treated with methadone or buprenorphine. J Anal Toxicol 35(1):32–39
Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Coggeshall M (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1459–1544
Welch MJ, Sniegoski LT, Tai S (2003) Two new standard reference materials for the determination of drugs of abuse in human hair. Anal Bioanal Chem 376(8):1205–1211
Wille SM, Raes E, Lillsunde P, Gunnar T, Laloup M, Samyn N, Verstraete AG (2009) Relationship between oral fluid and blood concentrations of drugs of abuse in drivers suspected of driving under the influence of drugs. Ther Drug Monit 31(4):511–519
Wilson ID, Brinkman UT (2003) Hyphenation and hypernation: the practice and prospects of multiple hyphenation. J Chromatogr A 1000(1-2):325–356
Wohlfarth A, Weinmann W, Dresen S (2010) LC-MS/MS screening method for designer amphetamines, tryptamines, and piperazines in serum. Anal Bioanal Chem 396(7):2403–2414
Wood M, Laloup M, Samyn N, Fernandez MDMR, de Bruijn EA, Maes RA, De Boeck G (2006) Recent applications of liquid chromatography–mass spectrometry in forensic science. J Chromatogr A 1130(1):3–15
World Health Organization (2004) Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention: WHO/UNODC/UNAIDS position paper. WHO, Geneva
Download references
Author information
Authors and affiliations.
Department of Forensic Sciences, College of Criminal Justice, Naif Arab University for Security Sciences, Riyadh, Saudi Arabia
Sachil Kumar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sachil Kumar .
Editor information
Editors and affiliations.
Biology and Serology Division, Regional Forensic Science Laboratory, Gwalior, Madhya Pradesh, India
Pankaj Shrivastava
University of Granada, Granada, Spain
Jose Antonio Lorente
School of Forensic Sciences, The West Bengal National University of Juridical Sciences, Kolkata, West Bengal, India
Ankit Srivastava
Department of Forensic Science, Government Institute of Forensic Science, Nagpur, Maharashtra, India
Ashish Badiye
Neeti Kapoor
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Kumar, S. (2023). Drugs of Abuse: Trends and Advanced Analytical Methods. In: Shrivastava, P., Lorente, J.A., Srivastava, A., Badiye, A., Kapoor, N. (eds) Textbook of Forensic Science . Springer, Singapore. https://doi.org/10.1007/978-981-99-1377-0_24
Download citation
DOI : https://doi.org/10.1007/978-981-99-1377-0_24
Published : 29 October 2023
Publisher Name : Springer, Singapore
Print ISBN : 978-981-99-1376-3
Online ISBN : 978-981-99-1377-0
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
NEWS... BUT NOT AS YOU KNOW IT
British dad stuck in one of the world’s most notorious and lawless prisons

Share this with

A British dad has been thrown into a notorious jail, where the prisoners are in charge and you have to pay for a cell.
John Henshaw, from Atherton, Greater Manchester , was backpacking through South America when he was stopped by police at La Paz airport in Bolivia on February 9.
Henshaw, 39, was reportedly arrested on a drug trafficking charge for the alleged possession of a ‘tiny’ amount of cannabis, the MailOnline reports.
He was then incarcerated in the San Pedro prison , which was featured on the TV show Behind Bars: The World’s Toughest Prisons.
The jail is known for its lawlessness, with allegedly no guards patrolling the inside the facility, only on the perimeters to make sure inmates don’t escape.
Henshaw’s worried daughter and ex-partner say he has no clean water, clothes or regular meals.
He told them he sleeps on the floor with around 30 inmates and has to share two toilets with some 120 people.

The prison is built to hold 600 inmates but reportedly has around 3,000 and if you want a bed and a cell you have to buy them from other prisoners.
Some of the ‘higher ranking’ prisoners have ‘suites’ with beds, access to wifi and hot tubs.
John is waiting for his case to be assessed and processed but this can reportedly take up to 90 days. So far he’s been at the prison for around 50.
According to his loved ones, he has struggled with illnesses during the stay, including ear infections due to insect infestations.

They have launched a GoFundMe to help him with his legal and living costs, including money for a cell
The page reads: ‘John has found himself in one of the worst prisons in South America.
‘He’s been thrown in San Pedro, La Paz, Bolivia which is featured on an episode of Worlds Toughest Jails.
‘There’s zero human rights, the prison is left to the prisoners. All the guards are on the outside, they never go inside.’

The page has raised almost £3,000.
John’s ex-partner Toni Rimmer and his daughter Kaitlyn Henshaw, 15, are worried about him.
Toni, who is helping with the legal process from the UK, said: ‘He’s a good person. It’s absolutely horrific in there.
‘He can only get money in drip feed from the embassy. The only food he has been able to buy for himself is a ham and cheese toastie. He gets a bowl of broth a day, that’s it.’
Sh e added: ‘He doesn’t deserve what’s happening to him in there, he’s had no previous charges.’

Kaitlyn, who has spoken to her dad on the phone a few times, said she was stressed about the situation and wants him home safe as soon as possible.
John is waiting to hear from a judge on a final decision on his charges and hopes he won’t have to stay in San Pedro for the full 90-day waiting period.
A member of the British Embassy in Bolivia confirmed that they were helping John and that he is in San Pedro prison.
Get in touch with our news team by emailing us at [email protected] .
For more stories like this, check our news page .
MORE : My partner told me I’d never see her or my son again unless I followed her one instruction
MORE : A hippie commune is fighting back against drug gangs
MORE : Huge crane ‘slices through roof’ after crashing into house
Sign Up for News Updates
Get your need-to-know latest news, feel-good stories, analysis and more.
Privacy Policy

Get us in your feed
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Harm Reduct J

An overview of forensic drug testing methods and their suitability for harm reduction point-of-care services
Lane harper.
1 University of Lethbridge, 4401 University Drive, Lethbridge, AB T1K 3M4 Canada
Jeff Powell
2 Carleton University, 1125 Colonel By Dr, Ottawa, ON K1S 5B6 Canada
Associated Data
Given the current opioid crisis around the world, harm reduction agencies are seeking to help people who use drugs to do so more safely. Many harm reduction agencies are exploring techniques to test illicit drugs to identify and, where possible, quantify their constituents allowing their users to make informed decisions. While these technologies have been used for years in Europe (Nightlife Empowerment & Well-being Implementation Project, Drug Checking Service: Good Practice Standards; Trans European Drugs Information (TEDI) Workgroup, Factsheet on Drug Checking in Europe, 2011; European Monitoring Centre for Drugs and Drug Addiction, An Inventory of On-site Pill-Testing Interventions in the EU: Fact Files, 2001), they are only now starting to be utilized in this context in North America. The goal of this paper is to describe the most common methods for testing illicit substances and then, based on this broad, encompassing review, recommend the most appropriate methods for testing at point of care.
Based on our review, the best methods for point-of-care drug testing are handheld infrared spectroscopy, Raman spectroscopy, and ion mobility spectrometry; mass spectrometry is the current gold standard in forensic drug analysis. It would be prudent for agencies or clinics that can obtain the funding to contact the companies who produce these devices to discuss possible usage in a harm reduction setting. Lower tech options, such as spot/color tests and immunoassays, are limited in their use but affordable and easy to use.
Given the current opioid crisis in Canada [ 1 – 3 ] and around the world [ 4 ], harm reduction agencies are seeking to help people who use drugs to do so more safely. Harm reduction sites and/or clinics are increasing in number and service provision across the world, making it crucial to provide point-of-care workers with the tools and knowledge necessary to provide proper care for people who use drugs. Drug, pill, and substance testing are increasingly being used as a harm reduction strategy throughout the world [ 5 – 8 ] to decrease the risk of adverse effects. Indeed, various approaches to drug testing have been around, even in North America, for decades [ 9 – 11 ]. More recently, in Canada, drug testing is becoming more common at music festivals [ 12 ]. In Canada, the Standing Committee on Health [ 13 ] recommended that the Government of Canada grant exemptions under the Controlled Drugs and Substances Act so that drug testing could occur at designated sites. While there are certainly legal hurdles to overcome when it comes to drug testing [ 6 ], there are three primary advantages to testing drugs before they are consumed: short- and long-term adverse effects (including overdose and fatality) can be avoided by the person using the substance; other institutions (such as hospitals) and public health authorities can be made aware when a lethal or novel substance begins to circulate; and, a global picture of drugs in circulation can be generated [ 5 , 14 – 16 ]. The goal of this paper is to describe the most common methods of testing chemical substances in both laboratory and point-of-care settings. We will conclude with recommendations for point-of-care testing of illicit substances. In this paper, we use the term “drug testing” to refer to the forensic testing of illicit substances in their intended consumption form. Please note that the legal issues surrounding, and the service models of, drug testing are beyond the scope of this paper.
Introduction to substance testing methods
The following methods have been validated by the Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG). Scientific Working Groups consist of scientific subject-matter experts who collaborate to determine best practices and develop consensus standards. As such, these methods have been proven to be effective in the analysis of unknown (forensic) examination of illicit substances and are therefore also the best methods to use in identifying unknown substances. Not all of these methods are easily accessible in a point-of-care framework, as some require high technical knowledge and/or a laboratory setting. Therefore, any of the following methods may be suitable on a case-by-case basis. This is due to the fact that some clinics may be able to easily access more discriminatory methods, through direct funding or industry partnership, whereas some clinics may have to rely on less precise testing methodologies and equipment due to lack of funding or support.
More discriminatory methods carry a much larger price tag to invest in the proper equipment. This may require community partnerships or a serious cost-benefit analysis or both. To keep the information precise and to attempt to interpret some of the associated technical details, the methods have been broken down into subheadings. Each method has three subheadings: “How does it work?” (a brief discussion of the theory behind the method), “What substances can be detected and how accurately?”, and “How easy is it to use?” The methods have also been broadly assigned into two larger categories: most discriminatory, or methods that will accurately identify a substance/mixture and that also have the potential to quantify the amount of substance, and least discriminatory, or methods that presumptively identify a substance and/or mixture without quantification. At the end of the paper, there will be a recommendation section that will focus strictly on the best methods/devices considering only point-of-care situations. The methods are summarized in Table Table1 1 .
Summary of drug testing technologies and methods, and definition of terms
None—requires absolutely no knowledge or training. Basic—requires simple (hours to days) training by someone who knows the technique or theory, but is not an expert in the field, i.e., someone with intermediate, advanced, or expert skill/knowledge. Intermediate—requires a higher level of knowledge or skill, although that may be obtained from either following previous instructions obtained (i.e., gaining experience) while a basic user, or from further instruction from an advanced or expert level user. Usually requires days to weeks of experience depending on technique. Advanced—requires some college or university level theory or experience. Usually taught directly or indirectly by an expert in the subject/field. Occasionally, an intermediate user may become advanced without advanced education through diligence and interest. Requires weeks to months (a typical semester). Expert—an expert in the area, almost always has post-secondary education related to the field. May be a bachelor, master, or PhD holder or very high specialized training. Instruction may also be provided by someone from a device manufacturing company who provides a seminar or some sort of direct training in usage of a technique or device. Typically always requires months to years depending on difficulty of the subject
Most discriminatory
Mass spectrometry, how does it work.
Mass spectrometry (MS) is the most discriminatory of the drug testing techniques. Mass spectrometry measures the precise molecular mass of ions as determined by their mass to charge ratio ( m / z ) and is the current gold standard in forensic drug analysis [ 17 ]. In general, mass spectrometry requires separation, ionization, and finally detection. Separation can be accomplished through gas chromatography (GC), liquid chromatography (LC), or capillary electrophoresis (CE). There are various ionization methods. The most commonly used in analysis of illicit substances are electron ionization (EI), atmospheric pressure chemical ionization (APCI), electrospray ionization (ESI), matrix-assisted laser desorption ionization (MALDI), atmospheric pressure photoionization (APPI), fast atom bombardment (FAB), and more recently direct analysis in real time (DART). Ionization methods can be grouped into hard or soft techniques.
Hard techniques like EI, FAB, and APCI cause molecules to fragment generating complex mass spectra. Fragmentation is useful in analysis because molecules have known fragmentation patterns. A spectral database allows for a computer to quickly match spectra and determine the molecular species. Hard techniques are limited to detecting small molecules. Most illicit drugs are small molecules with the exception of drugs of a biological nature being consumed in their raw form.
Soft ionization techniques such as MALDI and ESI minimize fragmentation and allow for the molecules being analyzed to remain intact. Soft ionization techniques are useful for large biomolecules such as proteins.
DART is of particular interest as it allows non-destructive testing, is fast, and can quickly quantify when used with an internal standard. A pill can be held in front of the gas stream and within seconds determine the molecular species present. DART does not require separation of each molecular species prior to analysis allowing untrained personnel to collect data [ 18 ].
What substances can be detected and how accurately?
Virtually, any substance can be identified using MS in combination with a separation (chromatographic) technique. Sensitivity of current mass spectrometers allows for detection of analytes at concentration in the attomolar range (10 −18 ) [ 19 ]. MS has increased sensitivity over some other analytical techniques as the analyzer, a mass-charge filter, reduces background interference (i.e., a clearer reading/analyte fingerprint can be produced). It demonstrates excellent specificity due to characteristic fragmentation patterns, high resolution, and unique filtering abilities available especially in tandem or higher order mass spectrometry [ 20 ].
MS provides information about molecular mass and isotopic abundance of elements and temporally resolved chemical data, allowing for highly accurate identification. Newer devices are easier to utilize and much smaller than older versions. Interfacing with computers allows for refined database searches, making the drug identification process easier.
A major drawback of MS is that the tested sample taken from the supply is destroyed by the testing process (DART being an exception). Only a very small sample size (milligrams) is required. There are also continuing costs due to consumable materials required, and some of these consumables are poisonous/hazardous. Complex mixtures must be separated with a chromatographic technique (either gas or liquid chromatography) to correctly identify each constituent (unless using DART).
How easy is it to use?
The expertise required to utilize this technology is intermediate to expert (for definitions of terms in context with this paper please, see Table Table1). 1 ). Individuals should have some theoretical knowledge of how the technology and specific instrument work and specialized training from an expert. The cost of a mass spectrometer can vary from US$5000 to US$1,000,000. While an older used mass spectrometer may be less expensive upfront, it is not necessarily suitable for point-of-care drug testing. There are also considerable ongoing operational costs, such as chromatography (separation) reagents, gas consumables (nitrogen, helium, etc.), sample preparation items, and routine maintenance and service. Some labs offer MS services with costs between US$5 and US$100 per sample.
Ion mobility spectrometry
Ion mobility spectrometry (IMS) separates and identifies ions based on their speed through a carrier gas. Ion mobility is dependent on three molecular characteristics: the charge, reduced mass, and the collision cross section of the ion. IMS requires ionization before samples are passed into the instrument. This can be accomplished by ESI, MALDI, APPI, and coronal discharge or by using radioactive sources such as nickel-63.
There are many designs for ion mobility spectrometers including drift tube, ion trap, traveling wave, high-field asymmetric waveform, and differential mobility types. Drift tube IMS determines the ion mobility based on the amount of time it takes for ions to reach the detector. Many modern instruments use a drift tube for analysis.
Of interest is the field asymmetric subtype of the high-field asymmetric waveform IMS. A field asymmetric ion mobility spectrometer (FAIMS) uses a high (strong) electric field to control the movement of the ions through a physical filter. A pulsing electric field can then be applied to select for ions with specific ion mobility. Only ions with the specifically selected mobility will be able to maintain a stable trajectory through the filter. The others will crash into the side walls and not reach the detector.
Any small molecule of illicit substance can be detected very quickly and accurately. FAIMS sensitivity is based on multiple characteristics of both the ion of interest and the physical environment. IMS can detect one molecule in a billion (ppb) and is very selective. IMS selectivity can be further enhanced when using FAIMS. FAIMS is able to operate in environments with high levels of interference with minimal adjustment to operating conditions [ 21 ]. IMS is non-destructive and only requires a very small sample if a quantitative method calls for destructive testing. Determination is very quick and can be accomplished in a few seconds even for a complex sample.
IMS instruments do not require a trained operator. They can be used to quickly analyze a sample. Identification does require a database of known molecules to compare the sample against. The process of building a database would require a trained chemist using another technique or a standard. Once built, a database could be referenced from any instrument without additional technical help [ 22 ]. Quantification is possible when using internal standards or prebuilt methods. IMS is regularly used by law enforcement agencies at airports to detect narcotics and explosives. Minimal maintenance, ease of use by non-technical personnel, low cost, fast and accurate determination, minimal cost of consumables, and robust methodologies make IMS one the best choices for drug identification.
Infrared spectrometry
Infrared (IR) spectroscopy is another highly discriminatory method and is based on the measurement of the amount of IR radiation which is absorbed or emitted by a sample as a function of wavelength. A spectrum is obtained by passing infrared radiation through a sample and determining the amount of the incident radiation (radiation that actually hits the molecule rather than passing through) that is absorbed at each IR frequency [ 23 ]. Interpretation of the spectra allows for determination of molecular functional groups. The IR spectra of a pure molecular compound provides a distinctive fingerprint which can be easily differentiated from the IR absorption pattern of other compounds, including compounds with the same chemical formula, but a different arrangement of atoms in the molecule (known as isomers) [ 23 ]. An advantage of IR techniques is that virtually, all compounds have IR active vibrational modes and can therefore be investigated both qualitatively and quantitatively. However, quantitative analysis can pose a problem with unknown samples and mixtures. The spectroscopic expertise required to forensically analyze and quantify a substance may be difficult or impossible to find in harm reduction clinics. Most papers that describe relatively simple quantification methods are carried out in pharmaceutical research with controlled standards, methodologies, and standards. While quantification of unknown substances is technically possible, it really comes down to a case-by-case basis and is generally a laborious process undertaken by advanced to expert level technicians and chemists in forensic laboratories. It is highly unlikely that quantification would be viable using this technology in this kind of setting. Recent advances in IR technology have allowed for the development of portable IR devices.
When reference spectra are available, most compounds can be unambiguously identified based on their IR spectra. Drugs can be identified through a searchable database (such as http://webbook.nist.gov /). IR cannot distinguish enantiomers (similar to MS) [ 24 ]. According to the SWGDRUG [ 24 ], IR can produce structural information that will provide sufficient selectivity that generates the highest discriminating capability. IR can discriminate between diastereomers (such as pseudoephedrine and ephedrine) and free base/acid and salt forms. Free base/acid and salt forms refer to differences in physical properties that can alter the application of the substance. Free base is usually more volatile and normally has a lower boiling point, allowing the substance to be smoked. The salt form is usually more stable and tends to be crystalline and dissolvable in water, allowing for ingestion, insufflation (inhaling through the nose), or injection. A common example is crack cocaine (free base) and cocaine (salt); they are in fact the same drug (cocaine), and the actual effect on the body is the same, but due to different absorption and dosages based on method of use, it is possible to observe a spectrum of differing responses to each of the drugs. One of the notable benefits of IR spectroscopy is that it does not destroy the sample provided—an important consideration when working with drugs and the people who use them. As well, it requires only a very small sample size in the range of milligrams or less. Additionally, samples can be studied in virtually any physical state (primarily solid or liquid). Interference is very common and causes difficulty in identification.
The level of expertise required to use this technology varies depending on the device. There are portable IR devices on the market that have been optimized for basic to intermediate knowledge base, such as by outreach workers. These devices can analyze the obtained spectrum and search internal databases to display the identified substance or substances in a mixture (to a certain concentration, based on the specifications of a given device). This is considered presumptive or qualitative testing, in that it may only give an accurate breakdown of the constituents of a substance or mixture and sometimes offer a semi-quantitative analysis (i.e., rank-ordered most to least in a mixture). For quantification (as percent mass by total mixture weight), Sorak et al. have shown that some portable IR devices may be used for low error quantitative analysis [ 25 ]; although in order to interpret the obtained spectrum in the these devices in a quantitative manner, advanced to expert level knowledge is required as the devices do not perform this task for the user. Many other IR devices also require at least an intermediate level understanding of the procedures and some require advanced to expert knowledge to correctly analyze and quantify the substances (including operation of the equipment and database searching). Costs of IR devices can be anywhere from the low thousands to US$60,000 and above.
Raman spectroscopy
Raman spectroscopy is an optical technique based on the inelastic scattering of radiation after it interacts with matter. The interaction of incident radiation with the molecules of the substance gives spectral vibrational information [ 26 ]. The technique involves shining a laser on a sample and detecting the scattered light. A small amount of the scattered light is shifted in energy from the laser frequency due to electromagnetic and molecular interactions in the sample [ 26 ]. Plotting the intensity of the shifted light versus frequency gives a Raman spectrum of the sample. An exciting breakthrough in this technology is the development of handheld, portable Raman spectrometers. Many of these devices, most notably the TruNarc device by Thermo Fisher Scientific, have been optimized for drugs of abuse detection with simple “point and shoot” action. These devices also search databases in real time at a device level and give a clear readout of what substance(s) were detected.
Virtually, any drug can be identified with Raman spectroscopy. It can be used to determine active pharmaceutical ingredients (APIs) as well as molecules with the same chemical formula but different molecular arrangement and polymorphs. This is important as many of the novel psychoactive substances that have been emerging are isomers, derivatives, and analogues of many of the classical drugs of abuse. Being able to differentiate between small differences in physical or chemical structure aids greatly in unambiguous identification. Portable Raman spectroscopy has even been reported to be able to detect the date-rape drug rohypnol (flunitrazepam) in spiked beverages [ 27 ].
Raman spectroscopy may have difficulty in identifying substances that exhibit strong fluorescence. These substances tend to be plant-based narcotics such as heroin. However, with proper sample preparation, it is possible to analyze even these substances. The TruNarc Raman spectroscopy device has been shown to have a very high level of agreement with laboratory results (MS) for cocaine, heroin, and methamphetamine; inconclusive results are generally related to illicit substances that are present at extremely low percentages of the total mixture. Some studies have indicated that cocaine can be detected at concentrations as low as 5% when the cocaine was cut with sorbitol [ 28 ]. Others have detected amphetamine residues (milli- to micrograms) on paper currency using Raman spectroscopy [ 29 ]. It must be stressed that the particular technology discussed (TruNarc by Thermo Fisher Scientific) does not offer quantitative data in its “point and shoot” identification action, although it does offer highly accurate and extremely easy-to-use qualitative testing. The Raman technique as a whole is able to identify and quantify (depending on the device) a wide range of illicit drugs, even in the presence of contaminants and adulterants [ 26 ]. Given that there are many substances used to “cut” illicit drugs, this feature is an important one.
RS is rapid and non-destructive, does not require chemical reagents, can detect separate substances in mixtures, is not subject to interference from water or moisture, and importantly, can detect substances through transparent packaging (such as plastic bags and glass containers). Little or no sample preparation is required, although some sample preparation is required for substances that exhibit high fluorescence (including some cutting agents). RS is ideal for both organic and inorganic species and can be used for both qualitative and quantitative analysis. Due to the similarity to IR (detecting forms of molecular movement to identify), Raman has similar issues with quantitative analysis. While quantitative analysis can absolutely be done with Raman spectroscopy, it can be a much more difficult process that may not be possible in a harm reduction setting. Due to the difficulty of quickly and easily performing quantitative analysis on many unknown samples, an important consideration for outreach is that portable handheld devices specifically designed to detect drugs of abuse are available. Qualitative results can be obtained in a fraction of seconds to several minutes.
The cost of a RS unit can vary widely (in the low thousands of dollars to US$50,000 and above). Like all of the previous devices, care must absolutely be taken in selecting the appropriate tool. Advanced knowledge is required for devices that are not optimized for drug testing.
The level of expertise required to use this technology varies depending on the device, similar to IR. Some Raman spectrometers have been optimized for “point and shoot” action, giving a clear interpretation/reread of the substance(s) analyzed, and thus require merely basic to intermediate expertise for presumptive analysis. The requirements for quantitative analysis for portable “point and shoot” Raman spectrometers are similar to IR. Sorak et al. have also shown that some portable Raman spectrometers can offer quantitative analysis to a high degree of precision [ 25 ], although it must be stressed that this comes with the exact same considerations as the portable IR, as stated above. Other bench top or lab specific devices are most often not as simple and may require some database searching and interpretation of results. This can push the level of expertise required to intermediate, advanced, or expert, depending on the chosen device.
X-ray diffractometry
In X-ray diffractometry (X-ray D), the drug sample is bombarded with high-energy X-ray radiation and crystalline atoms in the substance cause incident X-ray beams to diffract in various directions [ 30 ]. This allows for the determination of the spatial structure of molecules by measurement of how X-ray radiation is scattered by the molecular crystal lattice structure. By measuring the angles and intensities of the diffracted X-rays, it is possible to produce a three-dimensional picture of the density of electrons in the crystal, and, from this, it is possible to determine the positions of the atoms in the crystal as well as their chemical bonds and other structural information [ 30 ].
Any crystalline or partially crystalline substance (i.e., substances that are solid and usually either evidently crystalline or powder or pill, such as methamphetamine, ketamine, and cocaine) including those in mixtures and compounds with currently unidentified structure can be identified [ 31 , 32 ]. This method is generally restricted to solid substances. X-ray D is used to identify precise chemical forms but not to quantify them. It can be used to identify diluents or adulterants [ 31 ]. This method is sensitive to both polymorphs and contaminants (common in illicit drugs). X-ray diffractometry determines structural information of the substance, so the substance can be identified with a very high degree of accuracy. This method is specific because substances have unique diffraction lines or an “X-ray fingerprint.” It is also sensitive in that drug concentrations and any additional agents used in cutting can be discerned through the obtained data. Studies have shown that this method can be used to identify a specific drug at only 5% of the total pharmaceutical formulation [ 33 ].
One benefit of X-ray D is that it requires no sample preparation and does not destroy the substance being tested. As well, only a very small sample size is needed (milligrams to micrograms) [ 31 ]. While it is the most reliable structural determination method and can determine the structure of currently unknown molecules, it is not suitable outside of a laboratory environment.
X-rays are highly radioactive and very damaging to organic cells/DNA. Thus, this method requires a high level of training and safety procedures and is restricted to laboratory environments. The skill level involved in operation is advanced to expert.
Least discriminatory
Microcrystalline tests.
These chemical tests result in the formation of unique microcrystals of a given analyte when a specific reagent is applied. The unique crystal formation is compared to a reference standard/control using a common light microscope. Microcrystals are compared based on shape, size, color, and spatial arrangement [ 34 ].
Several commonly abused substances can be identified, including cocaine, heroin, methadone, GHB (gamma hydroxybutyrate ) , ketamine, phencyclidine, amphetamines, and methamphetamine [ 34 ]. With test reagents chosen to induce development of specific microcrystals with the analyte and a reference/control standard available, these tests can be highly specific as the crystals formed are a direct consequence of choice of reagent and analyte and are unique under these circumstances. This is provided that other substances do not react in a similar way, if at all, with the reagent, and provided that impurities, dilutents, and adulterants do not prevent or mask the formation of characteristic microcrystals for the drug tested. In these cases, a microcrystalline test can be considered highly characteristic but non-specific enough for a confirmatory test. Thus, this method is best suited to pure and/or separated samples. Sensitivity is high as samples require only micrograms of substance.
The benefit of microcrystalline tests is their relatively low cost. Minute amounts of reagents are required. Instrumentation is simple; however, this method does not quantify how much of a substance is present. Unfortunately, the sample that is tested is destroyed in the process, which may be less than ideal for people who are bringing the samples for identification.
The expertise required is intermediate to advanced and requires adept interpretation of results.
Thin-layer chromatography
Thin-layer chromatography (TLC) is a technique in which a sample is placed onto a planar stationary phase then a liquid mobile phase resulting in capillary action. The analyte is either adsorbed to the stationary phase or is in the mobile phase, and the time spent on the stationary phase or time spent in the mobile phase determines its retention time. Components of the sample travel at differing rates depending on the component’s size and affinity for the mobile phase [ 35 ]. The result is a plate of spots (separated components of the mixture) that have moved various distances on the stationary phase.
TLC can detect barbiturates, benzodiazepines, GHB, heroin, morphine, opium, oxycodone, and other opiates, amphetamines, cocaine, methamphetamine, MDMA (methylenedioxymethamphetamine or Ecstasy), ketamine, LSD, marijuana, mescaline, synthetic cannabinoids, and cathinones (commonly referred to as “bath salts”). Using TLC, it may be difficult to separate and identify novel psychoactive substances [ 36 ]. TLC performs fairly poorly at separating complex mixtures. Sensitivity is in the micro-nanogram range. Specificity can range from intermediate to high depending on the mixture, and measured retention factors can be used to make a preliminary identification of a substance but are not specific to a single compound [ 35 ]. In order to increase specificity in cases of similar retention factors, it must be used in conjunction with another technique such as Raman spectroscopy or colorimetric testing or in the case of UV active species, UV.
TLC is a relatively low-cost way to test substances and demonstrates good sensitivity and speed of separation. It can be used as a presumptive test with a fairly high degree of accuracy depending on sample purity. While TLC can identify some known substances in provided samples, it does not indicate (quantify) how much of a substance is present in the sample. TLC is best used in conjunction with a more discriminating technique such as Raman spectroscopy, MS, or IR.
TLC is relatively simple to use and interpret and is thus suitable for basic to advanced skill level. This means that someone with basic skill may be able to perform a test following instructions but have trouble interpreting the results, whereas someone with intermediate to advanced skill level would have greater ability to interpret a test and could supervise basic skill level users.
Spot/color tests
Spot/color tests offer presumptive testing based on chemical reactions between analytes and indicators. There are many possible indicator tests such as cobalt thiocyanate, Dille-Koppanyi, Duquenois-Levine, Mandelin, Marquis, nitric acid, para-dimethylaminobenzaldehyde, ferric chloride, Froehde, Mecke, Zwikker, and Simon’s (nitroprusside) [ 37 ]. The indicator chemically reacts with the analyte and causes a reaction that creates a certain color staining depending on the analyte tested. Spots are then compared visually with reference charts, the current standard being the Munsell color charts. There is a method that bypasses the human eye and its subjectivity by using a simple smartphone app to identify colors with high precision and accompanying software that matches the results in a searchable database [ 38 ]. This allows for a more precise quantitation of the color and therefore higher accuracy identification.
What substances can be detected, and how accurately?
Colorimetric tests exist for most drugs of abuse, including cocaine, various pharmaceutical opioids, amphetamines, LSD (lysergic acid diethylamide), cathinones (bath salts), heroin, and fentanyl. There may be other novel psychoactive substances that do not (yet) have any associated colorimetric tests. Each specific named test will have information on what analytes it can be used with. Unfortunately, the test also destroys the sample provided. That said, color tests do not require much sample: if it can be seen, it can be tested.
Colorimetric tests can be quite sensitive, with limits of detection in the microgram range depending on the spot test utilized and the analyte [ 37 ]. Multiple tests with multiple reagents can be used if a mixture of drugs is suspected, though each test requires in the low milligram range of substance and destroys the substance in testing. With the proper standards, these tests can be quite specific, although multiple analyses may be required for high specificity. Some knowledge about what the substance is supposed to be and about general appearance of certain substances can increase specificity. Colorimetric tests are considered presumptive, in that they can only identify presence or non-presence of a particular substance based on the test administered. A single test/reagent will only test for the presence or absence of a drug or class of drugs. A typical test is not sufficient for a suspected mixture or even an unsuspected mixture if there is any reason at all to have suspicion of the substance. An example battery test protocol for considerations of how to test a suspected mixture is included below.
Actual color results may vary depending on the concentration, whether the drug is in salt or free base form, additional diluents, or contaminants; positive result may indicate a specific drug or class of drugs present, but not always specific for a single drug or class. Colorimetric tests rely on simple chemical reactions and produce visible results that can be interpreted with the naked eye.
Reagents and laboratory materials needed are inexpensive and readily available and can be performed with minimal training. Because each individual perceives color uniquely and because lighting conditions are not always optimal in non-lab settings, accuracy can be greatly enhanced with the use of smartphone apps to report color test results quantitatively [ 38 ]. Overall skill level required is basic to intermediate. A basic user can run the simple test and obtain results, whereas an intermediate user would run a standard protocol. An example of an intermediate protocol would be to run a battery of tests based on how much sample can be obtained without objection from the user. The tests should be based on an educated guess system, narrowing down possibilities through analysis and questions. Potential questions would be as follows: What did the user think it was or was told it was? What are recent novel substances that have been appearing in the clinic or on the street lately? What is the most dangerous substances worth testing for (smallest window of dosage)? Is there any knowledge of common mixtures, such as opioid mixtures?
The tests should be interpreted within a maximal 10-min window. The tests can be analyzed via smartphone or at least under good lighting if using the naked eye in order to most accurately determine color. The tests can then be matched against a database if a computer or the internet is available. From a system such as this, a presumptive test can then become a much more powerful tool.
Immunoassay
Immunoassay involves the binding of an antibody that is selective for the drug or drug group of interest (antigen) and a label that will be part of the antibody-antigen complex that can be detected using some means (such as fluorescence). Antigen-antibody binding is based on a typical immune system response in which antibodies in biological tissue bind to antigens in order to neutralize or remove them. This technique is rarely used in drug analysis because these methods were originally designed for analysis in biological materials (primarily metabolites in urine). Thus, traditionally, immunoassay provides important patient information for clinicians but does not provide a determination of the type or amount of a drug prior to its ingestion/injection. ELISA can, however, be used to perform other types of biochemical assays in the detection of an analyte in a liquid sample. Very little scholarly information is easily accessible about which specific drugs ELISA can detect outside of biological samples (post ingestion/metabolization).
Various opioids and cocaine can be detected rapidly and somewhat effectively using immunoassay technology. There are problems with specificity regarding immunoassays, and there have been many instances of false positives due to similarity in drug structures or metabolites. Sensitivity is quite high with detection in the microgram range as antibody-antigen interactions occur on a molecular level [ 39 ].
Immunoassay is fast and relatively inexpensive and in most instances, does not require high-level scientific knowledge to perform and interpret. Running such tests can require intermediate skill level. However, there is very little information available that has been scientifically published or available for public access on the usage of immunoassays for whole drug analysis. Immunoassay is most often employed to detect drug usage after the fact, such as in urine drug screens.
Urine dipstick test
This method has recently come under attention as a relatively cheap, easy-to-use presumptive test for fentanyl [ 40 ]. A sample of the drug sample is dissolved in water, and if the drug contains fentanyl in a concentration above the cut-off levels, an indicator on the strip will appear. The methodology works via chromatographic immunoassay, and in the presence of an appropriate analyte, a strip on the indicator stick appears/changes color.
To date, fentanyl is the only drug for which this method of drug checking has been reported being used [ 25 ], and there is little published data about this methodology. There is no scientific data on sensitivity, although the strips have been developed to detect fentanyl in urine and are therefore specific to testing for fentanyl and/or fentanyl metabolites.
The provided sample is destroyed in the testing process. Urine dipsticks are very easy to use, quick to check, specific for fentanyl, proven in urine test situations, and recently been proven efficacious in testing unknown drug mixtures for the presence of fentanyl. However, dipsticks were designed for drug detection in urine, and therefore, due to low specific weight in other mediums, it may be possible that false positives occur.
Another potential concern with this method is that many retailers will only sell to health professionals, and thus, these items may be difficult to procure for harm reduction agencies unless they are affiliated with a health clinic. Some medical device companies may object to such a test being used in a harm reduction setting, even in the presence of qualified health professionals for liability reasons.
Ultraviolet spectroscopy
This method is based on the absorption of light energy in the ultraviolet (UV) wavelength range. Light in this range can raise the energy levels of the electrons within a molecule from ground state to higher energy levels. Each transition to a higher energy level requires a given amount of energy, provided by light of a particular wavelength. Using a particular wavelength of light, a characteristic UV absorption spectrum can be obtained based on the electronic structure of the whole molecule as this structure will determine what wavelength(s) are absorbed versus which pass through a sample. UV-vis (ultraviolet visible) spectrophotometers measure the intensity of light passing through a sample and compare it to the intensity of light before it passes through the sample and capture this information to create a characteristic spectrum.
Drugs with similar structures may provide the same UV spectra. UV-vis has been used to identify MDMA, ketamine hydrochloride, cocaine hydrochloride, diazepam, phenobarbital, and barbital concentrations in the microgram range, as well as specifically identify six different compounds and for the first time, accurately discriminate some mixtures [ 41 ]. Other substances may be identifiable although literature is sparse on confirmatory usage for a broad spectrum of illegal drugs. UV spectrometry can be used on solid samples and therefore can be non-destructive in nature, although some samples may need preparation that can make them unsuitable for use afterwards. UV can be used quantitatively (amounts) and qualitatively (identification) and yields rough structural information providing modest selectivity to allow for some discriminating capability [ 24 ].
UV can be combined with chromatographic techniques for greater selectivity and specificity. It is not suitable for detection of several drugs in a mixture. Samples must be diluted or the technique can yield saturated spectra. Compounds lacking suitable chromophore provide no signal (for example, GHB has a low wavelength chromophore which makes analysis by UV-vis much more difficult without further sample preparation), although most drugs of abuse have a suitable chromophore due to aromatic ring structures in their chemical structures. Additionally, UV spectrum can vary depending upon the pH of the sample solution, and it is possible for chemical composition to change during the analysis. The level of expertise involved in UV is basic to advanced. The technique may be easily taught to someone with little to know theoretical knowledge of the technique, although interpretation of results would require intermediate to advanced knowledge.
There are many variables to consider when selecting technology for drug checking on the front lines of harm reduction. Harm reduction agencies, if pursuing the addition of drug testing services, will need to consider not only the quantitative capabilities of the tests but whether the agency can afford the human and fiscal resources to support the use of the technology. Thus, the recommendations include a strong bias to cost-benefit and beg the important question of whether some of the less discriminatory interventions are better than no intervention at all. With these considerations in mind, the following recommendations will summarize the methods for drug testing at a point-of-care level.
The techniques that are the strongest candidates based on all considerations are IMS, IR, Raman spectroscopy, and spot/color tests, although these too have some associated drawbacks. Spot/color tests are purely presumptive. In most cases, quantitation is contingent on expert interpretation. In some cases, the therapeutic index is so small and such miniscule quantities can be used as an additive to mixtures that only the highest discriminatory techniques mentioned above are capable of proving unequivocally that the quantity present would fall in therapeutic index (i.e., would produce a high but not be fatal, barring extraneous circumstances).
In our review, the best methods for point-of-care drug testing are handheld IR or Raman spectroscopy. From a cost-to-benefit analysis, these methods (specifically the portable/handheld units) are superior in almost every way to every other method. Manufacturers have simply made these technologies extremely easy to use and effective at identification of unknown analytes. The major downsides of this technology are that quantitation may require advanced expertise and that these units are still fairly expensive. To use these units qualitatively usually requires very little technical expertise or training. Intended for use in the field, these units are small and portable and tend to be fairly rugged, while also being able to have near-lab identification ability [ 25 ]. While many of these devices are only currently in use in law/drug enforcement settings, use in harm reduction settings would be worth exploring.
IMS spectrometers are very robust and require minimal maintenance. They are routinely used in airports worldwide for narcotics detection. Training is easy and quick, and sensitivity and selectivity are very high. Consumables are cheap and have long lives. Sampling is non-destructive and quantification is possible without expert level understanding. Analysis is quick and accurate. IMS is the best option available for clinics with a moderate level of funding. Some gas analyzers allow online updating; rapid sample analysis of liquid, solid, and gas; and discrimination of multiple interfering species in a complex matrix. The capability to update online allows methodologies and new molecular species to be shared instantly among clinics enabling point-of-care testing to remain current.
Other methods worth considering for point-of-care drug testing are MS, TLC, and UV spectroscopy. MS is considered the current gold standard in forensic drug analysis. Since MS units have been in use for a long time, it is actually possible to obtain one for a decent price (low-to-mid thousands) in the used market. However, in order to obtain a newer device optimized for drug testing or for testing extremely low concentrations, it would come with a higher price tag, usually in the hundreds of thousands of dollars. This presents a difficulty of its own because of the wide range of machines available, it would take some considerable research at clinic level to determine the cost-benefit analysis of a new or used machine to ensure acquisition of a machine that is suitable for its intended purpose. Additionally, operation and maintenance of MS machines is still complex, so a clinic would have to assess training, operation, maintenance, and associated ongoing costs which may place such a device beyond the time and/or monetary costs to the clinic compared to the benefits provided.
UV spectroscopy and TLC are more affordable options, but also much less discriminatory. Both of these methods tend to be less technical in operation, maintenance, and interpretation of results, but also do not offer quantification at the same level of the more discriminatory methods. They are also less expensive than all of the more discriminatory techniques. However, when used in conjunction, TLC and UV can be quite powerful in identification of a wide variety of substances (including mixtures) and offer a more rudimentary quantification than the more discriminatory techniques.
A lower technology option is the spot/color tests, which are purely presumptive in nature, although they can be fairly specific at identification of a compound and/or mixture when utilizing a standardized procedure utilizing a battery of tests (as described above). Information about optimal technique can be easily accessed via the internet. Color tests are cost effective, fast to complete, and very easy to perform. The use of a smartphone app can aid in identifying the exact color profile. This can then be used in conjunction with a searchable database to perform the most accurate identifications. The fact that this technology is so cost-effective, easy to perform, and requires a very minute amount of substance makes it really stand out from many of the other presumptive methods [ 16 ]. This type of test is widely used in Europe [ 16 ]. These tests are not perfect and can be performed incorrectly. A proper standardization of technique should be implemented at the clinic level to maximize the accuracy of these tests.
Drug testing methods that are less suited to point-of-care drug testing situations include immunoassay, microcrystalline testing, and X-ray diffractometry. Immunoassays are traditionally designed for usage in biological samples as they work based on antibody-antigen interactions and as such are best suited for testing excreted metabolites (such as in urine). At best, an immunoassay can indicate the presence of drug(s), and at worst, they can give a high proportion of false positives. This may result in people using the substances anyways or serve to give the clinic a poor reputation, and users may soon stop going to the site for drug testing. That said, they are affordable and portable and can detect potentially fatal drugs like fentanyl.
Microcrystalline testing is a highly limited method as the drug needs to be mostly (or completely) pure. This testing has no quantification capabilities at all and requires high skills and knowledge to identify drugs based purely on crystal structure. X-ray diffractometry is a highly discriminating testing method; however, this method basically requires partnership with a specialized lab/institution. X-ray diffractometers are incredibly expensive (mid-to-high tens of thousands), difficult to maintain and operate, and have the added factor of using radioactivity which may present health and safety concerns.
There is a wide variety of techniques that have been validated for drug identification and/or quantification. Each of these techniques has a variety of associated pros and cons that must be considered. With this in mind, this review is not meant to be an in-depth rigorous scientific treatment of each of these methods, but a guide for the practical consideration of usage and recommendations for point-of-care harm reduction purposes. It is sincerely expected that this document will help to narrow down consideration of each of these techniques and that each clinic would then determine a smaller subset of techniques to consider implementing. It would be prudent for clinics that can obtain the funding to contact the companies who produce and design these devices and discuss possible usage in a harm reduction setting as many of the devices are only currently in use in law enforcement and research.
Acknowledgements
Availability of data and materials, abbreviations, authors’ contributions.
LH analyzed the extant literature, creating the basis for the paper. JP offered technical analysis and editorial support. EP worked with LH to make the text suitable to a non-technical audience. All authors read and approved the final manuscript.
Authors’ information
LH holds a Bachelors of Engineering, majoring in Biomedical Engineering and minoring in Biotechnology obtained from the University of Guelph in 2016. He is currently enrolled in a second degree program and participating in research in Biochemistry at the University of Lethbridge. Lane is also interested in the politics of sensible drug policies and associated programs, including, but not limited to, the implementation of harm reduction best practices in Canada.
JP received a Bachelors of Science in Chemistry from Carleton University. He currently works on automation and sensing technology.
EP holds degrees in nursing and is an assistant professor in the Faculty of Health Sciences at the University of Lethbridge. EP has a clinical background in outreach nursing and harm reduction and conducts research and evaluation studies with local harm reduction agencies.
Ethics approval and consent to participate
Consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lane Harper, Email: [email protected] .
Jeff Powell, Email: [email protected] .
Em M. Pijl, Email: [email protected] .

IMAGES
VIDEO
COMMENTS
Drug analysis is the testing of a suspected controlled substance to determine its composition. For information about forensic toxicology, or the testing of bodily fluids for controlled substances, click here. Understanding Test Results Every analysis of a suspected controlled substance should consist of at least two tests. The first is a presumptive or screening test which …
Illicit drug analysis is a critical function of forensic science laboratories that primarily serve a judicial system. At its core, illicit drug analysis is the analysis of unknown samples - commonly powders, crystalline material, liquids, tablets, capsules, paper squares and plant material to determine if an illicit substance is present. ...
The analysis of drug and its metabolite which may be either quantitative or qualitative is extensively applied in the pharmacokinetic studies. This review highlights the role of various analytical techniques and their corresponding analytical methods in the analysis of pharmaceuticals. 2.
2. Pharmaceutical analysis. Broadly speaking, this is the application of a process in order to identify a drug (single or combined) in its bulk or pharmaceutical dosage form. Testing pharmaceutical product involves chemical, physical and sometimes microbiological analysis [ 3 ].
Mass spectrometry How does it work? Mass spectrometry (MS) is the most discriminatory of the drug testing techniques. Mass spectrometry measures the precise molecular mass of ions as determined by their mass to charge ratio (m/z) and is the current gold standard in forensic drug analysis [].In general, mass spectrometry requires separation, ionization, and finally detection.
Drug Testing and Analysis is the unrivaled specialist journal for drug testing practitioners. The journal provides a focal point for the detection of illicit and controversial substances, covering sports doping, recreational drugs, pharmaceuticals, toxicologic pathology, forensics, and the environment.
Abstract. All pharmaceutical drugs, vaccines, cosmetic products, and many medical breakthroughs must first be approved through clinical research and trials before advancing to standard practice or entering the marketplace. Clinical trials are sets of tests that are required to determine the safety and efficacy of pharmaceutical compounds, drugs ...
Drug analysis refers to the detection and analysis of ingredients and contents in drugs. Drug analysis and testing involves the study of composition, physical and chemical properties, purity, and the determination of the content of active pharmaceutical ingredients and their preparations, to ensure that the medications are safe, rational, and effective.
Drug Development and Analysis Review. The drug discovery has now evolved into a much more scientific and rational process due to better understanding of biological processes and the underlying chemistry, owing to the progress made due to advances in high throughput experimental techniques and availability of high performance computation resources.
The analysis of drugs and their metabolites may be qualitative or quantitative and is widely used in pharmacokinetic research. This chapter highlights the stability study, its types, and importance as well as the role of several analytical techniques and methods in drug analysis.
Therapeutic drug monitoring (TDM) involves the optimization of therapy through adjustment of dose at the individual level by monitoring concentrations of drug or drug metabolite in body fluids (e.g. blood, plasma, or serum) or a suitable physiological matrix [1, 2].The scientific basis and practice of TDM date to 1946 with the establishment of a correlation between the pharmacological activity ...
Broadly defined, drug testing uses a biological sample to detect the presence or absence of a drug or its metabolites. This process can be completed in a variety of settings and with a variety of techniques. Many drug screening immunoassays were initially designed for use in the workplace as a drug screening tool for employees. As these tests have become cheaper, more readily available, and ...
Clinical drug testing analyzes plasma, serum, or urine to detect the presence or absence of a drug or its metabolites. As the metabolization rate of drugs differs, the detection window for specific drugs or metabolites varies. Clinical drug testing plays an essential role in managing poisoning because the self-report of the drugs taken is often unreliable. The same is true in treating ...
Laboratory Forensic Drug Analysis . Laboratory analysis of drug evidence is a critical component of all forensic analyses. Drug chemists are faced with a unique set of challenges and are required to balance safety and exposure probabilities with case backlog issues and a rapidly changing drug landscape.
Seized drug analysis is a complicated discipline with an expansive set of substances and matrices that could be encountered. Over the last decade, changes to the illicit drug market and the legal system have led to several new challenges—both analytical and philosophical. In this section, we highlight four of the broad challenges that are ...
Research Topics. Part of an innovative, multidisciplinary journal, exploring pharmaceutical analysis, drug mechanisms through in vitro and in vivo testing, and drug quality control.
Editorial on the Research Topic. Current Analytical Trends in Drug Testing in Clinical and Forensic Toxicology. The articles included in this collection cover novel analytical approaches, including chromatographic and spectrometric methods, and sample preparation techniques for the investigation and analysis of several classes of compounds.
Drug Identification. Analysis of controlled and uncontrolled substances is often fairly routine. After a visual assessment and measuring the mass of the exhibit, we will typically perform presumptive testing then use a different technique to confirm the identity of an unknown. While some labs or specialists may need to quantitatively ...
3.3 Phenome-wide MR analysis. Considering that many drugs exert their effects via the bloodstream, we investigated the potential impact of the 10 blood protein expressions on other health conditions. An extensive MR analysis was conducted on 1402 conditions and traits from the UK Biobank, detailed in eTables 18-27 in Appendix S1.
Pharmaceutical analysis of drug is totally dependent on the nature and/or properties of the drug to be analyzed. Drug that is to be analyzed possesses two types of properties that are as follows: 1.8.1 Physical Properties of Drugs. Physical properties are measured or observed without any change in the matter composition.
DOI: 10.1021/jacs.3c14359. Scientists from the University of Oulu (Finland) and Texas A&M University (U.S.), have developed a new method to study how proteins interact with small ligand molecules ...
Most PDP enrollees will face much higher cost sharing for brands than for generic drugs in 2024, as in prior years, including coinsurance for non-preferred drugs of 50% (the maximum coinsurance ...
Dopesick (2021) is the first TV series whose plot deals exclusively with the opioid crisis in the United States. The current study uses narrative analysis and framing theory to explore this series, discussing its portrayal of the people and themes involved in the opioid crisis. Our analysis found that although Dopesick attempts to portray multiple dimensions of the opioid crisis, its narrative ...
About the National Institute on Drug Abuse (NIDA): NIDA is a component of the National Institutes of Health, U.S. Department of Health and Human Services. NIDA supports most of the world's research on the health aspects of drug use and addiction. ... Analysis of social media language using AI models predicts depression severity for white ...
The Supreme Court on Tuesday signaled that it was likely to allow mifepristone, one of two drugs used in medication abortions, to remain widely available in the United States. During roughly 90 minutes of oral arguments, a majority of the justices appeared ready to throw out the dispute over the FDA's expansion of access to the drug in 2016 ...
Interpol Review of Drug Analysis 2019-2022. 1. Routine and improved analyses of abused substances. Improved methods of analysis, i.e., faster, more discriminatory, more sensitive, less costly, etc., are needed for all abused substances. Additionally, standard analytical data are required for previously unknown or rarely encountered substances ...
Based on the goal of analysis, DOA testing may target a limited number of drugs/drug classes, usually classic DOA, or more comprehensively encompass a large number of compounds with abuse potential. The targeted approach is generally applied when the law requires testing for a defined list of drugs/drug classes as in workplace drug testing.
Henshaw, 39, was reportedly arrested on a drug trafficking charge for the alleged possession of a 'tiny' amount of cannabis, the MailOnline reports. He was then incarcerated in the San Pedro ...
Mass spectrometry (MS) is the most discriminatory of the drug testing techniques. Mass spectrometry measures the precise molecular mass of ions as determined by their mass to charge ratio (m/z) and is the current gold standard in forensic drug analysis . In general, mass spectrometry requires separation, ionization, and finally detection.
The US Food and Drug Administration announced that Adderall was in shortage in mid-October 2022, and the share of people with ADHD who filled their prescriptions for Adderall and related ...