An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cold Spring Harb Perspect Med
- v.8(7); 2018 Jul

Health Benefits of Exercise
Gregory n. ruegsegger.
1 Department of Biomedical Sciences, University of Missouri, Columbia, Missouri 65211
Frank W. Booth
2 Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, Missouri 65211
3 Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, Missouri 65211
4 Dalton Cardiovascular Research Center, University of Missouri, Columbia, Missouri 65211
Overwhelming evidence exists that lifelong exercise is associated with a longer health span, delaying the onset of 40 chronic conditions/diseases. What is beginning to be learned is the molecular mechanisms by which exercise sustains and improves quality of life. The current review begins with two short considerations. The first short presentation concerns the effects of endurance exercise training on cardiovascular fitness, and how it relates to improved health outcomes. The second short section contemplates emerging molecular connections from endurance training to mental health. Finally, approximately half of the remaining review concentrates on the relationships between type 2 diabetes, mitochondria, and endurance training. It is now clear that physical training is complex biology, invoking polygenic interactions within cells, tissues/organs, systems, with remarkable cross talk occurring among the former list.
The aim of this introduction is briefly to document facts that health benefits of physical activity predate its readers. In the 5th century BC, the ancient physician Hippocrates stated: “All parts of the body, if used in moderation and exercised in labors to which each is accustomed, become thereby healthy and well developed and age slowly; but if they are unused and left idle, they become liable to disease, defective in growth and age quickly.” However, by the 21st century, the belief in the value of exercise for health has faded so considerably, the lack of exercise now presents a major public health problem ( Fig. 1 ) ( Booth et al. 2012 ). Similarly, the lack of exercise was classified as an actual cause of chronic diseases and death ( Mokdad et al. 2004 ).

Simplistic overview of how physical activity can prevent the development of type 2 diabetes and one of its complications, cardiovascular disease. Physical inactivity is an actual cause of type 2 diabetes, cardiovascular disease, and tens of other chronic conditions ( Table 1 ) via interaction with other factors (e.g., age, diet, gender, and genetics) to increase disease risk factors. This leads to chronic disease, reduced quality of life, and premature death. However, physical activity can prevent and, in some cases, treat disease progression associated with physical inactivity and other genetic and environmental factors.
Published in 1953, Jeremy N. Morris and colleagues conducted the first rigorous epidemiological study investigating physical activity and chronic disease risk, in which coronary heart disease (CHD) rates were increased in physically inactive bus drivers versus active conductors ( Morris et al. 1953 ). Since this pioneering report, a plethora of evidence shows that physical inactivity is associated with the development of 40 chronic diseases ( Table 1 ), including major noncommunicable diseases such as type 2 diabetes (T2D) and CHD, and as premature mortality ( Booth et al. 2012 ).
Worsening of 40 conditions caused by the lack of physical activity with growth, maturation, and aging throughout life span
The breadth of the list implies that a single molecular target will not substitute for appropriate daily physical activity to prevent the loss of all listed items.
In this review, we highlight the far-reaching health benefits of physical activity. However, note that the studies cited here represent only a fraction of the >100,000 studies showing positive associations between the terms “exercise” and “health.” In addition, we discuss how exercise promotes complex integrative responses that lead to multisystem responses to exercise, an underappreciated area of medical research. Finally, we consider how strategies that “mimic” parts of exercise training compare with physical exercise for their potential to combat metabolic disease.
EXERCISE IMPROVES CARDIORESPIRATORY FITNESS
There is arguably no measure more important for health than cardiorespiratory fitness (CRF) (commonly measured by maximal oxygen uptake, VO 2max ) ( Blair et al. 1989 ). For example, Myers et al. (2002 ) showed that each 1 metabolic equivalent (1 MET) increase in exercise-test performance conferred a 12% improvement in survival, stating that “VO 2max is a more powerful predictor of mortality among men than other established risk factors for cardiovascular disease (CVD).” Low CRF is also well established as an independent risk factor of T2D ( Booth et al. 2002 ) and CVD morbidity and mortality ( Kodama et al. 2009 ; Gupta et al. 2011 ). Similarly, Kokkinos et al. (2010) reported that men who transitioned from having low to high CRF decreased their mortality risk by ∼50% over an 8-yr period, whereas men who transitioned from having high to low CRF increased their mortality risk by ∼50%.
Importantly then, from the above paragraph, physical activity and inactivity are major environmental modulators of CRF, increasing and decreasing it, respectively, often through independent pathways. Findings from rats selectively bred for high or low intrinsic aerobic capacity show that rats bred for high capacity, which are also more physically active, have 28%–42% increases in life span compared to low-capacity rats ( Koch et al. 2011 ). Endurance exercise is well recognized to improve CRF and cardiometabolic risk factors. Exercise improves numerous factors speculated to limit VO 2max including, but not restricted to, the capacity to transport oxygen (e.g., cardiac output), oxygen diffusion to working muscles (e.g., capillary density, membrane permeability, muscle myoglobin content), and adenosine triphosphate (ATP) generation (e.g., mitochondrial density, protein concentrations).
Data from the HERITAGE Family Study has provided some of the first knowledge of genes associated with VO 2max plasticity because of endurance-exercise training. Following 6 wk of cycling training at 70% of pretraining VO 2max , Timmons et al. (2010) performed messenger RNA (mRNA) expression microarray profiling to identify molecules potentially predicting VO 2max training responses, and then assessed these molecular predictors to determine whether DNA variants in these genes correlated with VO 2max training responses. This approach identified 29 mRNAs in skeletal muscle and 11 single-nucleotide polymorphisms (SNPs) that predicted ∼50% and ∼23%, respectively, of the variability in VO 2max plasticity following aerobic training ( Timmons et al. 2010 ). Intriguingly, pretraining levels of these mRNAs were greater in subjects that achieved greater increases in VO 2max following aerobic training, and of the 29 mRNAs, >90% were unchanged with aerobic training, suggesting that alternative exercise intervention paradigms or pharmacological strategies may be needed to improve VO 2max in individuals with a low responder profile for the identified predictor genes ( Timmons et al. 2010 ). Keller et al. (2011) found that, in response to endurance training, improvements in VO 2max were associated with effectively up-regulating proangiogenic gene networks and miRNAs influencing the transcription factor–directed networks for runt-related transcription factor 1 (RUNX1), paired box gene 3 (PAC3), and sex-determining region Y box 9 (SOX9). Collectively, these results led the investigators to speculate that improvements in skeletal muscle oxygen sensing and angiogenesis are primary determinates in training responses in VO 2max ( Keller et al. 2011 ).
Clinically important concepts have emerged from the pioneering HERITAGE Family Study. One new clinical concept is that a threshold dose–response relationship influences the percentage of subjects responding with an increase in VO 2max to endurance training volumes (with volume being defined here as the product of intensity × duration), as previously published ( Slentz et al. 2005 , 2007 ). Ross et al. (2015) later extended the aforementioned Slentz et al. studies. After a 24-wk-long endurance training study ( Ross et al. 2015 ), percentages of women and men identified as nonresponders to the training (i.e., defined as not increasing their VO 2peak ) progressively fell inversely to a two stepwise progressive increase in endurance-exercise training volume, as described next. Thirty-nine percent (15 of 39) of training subjects did not increase their VO 2peak in response to the low-amount, low-intensity training; 18% (9 of 51) had no increase in VO 2peak in the group having high-amount, low-intensity training; and 0% (0 of 31) who underwent high-amount, high-intensity training did not increase their VO 2peak . A biological basis for the dose–response relationship in the previous sentence could be made from an analysis of interval training (IT) and IT/continuous-training studies published from 1965 to 2012 ( Bacon et al. 2013 ). A second older concept is being reinvigorated; Bacon et al. (2013) indicate that different endurance-exercise intensities and durations are needed for different systems in the body. They suggest that very short periods of high-intensity endurance-type exercise may be needed to reach a threshold for peripheral metabolic adaptations, but that longer training durations at lower intensities are required to see large changes in maximal cardiac output and VO 2max .
A comparable example exists for resistance training. Maximal resistance loads require a minimum of 2 min/per wk for each muscle group recruited by a specific maneuver to obtain a strength training adaptation [(8 contractions/set × 2 sec/contraction × 3 sets/day) × 2 days/wk) = 96 sec]. As of 2016, one opinion from Sarzynski et al. (2016) for the molecular mechanisms by which endurance exercise drives VO 2max include, but are not limited to, calcium signaling, energy sensing and partitioning, mitochondrial biogenesis, angiogenesis, immune functions, and regulation of autophagy and apoptosis.
Perhaps more importantly, lifelong aerobic exercise training preserves VO 2max into old age. CRF generally increases until early adulthood, then declines the remainder of life in sedentary humans ( Astrand 1956 ). The age-related decline in VO 2max is not trivial, as Schneider (2013) reported a ∼40% decline in healthy males and females spanning from 20 to 70 yr of age. However, cross-sectional data show that with lifelong aerobic exercise training, trained individuals often have the same VO 2max as a sedentary individual four decades younger ( Booth et al. 2012 ). Myers et al. (2002) found that low estimated VO 2max increases mortality 4.5-fold compared to high estimated VO 2max . They concluded, “Exercise capacity is a more powerful predictor of mortality among men than other established risk factors for cardiovascular disease.” Given the strong association between CRF, chronic disease, and mortality, we feel identifying the molecular transducers that cause age-related reductions in CRF may have profound implications for improving health span and delaying the onset of chronic disease. In two of our recent papers, transcriptomics was performed on the triceps muscle ( Toedebusch et al. 2016 ) and on the cardiac left ventricle ( Ruegsegger et al. 2017 ). We were addressing the question of what molecule initiates the beginning of the lifelong decline in aerobic capacity with aging. Aerobic capacity (VO 2max ) involves, at a minimum, the next systems/tissues, as oxygen travels through the mouth, airways, pulmonary membrane, pulmonary circulation, left heart, aorta/arteries/capillaries, and sarcoplasm/myoglobin to mitochondria. We allowed female rats access, or no access, to running wheels from 5 to 27 wk of age. Surprisingly, voluntary running had no effect on the delay in the beginning of the lifetime decrease in VO 2max . Our skeletal muscle transcriptomics elicited no molecular targets, whereas gene networks suggestive of influencing maximal stroke volume were identified in the left ventricle transcriptomics ( Ruegsegger et al. 2017 ).
Publications concerning the effects of exercise on the brain (from 54 to 216 papers listed on PubMed from 2007 to 2016) have increased 400%. In addition, a 2016 study ( Schuch et al. 2016 ) of three previous papers reported that humans with low- and moderate-CRF had 76% and 23%, respectively, increased risk of developing depression compared to high CRF in three publications. With this forming trend, the next section will consider exercise and brain health.
EXERCISE IMPROVES MENTAL HEALTH
Many studies support physical activity as a noninvasive therapy for mental health improvements in cognition ( Beier et al. 2014 ; Bielak et al. 2014 ; Tian et al. 2014 ), depression ( Kratz et al. 2014 ; McKercher et al. 2014 ; Mura et al. 2014 ), anxiety ( Greenwood et al. 2012 ; Nishijima et al. 2013 ; Schoenfeld et al. 2013 ), neurodegenerative diseases (i.e., Alzheimer’s and Parkinson’s disease) ( Bjerring and Arendt-Nielsen 1990 ; Mattson 2014 ), and drug addiction ( Zlebnik et al. 2012 ; Lynch et al. 2013 ; Peterson et al. 2014 ). In 1999, van Praag et al. (1999) showed the survival of newborn cells in the adult mouse dentate gyrus, a hippocampal region important for spatial recognition, is enhanced by voluntary wheel running. Similarly, spatial pattern separation and neurogenesis in the dentate gyrus are strongly correlated in 3-mo-old mice following 10 wk of voluntary wheel running ( Creer et al. 2010 ), and the development of new neurons in the dentate gyrus is coupled with the formation of new blood vessels ( Pereira et al. 2007 ). Many exercise-related improvements in cognitive function have been associated with local and systemic expression of growth factors in the hippocampus, notably, brain-derived neurotrophic factor (BDNF) ( Neeper et al. 1995 ; Cotman and Berchtold 2002 ). BDNF promotes many developmental functions in the brain, including neuronal cell survival, differentiation, migration, dendritic arborization, and synaptic plasticity ( Park and Poo 2013 ). In rat hippocampus, regular exercise promotes a progressive increase in BDNF protein for up to at least 3 mo ( Berchtold et al. 2005 ). In an opposite manner, BDNF mRNA in the hippocampus is rapidly decreased by the cessation of wheel running, suggesting BDNF expression is tightly related to exercise volume ( Widenfalk et al. 1999 ).
Findings by Wrann et al. (2013) highlight one mechanism by which endurance exercise may up-regulate BDNF expression. To summarize, Wrann et al. (2013) noted that exercise increases the activity of the estrogen-related receptor α (ERRα)/peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) complex, in turn increasing levels of the exercise-secreted factor FNDC5 in skeletal muscle and the hippocampus, whose cleavage products provide beneficial effects in the hippocampus by increasing BDNF gene expression. While future research should determine whether the FNDC5 cleavage-product was produced locally in hippocampal neurons or was secreted into the circulation, this finding eloquently displays one mechanism responsible for brain health benefits following exercise. Similarly, work by van Praag and colleagues suggests that exercise or pharmacological activation of AMP-activated protein kinase (AMPK) in skeletal muscle enhances indices of learning and memory, neurogenesis, and gene expression related to mitochondrial function in the hippocampus ( Kobilo et al. 2011 , 2014 ; Guerrieri and van Praag 2015 ).
Insulin-like growth factor 1 (IGF-1), is central to many exercise-induced adaptations in the brain. Like BDNF, physical activity increases circulatory IGF-1 levels and both exercise and infusion of IGF-1 increase BrdU + cell number and survivability in the hippocampus ( Trejo et al. 2001 ). Similarly, the protective effects of exercise on various brain lesions are nullified by anti-IGF-1 antibody ( Carro et al. 2001 ).
In 1979, Greist et al. (1979) provided evidence that running reduced depression symptoms similarly to psychotherapy. However, the precise mechanisms by which exercise prevents and/or treats depression remain largely unknown. Of the proposed mechanisms, increases in the availability of brain neurotransmitters and neurotrophic factors (e.g., BDNF, dopamine, glutamate, norepinephrine, serotonin) are perhaps the best studied. For example, tyrosine hydroxylase (TH) activity, the rate-limiting enzyme in dopamine formation, in the striatum, an area of the brain's reward system, is increased following 7 days of treadmill running in an intensity-dependent manner ( Hattori et al. 1994 ). Voluntary wheel running is also highly rewarding in rats, and voluntary wheel running in rats lowers the motivation to self-administer cocaine, suggesting exercise may be a viable strategy in the fight against drug addiction ( Larson and Carroll 2005 ).
Similar to the above examples, secreted factors from skeletal muscle have been linked to the regulation of depression. Agudelo et al. (2014) showed that exercise training in mice and humans, and overexpression of skeletal muscle PGC-1α1, leads to robust increases in kynurenine amino transferase (KAT) expression in skeletal muscle, an enzyme whose activity protects from stress-induced increases in depression in the brain by converting kynurenine into kynurenic acid. Additionally, overexpression of PGC-1α1 in skeletal muscle left mice resistant to stress, as evaluated by various behavioral assays indicative of depression ( Agudelo et al. 2014 ). Simultaneously, they report gene expression related to synaptic plasticity in the hippocampus, such as BDNF and CamkII, were unaffected by chronic mild stress compared to wild-type mice. Collectively, these findings suggest exercise-induced increases in skeletal muscle PGC-1α1 may be an important regulator of KAT expression in skeletal muscle, which, via modulation in plasma kynurenine levels, may alleviate stress-induced depression and promote hippocampal neuronal plasticity.
TYPE 2 DIABETES, MITOCHONDRIA, AND EXERCISE
T2d predictions show a pandemic.
In a 2001 Diabetes Care article ( Boyle et al. 2001 ), investigators at the U.S. Centers for Disease Control (CDC) predicted 29 million U.S. cases of T2D would be present in 2050. Unfortunately, the 2001 prediction of 29 million was reached in 2012! For 2012, the American Diabetes Association reported that 29 million Americans had diagnosed and undiagnosed T2D, which was 9% of the American population ( Dwyer-Lindgren et al. 2016 ). More rapid increases in T2D are now predicted by the CDC than in the previous estimate. The CDC now predicts a doubling or tripling in T2D in 2050. The tripling would mean that one out of three U.S. adults would have T2D in their lifetime by 2050 ( Boyle et al. 2010 ), which would be >100 million U.S. cases. The International Diabetes Federation (IDF) reports T2D cases worldwide. In 2015, the IDF reported that 344 and 416 million North American (including Caribbean) and worldwide adults, respectively, had T2D. Furthermore, the IDF predicts for 2040 that 413 and 642 million, respectively, will have T2D. In sum, T2D is now pandemic, and the pandemic will increase in numbers without current apparent action within the general public.
Type 2 Diabetes Prevalence Is Based on a Strong Genetic Predisposition
The Framingham study found that T2D risk in offspring was 3.5-fold and sixfold higher for a single and two diabetic parent(s), respectively, as compared to nondiabetic offspring ( Meigs et al. 2000 ). Thus, T2D is gene-based.
Noncoding regions of the human genome contain >90% of the >100 variants associated with both T2D and related traits that were observed in genome-wide association studies ( Scott et al. 2016 ). Another 2016 paper ( Kwak and Park 2016 ) lists at least 75 independent genetic loci that are associated with T2D. Taken together, T2D is a complex genetic disease ( Scott et al. 2016 ).
Type 2 Diabetes Is Modulated by Lifestyle, with Exercise as the More Powerful Lifestyle Factor
Three large-scale epidemiological studies have been performed on prediabetics, each in a different geographical location. The first study, and only study to have separate study arms for diet and exercise, was in China. The pure exercise intervention group had a 46% reduction in the onset of T2D, relative to the nontreated group, after 6 yr of the study ( Pan et al. 1997 ). Diet alone reduced T2D by 31% in the Chinese study. The second study on T2D was the Finnish Diabetes Prevention Study. It found a 58% reduction in T2D in the lifestyle intervention (combined diet and exercise) in its 522 prediabetic subjects after a mean study duration of 3.2 yr ( Tuomilehto et al. 2001 ). The latest of the three studies was in the U.S. Diabetes Prevention Program. The large randomized trial ( n = 3150 prediabetics) was stopped after 2.8 yr, because of harm to the control group. T2D prevalence in the high-risk adults was reduced by 58% with intensive lifestyle (diet and exercise) intervention, whereas the drug arm (metformin) of the study only reduced T2D by 31%, both compared to the noninnervation group ( Knowler et al. 2002 ). Thus, if differences in genetics in the above three differing ethnicities are not a factor, combined exercise and diet remain more effective in T2D prevention than the drug metformin two decades ago.
Exercise Increases Glucose by Signaling Independent of the Insulin Receptor
A single exercise bout increases glucose uptake by skeletal muscle, sidestepping the insulin receptor and thus insulin resistance in T2D patients ( Holloszy and Narahara 1965 ; Goodyear and Kahn 1998 ; Holloszy 2005 ). After insulin binding to its receptor, insulin initiates a downstream signaling cascade of tyrosine autophosphorylation of insulin receptor, insulin receptor substrate 1 (IRS-1) binding and phosphorylation, activation of a PI3K-dependent pathway, including key downstream regulators protein kinase B (Akt) and the Akt substrate of 160 kDa (AS160), ultimately promoting glucose transporter 4 (GLUT4) translocation to the plasma membrane ( Rockl et al. 2008 ; Stanford and Goodyear 2014 ). Despite normal GLUT4 levels, insulin fails to induce GLUT4 translocation in T2D ( Zierath et al. 2000 ). However, exercise activates a downstream insulin-signaling pathway at AS160 and TBC1 domain family member 1 (TBC1D1) ( Deshmukh et al. 2006 ; Maarbjerg et al. 2011 ), facilitating GLUT4 expression translocation to the plasma membrane independent of the insulin receptor. We contend that exercise could be considered as a very powerful tool to primarily attenuate the T2D pandemic.
Complex Biology of T2D Interactions with the Complex Biology of Exercise
An important consideration from the above is that T2D is such a genetically complex disease that a single gene has not been proven to be sufficiently causal to be effective, at this stage in time, to be a successful target for pharmacological treatment. The expectation for a single molecule target has been met for infectious diseases, which are often monogenic diseases. For example, a vaccine against smallpox was highly successful. Edward Jenner in 1796 produced the first successful vaccine. An important fact is that exercise is genetically complex. The literature allows us to speculate that exercise is at least as genetically complex as the approximately 75 genes associated with T2D ( Kwak and Park 2016 ). An example indicating that exercise is complex biology follows. RNA sequencing analysis of all 119 vastus lateralis muscle biopsies found that endurance training for 4 days/wk for 12 wk produced the differential expression of 3404 putative isoforms, belonging to 2624 different genes, many associated with oxidative ATP production in 23 women and men aged 29 yr old ( Lindholm et al. 2016 ). Our notion is that over 2600 genes suggests complex biology.
A “Case-Type” Study of the Molecular Underpinnings of Exercise, Mitochondria, and T2D Interactions
A PubMed search for the terms “diabetes mitochondria exercise molecular” elicited 74 papers. We arbitrarily selected some of the most recent 50 (spanning from mid-2014 into January 2017), with the assumption they would be representative of any other papers that we did not find in our search. Papers fell into our two arbitrary categories of single gene studies versus “omic”-type studies. First, subcategories of studies that develop themes will be arbitrarily presented.
Recent Studies Show Single Gene Manipulation Alters Mitochondrial Level and Running Performance
Numerous reports in the past couple of years observed that single gene manipulations increase mitochondrial gene expression and activity, which was also associated with increased exercise performance/capacity. A few of these are presented below:
- Irisin was shown to increase oxidative metabolism in myocytes and increase PGC-1α mRNA and protein ( Vaughan et al. 2014 ), which extends the first observation made earlier in adipose tissue by Spiegelman ( Bostrom et al. 2012 ).
- Patients with impaired glucose tolerance underwent low-intensity exercise training. Patients whose mitochondrial markers increased to levels that were measured in a separate cohort of nonexercised healthy individuals recovered normal glucose tolerance ( Osler et al. 2015 ). In opposition, those patients whose mitochondria markers did not improve, remained with impaired glucose tolerance.
- In 2003, muscle PGC-1α mRNA was shown to be induced by endurance-exercise training in human skeletal muscle ( Short et al. 2003 ). PGC-1α was shown to have multiple isoforms ( Lin et al. 2002 ). After a 60-min cycling bout, human vastus lateralis biopsies were taken from both sexes in their mid-20s. Additional biopsies were taken 30 min, and at 2, 6, and 24 hr postexercise. At 30 min postexercise, PGC-1α-ex1b mRNA and PGC-1α mRNA increased 468- and 2.4-fold, respectively, whereas PGC-1α-ex1b protein and PGC-1α protein increased 3.1-fold and no change, respectively. Gidlund et al. (2015 ) interprets the above data as implying PGC-1α-ex1b could be responsible for other changes that have previously been recorded before the increase in total PGC-1α postexercise.
- Mice with knockout of the kinin B1 receptor gene had higher mitochondrial DNA quantification and of mRNA levels of genes related to mitochondrial biogenesis in soleus and gastrocnemius muscles and had higher exercise times to exhaustion, but did not have higher VO 2max ( Reis et al. 2015 ).
- Mice do not normally express cholesteryl ester transfer protein (CETP), which is a lipid transfer protein that shuttles lipids between serum lipoproteins and tissues. Overexpression of CETP in mice after 6 wk on a high-fat diet increased treadmill running duration and distance, mitochondrial oxidation of glutamate/malate, but not palmitoylcarnitine oxidation, and doubled PGC-1α mRNA concentration ( Cappel et al. 2015 ).
- The myokine musclin is a peptide secreted from exercising muscle during treadmill running. Removal of musclin release during running results in lowered VO 2max , lower skeletal muscle mitochondrial content and respiratory complex protein expression, and reduced exercise tolerance ( Subbotina et al. 2015 ).
- Lactate dehydrogenase B (LDHB), which produces pyruvate from lactate, was overexpressed in mouse skeletal muscle. Increases in markers of skeletal muscle mitochondria were associated with increased running distance in a progressive speed test, and increased peak VO 2 ( Liang et al. 2016 ).
- Another example of endurance-type exercise adaptations is the 2016 paper that transcription factor EB (TFEB) regulates metabolic flexibility in skeletal muscle independent of PGC-1α during endurance-type exercise ( Mansueto et al. 2017 ). Lack of metabolic flexibility, termed “metabolic inflexibility,” is important because it is common in T2D. One definition of metabolic inflexibility is its inability to rapidly switch between glucose and fatty acid substrates for ATP production when nutrient availability changes from high blood glucose levels immediately after a meal to decreasing below 100 mg/dl when not eating for hours after a meal. A clinical consequence of T2D-induced metabolic inflexibility is prolonged periods of hyperglycemia, because skeletal muscle is more insulin insensitive in T2D. In contrast, after sufficient endurance exercise, skeletal muscle increases its insulin sensitivity by a second pathway that is independent of proximal postreceptor insulin signaling (see Stephenson et al. 2014 for further discussion).
Studies Showing that Manipulation of One Signaling Molecule Does Not Alter Expression of All Genes with Mitochondrial Functions Found in Skeletal Muscles of Wild-Type Animals to Exercise Training
A 2010 review article ( Lira et al. 2010 ) concludes from gene-deletion studies that p38γ MAPK/PGC-1α signaling controls mitochondrial biogenesis’ adaptation to endurance exercise in skeletal muscle. Two studies do not completely agree with the conclusion in the review article. The Pilegaard laboratory published a 2008 study ( Leick et al. 2008 ) that did not confirm their hypothesis that PGC-1α was required for every metabolic protein adaptive increase after endurance-exercise training by skeletal muscle. They reported that PGC-1α was not required for endurance-training-induced increases in ALAS1, COXI, and cytochrome c expression ( Leick et al. 2008 ). Their interpretation, at that time, was that molecules other than PGC-1α can exert exercise-induced mitochondrial adaptations. A second study published in 2012 rendered a similar verdict. A 12-day program of endurance training led to the middle portion of the gastrocnemius muscle demonstrating a similar 60% increase in mitochondrial density in both wild-type and PGC-1α muscle-specific knockout mice (Myo-PGC-1αKO) ( Rowe et al. 2012 ). The paper concludes that PGC-1α is dispensable for endurance-exercise’s induction of skeletal muscle mitochondrial adaptations.
Exercise signaling targets have actions that are independent of PGC-1α, which is specific to endurance exercise. In 2002, two groups identified PGC-1β, a transcriptional coactivator closely related to PGC-1α ( Kressler et al. 2002 ; Lin et al. 2002 ). Later in 2012, the PGC-1α4 variant of PGC-1α was found to induce skeletal muscle hypertrophy and strength ( Ruas et al. 2012 ). The importance of the finding of a PGC-1α variant is that it partially explains the phenotypic variation for differing types of exercise. Since the 1970s ( Holloszy and Booth 1976 ), it has been appreciated that the biochemical and anatomical observations between endurance and resistance differed. For example, Holloszy and Booth (1976) noted in 1976 that, whereas endurance-type exercise markedly increased skeletal muscle mitochondrial density with very minor increases in muscle fiber diameter, strength-type exercise, in contrast, increased muscle fiber diameter without increases in skeletal muscle mitochondrial density. Taken together, a drug specific for PGC-1α will not likely mimic separate physical training for endurance, strength/resistance, and coordination types of exercise in the same subject. Thus, the common usage of the term exercise capacity is a misnomer because endurance training and resistance training were shown to have different exercise capacity phenotypes very long ago.
In a 2015 Diabetes paper ( Wong et al. 2015 ), Muoio’s laboratory concluded that changes in glucose tolerance and total body fat depended upon how much energy is expended in contracting muscle rather than muscle mitochondrial content or substrate selection. A finding to support the previous sentence was the glucose tolerance tests (GTTs). MCK-PGC-1α mice and their nontransgenic (NT) littermates were not different in GTT, with both being the most glucose intolerant after 10 wk of high-fat feeding. Adding 10 wk of voluntary wheel running to the two high-fat-feed groups during the next 10-wk period (weeks 11–20 of the experiment) lowered the glucose intolerance, and then during weeks 21–30 of the experiment, glucose intolerance was further lowered by adding 25% caloric restriction with the high-fat food and running during the final 10 wk. The percentage weight lost after 30 wk of high-fat feeding was positively related to greater running distances. No single front-runner gene candidate could be identified by principle component analysis. Taken together, the paper suggests “doubts” that pharmacological exercise mimetics that increase muscle oxidative capacity will be effective antiobesity and/or antidiabetic agents. Rather, Muoio and investigators suggest energy expenditure by muscle contraction induces localized shifts in energy balance inside the muscle fiber, which then initiates a broad network of metabolic intermediates regulating nutrient sensing and insulin action. A further discussion of complex biology produced by polygenicity continues next.
POLYGENICITY OF EXERCISE LEADS TO COMPLEX MULTISYSTEM RESPONSES TO IMPROVE HEALTH OUTCOMES
Multiples tissues, organs, and systems are influenced by physical activity, or the lack thereof ( Table 2 ).
Worsening of maximal functioning in selected major organ/tissue/systems that are caused by the lack of physical activity with growth, maturation, and aging
The higher their maximal function is before the end of each item’s maturation, the longer chances are that the quality of life will remain optimal. The breadth of the list implies that a single molecular target will not substitute for appropriate daily physical activity to prevent the loss of all listed items.
To present one extreme, that most will agree, one molecule will not describe the 1000s of molecules adapting to aerobic, resistance, and coordination exercise training. On the opposite extreme, many could likely agree that usage of the various “omics” underlying all adaptations to physical activity will differ (i.e., not be identical in most aspects) among the next list: various cell types within a tissue/organ, tissues/organs, and various intensities of physical activity (i.e., the thresholds among gene responses for health benefits will differ because of the presence of responders and nonresponders, or protein isoform type); during various types cycling (circadian or menstrual); postprandial versus fasting between meals; male and female; child, adult, and the elderly; trained and untrained; aerobic- and resistance-exercise types; and so forth. Others have repetitively written that only ∼59% of the risk reduction for all forms of CVD have been shown to be caused by effects through traditional factors ( Mora et al. 2007 ; Joyner and Green 2009 ). Thus, we pose the next question: what is the identity of all molecules in the yet-to-be-discovered gap between our knowledge of single gene functions and the totality of personalized prescription of physical activity to maximize the period of life free of any chronic disease, termed health span?
While approaches using single-gene manipulations are valuable tools, research must also focus on integrating exercise-responsive molecules into networks that maintain or improve health. This process will reveal complex, multisystem, polygenic networking essential for the advancement of many goals pertaining to exercise physiology, such as tailoring exercise prescriptions and implementing personalized medicine. One example is the developing myokine network with auto-, para-, and endocrine molecules. The first myokine interleukin (IL)-6 began to be described as early as 1994 by the Pedersen laboratory ( Ullum et al. 1994 ), with a history of its development as the first exercise myokine recounted in 2007 ( Pedersen et al. 2007 ). Since their discovery, myokine action within and at a distance from their origins in skeletal muscle have been increasingly studied, as schematically illustrated by Schnyder and Handschin (2015) ( Fig. 2 ).

Figure provides an illustration of myokine production by skeletal muscle for actions within or at a distance. Myokine release promotes a high degree of intertissue cross talk. CNTF, Ciliary neurotrophic factor; OSM, oncostatin M; IL, interleukin; BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor. (From Schnyder and Handschin 2015 ; reprinted, with permission, courtesy of PMC Open Access.)
Similarly, maximal aerobic exercise is accompanied by tremendous stress on many systems, yet whole-body homeostasis is remarkably maintained. For example, world-class endurance athletes can increase whole-body energy production well over 20-fold ( Joyner and Coyle 2008 ), whereas maintaining blood glucose concentrations at resting levels ( Wasserman 2009 ). Intuitively, such effort would require sophisticated interorgan cross talk and polygenic integration of numerous functions.
Exercise Provides Too Many Benefits to “Fit into a Single Pill”
Despite the well-known benefits of exercise, most adults and many children lead relatively sedentary lifestyles and are not active enough to achieve the health benefits of exercise ( Warburton et al. 2006 ; Fried 2016 ). Accelerometry measurements suggest that >90% of U.S. individuals >12 yr of age and ∼50% of children aged 6–11 yr old fail to meet U.S. Federal physical activity guidelines ( Troiano et al. 2008 ). Given this incredibly low compliance, the identification of genetic and/or orally active agents that mimic the effects of endurance exercise might have high appeal for a majority of sedentary individuals. This high appeal has led to recent identification/development of exercise “mimetics.” In 2009, we set criteria for proper usage of the term “exercise mimetic,” based upon its common usage ( Booth and Laye 2009 ). We gave the Oxford English Dictionary’s definition of mimetic, “A synthetic compound that produces the same (or a very similar) effect as another (especially a naturally occurring) compound.” While many exercise “mimetics” activate signaling pathways commonly associated with muscle endurance, these agents have not completely mimicked all effects for all types of exercise. For example, the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), when given daily to rats over a 5-wk-period, did not increase maximal oxygen consumption (VO 2peak ) in the sedentary group of rats that were forced to run to VO 2peak on treadmills, as compared to sedentary rats receiving the vehicle ( Toedebusch et al. 2016 ). Thus, in our opinion, the published claim ( Narkar et al. 2008 ) that AICAR is an exercise mimetic is invalidated because it did not increase VO 2peak . While these agents may undoubtedly have specific health benefits, it is currently impractical to assume that all of the benefits of exercise can be replaced by “exercise mimetics.”
CONCLUDING REMARKS
Exercise is a powerful tool in the fight to prevent and treat numerous chronic diseases ( Table 1 ). Given its whole-body, health-promoting nature, the integrative responses to exercise should surely attract a great detail of interest as the notion of “exercise is medicine” continues to its integration into clinical settings.
ACKNOWLEDGMENTS
The authors disclose no conflicts of interest. Partial funding for this project was obtained from grants awarded to G.N.R. (AHA 16PRE2715005).
Editors: Juleen R. Zierath, Michael J. Joyner, and John A. Hawley
Additional Perspectives on The Biology of Exercise available at www.perspectivesinmedicine.org
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. 2014. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression . Cell 159 : 33–45. [ PubMed ] [ Google Scholar ]
- Astrand PO. 1956. Human physical fitness with special reference to sex and age . Physiol Rev 36 : 307–335. [ PubMed ] [ Google Scholar ]
- Bacon AP, Carter RE, Ogle EA, Joyner MJ. 2013. VO 2max trainability and high intensity interval training in humans: A meta-analysis . PLoS ONE 8 : e73182. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Beier M, Bombardier CH, Hartoonian N, Motl RW, Kraft GH. 2014. Improved physical fitness correlates with improved cognition in multiple sclerosis . Arch Phys Med Rehabil 95 : 1328–1334. [ PubMed ] [ Google Scholar ]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. 2005. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus . Neuroscience 133 : 853–861. [ PubMed ] [ Google Scholar ]
- Bielak AA, Cherbuin N, Bunce D, Anstey KJ. 2014. Preserved differentiation between physical activity and cognitive performance across young, middle, and older adulthood over 8 years . J Gerontol B Psychol Sci Soc Sci 69 : 523–532. [ PubMed ] [ Google Scholar ]
- Bjerring P, Arendt-Nielsen L. 1990. Inhibition of histamine skin flare reaction following repeated topical applications of capsaicin . Allergy 45 : 121–125. [ PubMed ] [ Google Scholar ]
- Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. 1989. Physical fitness and all-cause mortality. A prospective study of healthy men and women . JAMA 262 : 2395–2401. [ PubMed ] [ Google Scholar ]
- Booth FW, Laye MJ. 2009. Lack of adequate appreciation of physical exercise’s complexities can preempt appropriate design and interpretation in scientific discovery . J Physiol 587 : 5527–5539. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. 2002. Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy . J Appl Physiol (1985) 93 : 3–30. [ PubMed ] [ Google Scholar ]
- Booth FW, Roberts CK, Laye MJ. 2012. Lack of exercise is a major cause of chronic diseases . Compr Physiol 2 : 1143–1211. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. 2012. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis . Nature 481 : 463–468. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. 2001. Projection of diabetes burden through 2050: Impact of changing demography and disease prevalence in the U.S . Diabetes Care 24 : 1936–1940. [ PubMed ] [ Google Scholar ]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. 2010. Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence . Popul Health Metr 8 : 29. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cappel DA, Lantier L, Palmisano BT, Wasserman DH, Stafford JM. 2015. CETP expression protects female mice from obesity-induced decline in exercise capacity . PLoS ONE 10 : e0136915. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy . J Neurosci 21 : 5678–5684. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cotman CW, Berchtold NC. 2002. Exercise: A behavioral intervention to enhance brain health and plasticity . Trends Neurosci 25 : 295–301. [ PubMed ] [ Google Scholar ]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. 2010. Running enhances spatial pattern separation in mice . Proc Natl Acad Sci 107 : 2367–2372. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. 2006. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle . Diabetes 55 : 1776–1782. [ PubMed ] [ Google Scholar ]
- Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, Flaxman AD, Mokdad AH. 2016. Diagnosed and undiagnosed diabetes prevalence by county in the U.S., 1999–2012 . Diabetes Care 39 : 1556–1562. [ PubMed ] [ Google Scholar ]
- Fried LP. 2016. Interventions for human frailty: Physical activity as a model . Cold Spring Harb Perspect Med doi: 10.1101/cshperspect.a025916. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gidlund EK, Ydfors M, Appel S, Rundqvist H, Sundberg CJ, Norrbom J. 2015. Rapidly elevated levels of PGC-1α-b protein in human skeletal muscle after exercise: Exploring regulatory factors in a randomized controlled trial . J Appl Physiol (1985) 119 : 374–384. [ PubMed ] [ Google Scholar ]
- Goodyear LJ, Kahn BB. 1998. Exercise, glucose transport, and insulin sensitivity . Annu Rev Med 49 : 235–261. [ PubMed ] [ Google Scholar ]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M. 2012. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise . Behav Brain Res 233 : 314–321. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Greist JH, Klein MH, Eischens RR, Faris J, Gurman AS, Morgan WP. 1979. Running as treatment for depression . Compr Psychiatry 20 : 41–54. [ PubMed ] [ Google Scholar ]
- Guerrieri D, van Praag H. 2015. Exercise-mimetic AICAR transiently benefits brain function . Oncotarget 6 : 18293–18313. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gupta S, Rohatgi A, Ayers CR, Willis BL, Haskell WL, Khera A, Drazner MH, de Lemos JA, Berry JD. 2011. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality . Circulation 123 : 1377–1383. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hattori S, Naoi M, Nishino H. 1994. Striatal dopamine turnover during treadmill running in the rat: Relation to the speed of running . Brain Res Bull 35 : 41–49. [ PubMed ] [ Google Scholar ]
- Holloszy JO. 2005. Exercise-induced increase in muscle insulin sensitivity . J Appl Physiol (1985) 99 : 338–343. [ PubMed ] [ Google Scholar ]
- Holloszy JO, Booth FW. 1976. Biochemical adaptations to endurance exercise in muscle . Annu Rev Physiol 38 : 273–291. [ PubMed ] [ Google Scholar ]
- Holloszy JO, Narahara HT. 1965. Studies of tissue permeability. X: Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle . J Biol Chem 240 : 3493–3500. [ PubMed ] [ Google Scholar ]
- Joyner MJ, Coyle EF. 2008. Endurance exercise performance: The physiology of champions . J Physiol 586 : 35–44. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Joyner MJ, Green DJ. 2009. Exercise protects the cardiovascular system: Effects beyond traditional risk factors . J Physiol 587 : 5551–5558. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Keller P, Vollaard NB, Gustafsson T, Gallagher IJ, Sundberg CJ, Rankinen T, Britton SL, Bouchard C, Koch LG, Timmons JA. 2011. A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype . J Appl Physiol (1985) 110 : 46–59. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin . N Engl J Med 346 : 393–403. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kobilo T, Yuan C, van Praag H. 2011. Endurance factors improve hippocampal neurogenesis and spatial memory in mice . Learn Mem 18 : 103–107. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. 2014. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice . Learn Mem 21 : 119–126. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, et al. 2011. Intrinsic aerobic capacity sets a divide for aging and longevity . Circ Res 109 : 1162–1172. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. 2009. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis . JAMA 301 : 2024–2035. [ PubMed ] [ Google Scholar ]
- Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, et al. 2010. Exercise capacity and mortality in older men: A 20-year follow-up study . Circulation 122 : 790–797. [ PubMed ] [ Google Scholar ]
- Kratz AL, Ehde DM, Bombardier CH. 2014. Affective mediators of a physical activity intervention for depression in multiple sclerosis . Rehabil Psychol 59 : 57–67. [ PubMed ] [ Google Scholar ]
- Kressler D, Schreiber SN, Knutti D, Kralli A. 2002. The PGC-1-related protein PERC is a selective coactivator of estrogen receptor α . J Biol Chem 277 : 13918–13925. [ PubMed ] [ Google Scholar ]
- Kwak SH, Park KS. 2016. Recent progress in genetic and epigenetic research on type 2 diabetes . Exp Mol Med 48 : e220. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Larson EB, Carroll ME. 2005. Wheel running as a predictor of cocaine self-administration and reinstatement in female rats . Pharmacol Biochem Behav 82 : 590–600. [ PubMed ] [ Google Scholar ]
- Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. 2008. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle . Am J Physiol Endocrinol Metab 294 : E463–E474. [ PubMed ] [ Google Scholar ]
- Liang X, Liu L, Fu T, Zhou Q, Zhou D, Xiao L, Liu J, Kong Y, Xie H, Yi F, et al. 2016. Exercise inducible lactate dehydrogenase B regulates mitochondrial function in skeletal muscle . J Biol Chem 291 : 25306–25318. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. 2002. Peroxisome proliferator-activated receptor γ coactivator 1 β (PGC-1β ), a novel PGC-1-related transcription coactivator associated with host cell factor . J Biol Chem 277 : 1645–1648. [ PubMed ] [ Google Scholar ]
- Lindholm ME, Giacomello S, Werne Solnestam B, Fischer H, Huss M, Kjellqvist S, Sundberg CJ. 2016. The impact of endurance training on human skeletal muscle memory, global isoform expression and novel transcripts . PLoS Genet 12 : e1006294. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lira VA, Benton CR, Yan Z, Bonen A. 2010. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity . Am J Physiol Endocrinol Metab 299 : E145–E161. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. 2013. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis . Neurosci Biobehav Rev 37 : 1622–1644. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maarbjerg SJ, Sylow L, Richter EA. 2011. Current understanding of increased insulin sensitivity after exercise—Emerging candidates . Acta Physiol (Oxf) 202 : 323–335. [ PubMed ] [ Google Scholar ]
- Mansueto G, Armani A, Viscomi C, D’Orsi L, De Cegli R, Polishchuk EV, Lamperti C, Di Meo I, Romanello V, Marchet S, et al. 2017. Transcription factor EB controls metabolic flexibility during exercise . Cell Metab 25 : 182–196. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mattson MP. 2014. Interventions that improve body and brain bioenergetics for Parkinson’s disease risk reduction and therapy . J Parkinsons Dis 4 : 1–13. [ PubMed ] [ Google Scholar ]
- McKercher C, Sanderson K, Schmidt MD, Otahal P, Patton GC, Dwyer T, Venn AJ. 2014. Physical activity patterns and risk of depression in young adulthood: A 20-year cohort study since childhood . Soc Psychiatry Psychiatr Epidemiol 49 : 1823–1834. [ PubMed ] [ Google Scholar ]
- Meigs JB, Cupples LA, Wilson PW. 2000. Parental transmission of type 2 diabetes: The Framingham Offspring Study . Diabetes 49 : 2201–2207. [ PubMed ] [ Google Scholar ]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. 2004. Actual causes of death in the United States, 2000 . JAMA 291 : 1238–1245. [ PubMed ] [ Google Scholar ]
- Mora S, Cook N, Buring JE, Ridker PM, Lee IM. 2007. Physical activity and reduced risk of cardiovascular events: Potential mediating mechanisms . Circulation 116 : 2110–2118. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. 1953. Coronary heart-disease and physical activity of work . Lancet 265 : 1053–1057. [ PubMed ] [ Google Scholar ]
- Mura G, Moro MF, Patten SB, Carta MG. 2014. Exercise as an add-on strategy for the treatment of major depressive disorder: A systematic review . CNS Spectr 19 : 496–508. [ PubMed ] [ Google Scholar ]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. 2002. Exercise capacity and mortality among men referred for exercise testing . N Engl J Med 346 : 793–801. [ PubMed ] [ Google Scholar ]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. 2008. AMPK and PPARδ agonists are exercise mimetics . Cell 134 : 405–415. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. 1995. Exercise and brain neurotrophins . Nature 373 : 109. [ PubMed ] [ Google Scholar ]
- Nishijima T, Llorens-Martin M, Tejeda GS, Inoue K, Yamamura Y, Soya H, Trejo JL, Torres-Aleman I. 2013. Cessation of voluntary wheel running increases anxiety-like behavior and impairs adult hippocampal neurogenesis in mice . Behav Brain Res 245 : 34–41. [ PubMed ] [ Google Scholar ]
- Osler ME, Fritz T, Caidahl K, Krook A, Zierath JR, Wallberg-Henriksson H. 2015. Changes in gene expression in responders and nonresponders to a low-intensity walking intervention . Diabetes Care 38 : 1154–1160. [ PubMed ] [ Google Scholar ]
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, et al. 1997. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study . Diabetes Care 20 : 537–544. [ PubMed ] [ Google Scholar ]
- Park H, Poo MM. 2013. Neurotrophin regulation of neural circuit development and function . Nat Rev Neurosci 14 : 7–23. [ PubMed ] [ Google Scholar ]
- Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. 2007. Role of myokines in exercise and metabolism . J Appl Physiol (1985) 103 : 1093–1098. [ PubMed ] [ Google Scholar ]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. 2007. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus . Proc Natl Acad Sci 104 : 5638–5643. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Peterson AB, Hivick DP, Lynch WJ. 2014. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: Impact of sex and estrous cycle in rats . Psychopharmacology (Berl) 231 : 2661–2670. [ PubMed ] [ Google Scholar ]
- Reis FC, Haro AS, Bacurau AV, Hirabara SM, Wasinski F, Ormanji MS, Moreira JB, Kiyomoto BH, Bertoncini CR, Brum PC, et al. 2015. Deletion of kinin B2 receptor alters muscle metabolism and exercise performance . PLoS ONE 10 : e0134844. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rockl KS, Witczak CA, Goodyear LJ. 2008. Signaling mechanisms in skeletal muscle: Acute responses and chronic adaptations to exercise . IUBMB Life 60 : 145–153. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ross R, de Lannoy L, Stotz PJ. 2015. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response . Mayo Clin Proc 90 : 1506–1514. [ PubMed ] [ Google Scholar ]
- Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. 2012. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle . PLoS ONE 7 : e41817. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, et al. 2012. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy . Cell 151 : 1319–1331. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ruegsegger GN, Toedebusch RG, Braselton JF, Childs TE, Booth FW. 2017. Left ventricle transcriptomic analysis reveals connective tissue accumulation associates with initial age-dependent decline in VO 2peak from its lifetime apex . Physiol Genomics 49 : 53–66. [ PubMed ] [ Google Scholar ]
- Sarzynski MA, Ghosh S, Bouchard C. 2016. Genomic and transcriptomic predictors of response levels to endurance exercise training . J Physiol 10.1113/JP272559. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schneider J. 2013. Age dependency of oxygen uptake and related parameters in exercise testing: An expert opinion on reference values suitable for adults . Lung 191 : 449–458. [ PubMed ] [ Google Scholar ]
- Schnyder S, Handschin C. 2015. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise . Bone 80 : 115–125. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. 2013. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus . J Neurosci 33 : 7770–7777. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schuch FB, Vancampfort D, Sui X, Rosenbaum S, Firth J, Richards J, Ward PB, Stubbs B. 2016. Are lower levels of cardiorespiratory fitness associated with incident depression? A systematic review of prospective cohort studies . Prev Med 93 : 159–165. [ PubMed ] [ Google Scholar ]
- Scott LJ, Erdos MR, Huyghe JR, Welch RP, Beck AT, Wolford BN, Chines PS, Didion JP, Narisu N, Stringham HM, et al. 2016. The genetic regulatory signature of type 2 diabetes in human skeletal muscle . Nat Commun 7 : 11764. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. 2003. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity . Diabetes 52 : 1888–1896. [ PubMed ] [ Google Scholar ]
- Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ, Duscha BD, Kraus WE. 2005. Inactivity, exercise, and visceral fat. STRRIDE: A randomized, controlled study of exercise intensity and amount . J Appl Physiol (1985) 99 : 1613–1618. [ PubMed ] [ Google Scholar ]
- Slentz CA, Houmard JA, Kraus WE. 2007. Modest exercise prevents the progressive disease associated with physical inactivity . Exerc Sport Sci Rev 35 : 18–23. [ PubMed ] [ Google Scholar ]
- Stanford KI, Goodyear LJ. 2014. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle . Adv Physiol Educ 38 : 308–314. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stephenson EJ, Smiles W, Hawley JA. 2014. The relationship between exercise, nutrition and type 2 diabetes . Med Sport Sci 60 : 1–10. [ PubMed ] [ Google Scholar ]
- Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, Stepniak E, Walsh SA, Acevedo MR, Perez-Terzic CM, et al. 2015. Musclin is an activity-stimulated myokine that enhances physical endurance . Proc Natl Acad Sci 112 : 16042–16047. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tian Q, Erickson KI, Simonsick EM, Aizenstein HJ, Glynn NW, Boudreau RM, Newman AB, Kritchevsky SB, Yaffe K, Harris TB, et al. 2014. Physical activity predicts microstructural integrity in memory-related networks in very old adults . J Gerontol A Biol Sci Med Sci 69 : 1284–1290. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, et al. 2010. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans . J Appl Physiol (1985) 108 : 1487–1496. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Toedebusch RG, Ruegsegger GN, Braselton JF, Heese AJ, Hofheins JC, Childs TE, Thyfault JP, Booth FW. 2016. AMPK agonist AICAR delays the initial decline in lifetime-apex VO 2peak , while voluntary wheel running fails to delay its initial decline in female rats . Physiol Genomics 48 : 101–115. [ PubMed ] [ Google Scholar ]
- Trejo JL, Carro E, Torres-Aleman I. 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus . J Neurosci 21 : 1628–1634. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. 2008. Physical activity in the United States measured by accelerometer . Med Sci Sports Exerc 40 : 181–188. [ PubMed ] [ Google Scholar ]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. 2001. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance . N Engl J Med 344 : 1343–1350. [ PubMed ] [ Google Scholar ]
- Ullum H, Haahr PM, Diamant M, Palmo J, Halkjaer-Kristensen J, Pedersen BK. 1994. Bicycle exercise enhances plasma IL-6 but does not change IL-1α, IL-1β, IL-6, or TNF-α pre-mRNA in BMNC . J Appl Physiol (1985) 77 : 93–97. [ PubMed ] [ Google Scholar ]
- van Praag H, Kempermann G, Gage FH. 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus . Nat Neurosci 2 : 266–270. [ PubMed ] [ Google Scholar ]
- Vaughan RA, Gannon NP, Barberena MA, Garcia-Smith R, Bisoffi M, Mermier CM, Conn CA, Trujillo KA. 2014. Characterization of the metabolic effects of irisin on skeletal muscle in vitro . Diabetes Obes Metab 16 : 711–718. [ PubMed ] [ Google Scholar ]
- Warburton DE, Nicol CW, Bredin SS. 2006. Health benefits of physical activity: The evidence . CMAJ 174 : 801–809. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wasserman DH. 2009. Four grams of glucose . Am J Physiol Endocrinol Metab 296 : E11–E21. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Widenfalk J, Olson L, Thoren P. 1999. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain . Neurosci Res 34 : 125–132. [ PubMed ] [ Google Scholar ]
- Wong KE, Mikus CR, Slentz DH, Seiler SE, DeBalsi KL, Ilkayeva OR, Crain KI, Kinter MT, Kien CL, Stevens RD, et al. 2015. Muscle-specific overexpression of PGC-1α does not augment metabolic improvements in response to exercise and caloric restriction . Diabetes 64 : 1532–1543. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. 2013. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway . Cell Metab 18 : 649–659. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zierath JR, Krook A, Wallberg-Henriksson H. 2000. Insulin action and insulin resistance in human skeletal muscle . Diabetologia 43 : 821–835. [ PubMed ] [ Google Scholar ]
- Zlebnik NE, Anker JJ, Carroll ME. 2012. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats . Psychopharmacology 224 : 387–400. [ PMC free article ] [ PubMed ] [ Google Scholar ]
Advertisement
The Health Benefits of Exercise and Physical Activity
- Gastroenterology, Critical Care, and Lifestyle Medicine (SA McClave, Section Editor)
- Published: 14 July 2016
- Volume 5 , pages 204–212, ( 2016 )
Cite this article
- Keith R. Miller 1 ,
- Stephen A. McClave 2 ,
- Melina B. Jampolis 3 ,
- Ryan T. Hurt 4 ,
- Kristine Krueger 2 ,
- Sarah Landes 2 &
- Bryan Collier 5
24k Accesses
24 Citations
51 Altmetric
Explore all metrics
Physical inactivity is a modifiable risk factor (similar to dyslipidemia and hypertension) for a variety of chronic diseases, including cancer and cardiovascular disease. Exercise provides a clear health benefit, which serves in the primary and secondary prevention of these disease processes (the most important being a reduction in cardiovascular disease and premature death). The physiologic mechanisms for such a benefit occur at both a cellular and multisystem level. Prolonged periods of occupational or leisure-time sitting have adverse health effects independent of exercise performed before or after. Almost any form of physical activity (PA) is beneficial, whether part of a regular exercise program or as a series of intermittent, incidental, non-purposeful, lifestyle-embedded activity (causing non-exercise activity thermogenesis or NEAT). The health benefits of exercise appear to be dose-dependent. Physicians should recommend near daily exercise which includes at various times strength training, stretching, and aerobic activity in addition to emphasizing adjustments that allow for reduced sitting and increased activity during daily routines. Patients should understand that for optimal health, exercise is no longer optional.
Similar content being viewed by others

Physical Activity Strategies

Physical Activity, Fitness, and Coronary Heart Disease

Physical Inactivity and the Economic and Health Burdens Due to Cardiovascular Disease: Exercise as Medicine
Avoid common mistakes on your manuscript.
Introduction
Physical inactivity is a modifiable risk factor for cardiovascular disease, obesity, depression, cancer, diabetes mellitus, hypertension, and osteoporosis. Physical exercise reduces the risk of premature death and prolongs longevity, and is an important treatment modality in the primary and secondary prevention of the above disorders [ 1 ]. For most states in this country, less than half of the population meets CDC exercise recommendations [ 2 •]. The decline in physical activity (PA) occurs both at work and in leisure time, and may have at least partially contributed to the increase in obesity over the past 30 years. Low recreational physical activities have been associated with a threefold increase for major weight gain in men and a fourfold increase in women [ 3 ]. Surveys of PA across the lifecycle show that physical exercise peaks in the middle high-school age range and begins declining through high school and into adult life. A vicious cycle of decline occurs between inactivity and loss of skeletal muscle mass which accelerates with age. With avoidance of activity requiring effort, there is increased loss of exercise capacity. This loss causes the perception of effort associated with even sub-maximal work to worsen, as the anaerobic threshold decreases. The vicious cycle contributes to further inactivity and deterioration of physical function. The only treatment that can break the cycle is exercise.
Impact of Exercise on Aging
Exercise provides powerful health benefits for quality of life, physical function, and independent living throughout the life cycle. Exercise impedes the aging process and promotes longevity. Observational studies have shown that even in the presence of disease processes such as hypertension (HTN), chronic obstructive pulmonary disease (COPD), diabetes, smoking, high body mass index (BMI), and hypercholesterolemia, increasing PA has a dose-dependent effect in decreasing relative risk of death [ 4 ]. In a study of subjects over a 13-year time period, both baseline fitness, and improvement in physical fitness through exercise and PA was associated with significant increases in longevity [ 4 ]. Functional independence with advanced age relates to the overall level of physical fitness. Physical fitness is most affected by the status of the cardiorespiratory and vascular systems, as well as muscle function [ 5 •].
Garatachea et al. provide an excellent review of the effect of exercise on the physiologic changes associated with aging [ 5 •]. Exercise exerts its positive influence on both a cellular level and at the level of organ systems. At the cellular level, exercise helps reduce genomic instability, epigenetic alteration, loss of proteostasis, dysregulated nutrient sensing, cellular senescence, and altered intracellular communication that leads to inflammation. These effects alter the way the body performs transcellular signaling in the skeletal muscle, the turning on and off of genes through epigenetics, and the manner in which the system manages reactive oxygen species [ 5 •]. On a multisystem level, the benefits of exercise include improvement in brain, cardiovascular, lung, and muscle function, favorable alterations in body composition, and advantageous changes in metabolic responses. The report concludes by suggesting that in the future, pharmaceuticals should be designed which mimic the effects of exercise on the aging process [ 5 •].
Effect of Exercise on Treatment of Disease
Robert Butler from the National Institute on Aging has said that “If exercise could be put in a bottle, it would be the strongest medicine money could buy” [ 6 ]. Exercise helps prevent common chronic diseases (primary prevention), and often plays an important role in the treatment of these disease processes (secondary prevention). Specific benefits from exercise have been seen with cardiovascular disease, stroke, diabetes mellitus, depression, cancer, obesity, and osteoporosis [ 7 , 8 ]
Cardiovascular Disease
Increased levels of PA and physical fitness have a graded effect in reducing the risk of death from cardiovascular disease. The relative risk from all cause and cardiovascular disease mortality is reduced 20–35 % by exercise and PA [ 9 ]. In an observational study, subjects in the lowest quintile of exercise had a relative risk of 3.4 in men and 4.7 in women for death compared to those in the highest quintile [ 10 ]. An increase in activity-related energy expenditure by as little as 1000 kcal or 1 metabolic equivalent (MET)-hour of exercise per week has a mortality benefit of 20 % [ 11 ]. Physically inactive women have a 52 % increase in death, a cardiovascular disease-related death that is doubled, and a cancer-related death rate that is increased by 29 % [ 11 ]. These risks on mortality from inactivity are similar to other modifiable risk factors such as HTN, hypercholesterolemia, and obesity. In randomized controlled trials (RCT)s, exercise and PA are valuable for the secondary prevention of cardiovascular disease. Whereas in the past, traditional recommendations for patients with a heart attack included rest and physical inactivity. Newer information demonstrates that exercise actually attenuates or reverses risk of cardiovascular disease [ 12 ]. The benefit of exercise is seen in cardiac rehabilitation, where increasing PA reduces the risk of premature death following a myocardial infarction [ 12 ]. Added energy expenditure of 1600 kcal/week from exercise may halt the progression of heart disease and energy expenditure of >2200 kcal/week can lead to plaque reduction [ 13 ]. The minimum training recommendation for patients following myocardial infarction is to reach 45 % of their heart rate reserve through cardiac rehabilitation [ 12 , 13 ].
Multiple mechanisms have been identified whereby exercise reduces the risk of premature death [ 4 ]. Exercise affects body composition by decreasing abdominal adiposity and improving weight control. Exercise enhances lipid profiles by reducing serum triglyceride levels, raising HDL, and reducing the LDL/HDL ratio. In addition, a recent meta-analysis showed beneficial changes in lipoprotein subclasses associated with regular exercise including a reduction in small LDL-p and an increase in large LDL-p [ 14 ]. Exercise enhances hemodynamics by decreasing blood pressure, increasing cardiac function, and improving coronary blood flow. Autonomic tone is enhanced and shear stress-mediated endothelial function is improved. Exercise reduces systemic inflammation, as evidenced by reduced C-reactive protein (CRP) levels. Improved psychological well-being in response to exercise is associated with reduced stress, anxiety, and depression [ 4 ].
PA is inversely correlated with risk of incident stroke as shown in a large nurses’ health study [ 15 ]. Habitual exercise reduces risk of stroke by 40–50 % at the highest level of PA. Change in PA is protective against stroke as evidenced by the fact that an increase of 3.5 h of exercise or PA per week is associated with a 29 % reduction in ischemic stroke [ 15 ].
Diabetes Mellitus
Exercise is valuable in both the primary and secondary prevention of diabetes mellitus. Aerobic and resistant-type exercise reduces the likelihood of developing type-2 diabetes mellitus. For each 500 kcal of energy expended per week, there is an associated 6 % reduction in the likelihood of type-2 diabetes (which may be even greater with increasing BMI) [ 16 ]. In patients already diagnosed to have diabetes mellitus, walking 2 h per week is associated with a 39–54 % reduction in all-cause mortality from diabetes mellitus, and a 34–53 % reduction in mortality related to cardiovascular disease [ 17 ]. The benefit of exercise on glycemic control appears to be greater with resistance training than aerobic exercise. A meta-analysis of exercise and PA in diabetes showed that exercise reduces hemoglobin A1C by 0.66 %, an effect similar to intensive glucose-lowering pharmacologic therapy [ 18 ]. The mechanisms by which exercise benefits diabetes relate to the fact that exercise increases glycogen synthetase and hexokinase activity [ 4 ]. Exercise reduces GLUT-4 protein and messenger RNA expression and increases muscle capillary density, which helps improve glucose delivery to the muscle [ 4 ].
Increasing PA, either occupational or at leisure, has been shown to exert a primary preventative effect on two cancers—breast and colon cancer [ 19 ]. Moderate exercise of as little as 4–5 METs (equivalent to mowing the lawn or brisk walking), is required to achieve this effect [ 20 ]. Exercise is associated with a 20–30 % reduction in the incidence of breast cancer in women, and a 30–40 % reduction in the incidence of colon cancer in both men and women [ 20 ]. In those patients already diagnosed to have one of these cancers, exercise reduces the likelihood for cancer recurrence and reduces risk from cancer death by as much as 26–40 % [ 21 ]. PA improves quality of life and overall health status in cancer patients. The mechanisms by which exercise improves risk from cancer may relate to reduced fat stores, an increase in energy expenditure offsetting a high-fat diet, activity-related changes in sex hormone levels, improvement in immune function, and reduced generation of free oxygen radicals [ 4 ].
Osteoporosis
Exercise has a valuable effect in the primary prevention of osteoporosis. Routine PA minimizes age-related bone loss. Weight-bearing exercise (especially resistance exercise) increases bone density compared to low impact non-weight-bearing exercise. Exercise prevents 1 % of bone loss per year, an effect which is greater in post-menopausal than pre-menopausal women [ 22 ]. In RCTs, exercise reduces the risk and number of falls, as well as the risk of fracture [ 22 ]. Even in men, PA reduces the risk of fracture by 62 % over the age of 21 years [ 23 ]. Exercise is also valuable in the secondary prevention of osteoporosis. RCTs in the past have shown that exercise with resistance training increases bone density in older osteoporotic women by as much as 1.4 %, while agility training alone increases bone density by 0.5 % [ 24 ]. Stretching, which was used as sham control, was shown to have no effect on the expected decrease in bone density with age [ 24 ]. In a 12-year follow up of over 60,000 post-menopausal women, risk of hip fracture was lowered 6 % for each increase of three MET-hours per week of activity (the equivalent of walking three miles in 1 h) [ 25 ]. Active women with at least 24 met-hours of exercise per week had a 55 % lower risk of hip fracture than sedentary women with no other exercise. Walking at least 4 h per week was associated with a 41 % lower risk of hip fracture than walking less than one hour per week [ 25 ].
Exercise has a valuable therapeutic effect on the treatment on multiple types of depression, including dysthymic, seasonal, bipolar, post-natal, pre-menstrual, atypical, and major depression [ 26 ]. The value in treating depression comes from an innate anti-depressive effect from exercise. Combining exercise with psychotropic medications achieves better treatment results than the same medications alone [ 26 ]. Exercise is relatively inexpensive, safe, and has minimal side effects when done correctly. Exercise may help reduce the dose of anti-depressive medications required. Subjects are less likely to relapse with an active exercise program [ 26 ].
The patients with depression who are most likely to benefit from exercise include those with age <20 or >40 years, higher education, higher baseline physical status, females, untrained subjects, and those with mild to moderate depression [ 26 ]. There are a number of aspects of exercise that get the optimal results in treating depression including programs that are structured, individually tailored to the patient, low to moderate intensity, when it is used as an adjunct to medication therapy, and exercise that is a combination of aerobic or resistive training performed 3–4 times per week [ 26 ]. The mechanism of effect from exercise on depression occurs on a systemic level as well as a direct effect on central nervous system (CNS) function. Exercise appears to increase serotonin, ACTH, endorphins, and endocannabinoids within the CNS. On a systemic level, exercise increases norepinephrine and reduces cortisol, tumor necrosis factor (TNF), and interleukin-6 [ 26 ].
In a controversial article that appeared in Time magazine in 2009, the journalist John Cloud wrote about “The Myth of Exercise” and its effect on treating obesity [ 27 ]. The article suggested that exercise was not good for weight management in obesity. The author pointed out that exercise leads to increased appetite and intake of food and causes a decrease in non-exercise energy expenditure, and therefore that exercise was a poor strategy for weight loss [ 27 ]. A number of letters to the editor of Time magazine followed the publication of this article, including letters from the American Society for Sports Medicine, arguing that facts were misrepresented and that the article gave the wrong message about the health benefits of exercise.
A recent review by Swift clarified the role of exercise in managing or preventing obesity, and suggested that Cloud’s article was in fact an accurate portrayal of the facts [ 28 ]. The key issue of Swift’s review is that exercise without caloric restriction is unlikely to succeed in weight loss [ 28 ]. Increasing PA can prevent weight gain, but it requires 150–250 min per week of moderate to vigorous exercise or 1200–2000 kcal/week expended through exercise to accomplish this feat [ 29 ]. Aerobic exercise by itself is minimally helpful in promoting weight loss, successful in loss of only 0–2 kg total [ 29 ]. Extreme high-volume aerobic exercise can achieve significant weight loss, but this is usually unsustainable by most obese patients. Moderate intensity, surprisingly, is no different than vigorous intensity in achieving weight loss, unless subjects are matched for exercise duration. Resistance training by itself has no impact on weight loss, and aerobic training combined with resistant training has no greater effect than aerobic training alone. However, adding caloric restriction to aerobic training does result in successful weight loss of 9–13 kg, and higher intensity of exercise has the potential for even greater weight loss [ 29 ]. Some obese subjects do experience weight compensation in response to exercise, defined by the circumstances where less weight is lost than expected with the amount of exercise sustained, often a factor related to an increase in caloric intake [ 28 , 30 ]. This is more likely to occur in women performing 150 % of weekly recommendations (compared to women performing only 100 % or 50 % of weekly recommendations) [ 28 , 30 ]. Even if minimal or no weight loss occurs in response to exercise, obese subjects still benefit from the increase in PA due to increased cardiorespiratory fitness, glucose control, endothelial function, improvements in hyperlipidemia, quality of life, and a reduction in future weight gain [ 28 ].
Caloric restriction is better than exercise for significant weight loss initially, and the weight loss is not necessarily enhanced significantly by adding exercise [ 28 ], although exercise training plus caloric restriction does improve body composition by increasing fat loss and decreasing loss of lean body mass [ 31 ]. The greatest value of exercise in the management of obesity occurs not in the initial weight loss, but in the situation where obese patients have lost weight successfully and now require substantial PA to maintain that weight loss [ 28 ]. Interestingly, an “energy gap” has been identified as the difference in energy expenditure before and after weight loss [ 32 ]. The energy gap is estimated to be approximately 8 kcal per day per pound of weight lost. An energy gap, for example, of 40 lbs lost would be associated with 320 kcal of energy. Sustaining this weight loss successfully would require either a continued reduction in energy consumption by 320 kcal per day, or increasing activity-associated energy expenditure by the same amount [ 32 ]. Based on the Set Point theory, both biological and environmental pressures oppose the strategy of food restriction in keeping weight off, but the same effect does not occur with increased PA [ 32 ]. Therefore, while food restriction is the key to weight loss, PA is the key to successful maintenance of the weight lost [ 32 ]. The ACSM has identified that people who successfully maintain weight loss average at least 250 min of PA per week [ 29 ].
Low Back Pain
A 2016 systematic review and meta-analysis reviewed 23 randomized controlled trials evaluating the prevention of low back pain [ 33 ]. Over 30,000 patients were involved in these studies. Ultimately, the combination of exercise (varying regimens of abdominal strengthening, core stability, cardiovascular, and isometrics) plus education regarding prevention of low back pain was found to reduce the risk of low back pain as well as sick leave related to low back pain. Exercise alone was also found to have an impact but had a more short term effect (<12 months), thought to be due to cessation of exercise following the intervention. Other interventions, including back belts, insoles, and education alone were not found to have any impact [ 33 ].
Not All Exercise is Created Equal
Physical activity versus physical fitness.
The lay public tends to use the terms PA and physical fitness interchangeably, but subtle differences between the two exist. Physical fitness is a physiologic state of being with regard to daily living and/or sports performance [ 4 ]. Physical fitness is comprised of cardiovascular, musculoskeletal, body composition, and metabolic components [ 4 ]. Physical fitness is similar to PA, but is more predictive of health outcomes. For example, a high-fit versus a low-fit person is estimated to have a 50 % lower mortality [ 34 ]. Physical fitness, therefore, becomes a better measure of PA than self-reporting. From a public health standpoint, however, it is better and more productive to encourage the public to be physically active and not push the need to be physically fit. Eventually, increased activity should lead to physical fitness.
In the past, guidelines for optimal health seemed to have had a singular focus on aerobic fitness. But a new paradigm shift has occurred with the addition of the concept of musculoskeletal fitness [ 4 ]. In other words, health status can improve due to increased PA in the absence of changes in aerobic fitness. Regular PA can decrease risk factors from chronic disease and disability without changing cardiac output or oxidative potential, especially in the elderly [ 4 ]. The shift has been to focus on the health benefits of musculoskeletal fitness, which may be a critical factor in the functional threshold for dependence with the aging population. Loss of muscular fitness can result in loss of capacity for daily living, and a cycle of decline can ensue [ 4 ]. Improvement in musculoskeletal function can delay the onset of disability, dependence, and chronic disease [ 35 ]. Musculoskeletal fitness is associated with fewer functional limitations and a reduced incidence of cardiovascular disease, diabetes, degenerative joint disease, and coronary artery disease [ 35 ]. Therefore, resistance training that works all the major muscle groups (including legs, hips, back, abdomen, chest, shoulders, and arms) and flexibility exercise, which are necessary to achieve musculoskeletal fitness, are recommended to be done at least twice weekly, to complement aerobic fitness and optimize overall health status.
Adverse Health Risk from Sitting
In an effort to delineate those factors which contribute to the obesity epidemic, researchers are increasingly focused on the adverse health risk from prolonged sitting [ 36 •]. A newly recognized occupational hazard has evolved because of workers needing to sit at a computer screen throughout the workday. Each mean hour of sitting after a total mean of 7 h per day is associated with a 5 % increase in premature death [ 36 •]. More time sitting at work has been shown to correlate with more sitting in leisure time. Prolonged sitting while watching TV at home, for example, has adverse effects on mental health, well-being, and muscle strength. Long sedentary hours have been linked to a twofold increase in diabetes, a twofold increase in cardiovascular disease, a 13 % increase in the incidence of cancer, and a 17 % increase in mortality related to cancer [ 36 •]. It is estimated that the average worker in the USA and England spends 60–70 % of waking hours in a sedentary sitting position. The effect of sitting has been likened to the transmission of a car. Sitting for such a prolonged period is like putting a car in reverse, causing one’s overall health status to go in the wrong direction [ 36 •]. Approximately 20–30 % of the time is spent in light intensity activity, described as postural changes, standing and movement, or ambulation. For less than 5–10 % of waking hours, individuals spend in moderate to vigorous PA. The adverse effect of sitting on health status is independent of the exercise or PA done before or after [ 36 •]. In other words, no amount of PA later can overcome the negative health effects of prolonged sitting.
Changes in the workplace environment may be the key issue to minimizing the negative effects of prolonged sitting. Particularly, in the UK, recommendations and guidelines have been developed to avoid this health hazard [ 36 •]. Workers are encouraged to accumulate up to 2 h per day at work standing or performing light walking, with the goal to progress ultimately to 4 h per day. Workers should interrupt seat-based work with standing-based work. However, workers should avoid both prolonged periods of standing as well as prolonged periods of sitting. Adaptation of these guidelines may lead to musculoskeletal complaints and fatigue, which should be monitored by managers in the workplace. Such health promotion strategies should eventually extend from the workplace to the leisure time [ 36 •].
Non-Exercise Activity Thermogenesis
Non-exercise activity thermogenesis (NEAT) has been described as unstructured PA, energy expended unrelated to sleeping, eating, or sports exercise. NEAT is energy expended outside of purposeful exercise [ 37 ]. Surprisingly, this incidental, non-purposeful lifestyle-embedded PA can have tremendous health benefits. Three components of NEAT include body posture, ambulation, and all other movements (the most important of which may be fidgeting) [ 38 ]. Researchers involved in the study of obesity are finding that in some cases what delineates the lean subject from an obese one is a difference in NEAT, not exercise-associated activity thermogenesis [ 38 ]. Early experiments which helped identify NEAT came from studies where energy requirements were measured and all subjects were placed on a diet of 1000 cal over requirements [ 39 ]. Subjects were then videotaped, and in a blinded fashion designated as fidgeters or non-fidgeters. At the end of the trial, those patients who were designated as fidgeters failed to gain weight, while those identified to be non-fidgeters sustained significant weight gain. The increase in kilocalories of energy expenditure attributed to NEAT was inversely proportional to fat gain in pounds [ 39 ]. NEAT ranges from 15 % of total energy expenditure (TEE) in sedentary subjects to as much as 50 % of TEE in fidgeting physically active people [ 39 ]. Fidgeting has been shown in twin studies to be genetic, with an estimated >62 % heritability [ 40 ]. Simply standing or lightly ambulating can increase energy expenditure by an average of 350 kcal/day (range 269–477 kcal/day) [ 37 ]. NEAT tends to be greater in men than women, in obese subjects rather than lean, and in those with more education than those with less [ 38 , 39 ]. NEAT tends to be seasonal and overall, declines with age [ 39 ]. The concept of an energy gap is pertinent to NEAT. An average citizen in the USA has been shown to gain 1–2 lbs each year through their adult life. An energy gap of 100 kcal additional energy consumed each day would account for this weight gain [ 41 ]. NEAT can be an important contributor to TEE, such that increases in NEAT of as little as 100–150 kcal of activity per day could prevent such weight gain (by offsetting the energy gap) in the vast majority of people [ 41 ]. Recommendations now suggest that if you were not lucky enough to inherit fidgeting, you should “act like a fidgeter,” standing often, getting up from sitting, pacing, parking at the back of a parking lot, and taking stairs instead of elevators [ 40 ].
Continuous Versus Interval Exercise
Long bouts of continuous exercise as a strategy for weight loss or weight maintenance can be a contentious and challenging recommendation for the general public. Longer duration, continuous exercise may be difficult and not particularly enjoyable for patients and may not fit as well with work or home schedules. Research now has shown that interval exercise, which involves alternating short bouts of high-intensity exercise with lower-intensity exercise that allows for partial recovery, can match the health benefits of continuous exercise [ 42 ]. Studies in patients with class-1 obesity (BMI 30–34.9 kg/m 2 ), walking at a moderate level of intensity, randomized to two 15-min intervals of walking versus one 30-min interval, showed essentially the same improvements in overall health status [ 42 ]. Both intermittent and continuous exercise resulted in improvement of maximum oxygen consumption, body composition, and lipid profiles. In some categories, interval exercise even exceeded the benefit seen with continuous exercise (such as VLDL levels and percent fat lost) [ 42 ]. The value of these findings for intermittent exercise stems from three factors: there is less attrition with recommendations for interval exercise, time constraints, and short periods of interval exercising may allow for greater intensity of PA [ 42 ]. An additional study involving 28 sedentary overweight or obese men compared five 45- to 60-min sessions of continuous moderate intensity cycling per week for 6 weeks with three 20-min sessions of high-intensity interval exercise per week (for a total of 60 min) for 6 weeks. Similar improvements in cardio-metabolic risk factors including improved insulin sensitivity, cardiovascular fitness, and a reduction in blood lipids and body fat percentage were observed in the groups [ 43 ]. While cardiovascular fitness was improved to a greater extent in the continuous exercise group, this study, along with numerous other studies of interval exercise showing similar outcomes in different populations, are encouraging in that they show many of the same improvements in overall health with a substantially reduced time commitment [ 43 ]. This is especially relevant as lack of time is cited as the most common reason for not exercising by many. In addition, interval exercise can be easily adapted to an individual’s starting fitness level by adjusting either the duration or intensity (or both) of the high-intensity component of exercise. This may be especially beneficial for sedentary overweight or obese individuals who are new to exercise. In light of both the potential health and time saving benefits, interval exercise training appears to be an appealing and worthwhile exercise option in addition to, or instead of, continuous exercise. The good news for public health is that short walks on a subject’s lunch break or brief periods of activity before and after work all count, and the sum of their duration may have similar benefits to a single continuous interval of exercise of the same duration.
Success of Pedometers
The use of pedometers to increase PA was generated years ago in Japanese walking clubs. The rationalization for the pedometer was that the average stride was estimated to be 2.5 ft. Therefore, 2000 steps should approximately equal a mile, 10,000 equaling about 5 miles [ 44 ]. Based on this rationalization, PA can be classified as sedentary (<5000 steps per day), low active (5000 to 7500 steps), somewhat active (7500 to 10,000 steps), and active (>10,000 steps per day). Highly active physical exercise is associated with >12,500 steps per day [ 44 ]. This is an arbitrary categorization, however, and 10,000 steps per day may be too little for children or too much for the elderly. Weight loss using a pedometer without caloric restriction is associated with minimal to modest weight loss of <2 kg [ 44 ]. Health benefits associated with use of the pedometer may be limited to a reduction in blood pressure, with not much change in cholesterol, triglycerides, or fasting glucose [ 44 ].
Exercise in the Intensive Care Unit
Exercise is becoming increasingly important in one of the least expected circumstances, that of a critically ill patient in the intensive care unit (ICU). Researchers have found that exercising muscle increases the uptake of amino acid fuel and promotes greater protein synthesis [ 45 , 46 ]. Patients in the ICU on a ventilator in some centers are gotten out of bed and encouraged to walk with assistance in the hallway. Other centers have used a pedaling device, some of which can even be adapted for passive activity in a patient who is otherwise sedated and minimally responsive. Exercise in the critical care setting helps maintain muscular strength, reduces the risk for long-term neuromuscular weakness, shortens rehabilitation, and is more likely to result in the patient being discharged to their home [ 45 , 46 ].
Recommendations for Public Health
Similar to the Food Guide Pyramid designed by the USDA, an activity pyramid has been created to guide the public in strategies to increase flexibility, muscular strength, and aerobic capacity ( www.wellspan.org/media/3648/activitypyramid-2009.pdf ). Every day, subjects are encouraged to increase activity in leisure and at work. Three to five times per week, aerobic activity should occur, accumulating 150 min each week ( www.wellspan.org/media/3648/activitypyramid-2009.pdf ). Two to three times per week, muscular activity focusing on flexibility and strength training should be scheduled. Sitting more than 30 min at a time, watching TV, or staring at a computer screen should be minimized or reduced as much as possible ( www.wellspan.org/media/3648/activitypyramid-2009.pdf ).
Guidelines differentiate between moderate and vigorous intensity of PA. Moderate intensity is defined by a 3–5 MET level of effort, and includes activities that cause some increase in breathing and heart rate (such as walking 3–4 miles per hour, bicycling on level ground, light swimming, gardening, or mowing a lawn) [ 4 ]. Vigorous intensity is defined by ≥6 METs, and is exemplified by activities causing large increases in breathing, heart rate, and sweating. Such activities of vigorous intensity would include jogging or running at faster than a 10 min mile, aerobic dancing, competitive sports, heavy yard or construction work, brisk swimming, or fast bicycling [ 4 ].
The amount of PA needed to optimize health is not clear. The particular dose of exercise required to achieve benefits with regard to a particular disease process is difficult to ascertain. For cardiovascular disease, the intensity of PA is inversely and linearly associated with increased mortality, with the biggest effect seen as a reduction of premature death [ 47 ]. PA of >2000 kcal per week extends life by 1–2 years by age 80 [ 47 ]. An average energy expenditure of 1000 kcal per week is associated with a 20–30 % decrease in all-cause mortality. Beginning at a minimum of 1000 kcal per week, increasing benefits are seen with increasing energy expenditure, suggesting a dose-response gradient to the effect of exercise on cardiovascular health [ 47 ]. For diabetes mellitus, there is decreased risk from this disease process with PA of >5.5 METs for at least 40 min per week [ 48 ]. Walking 2 h per week decreases the risk of premature death from diabetes [ 48 ]. Moderate exercise defined by a >4.5 METs for 30–60 min per day reduces both the risk of colon cancer and breast cancer [ 19 ]. For women in particular, >7 h per week of moderate exercise has been shown to be successful in reducing risk of breast cancer (TI01). For osteoporosis, the dose-response gradient is less clear, with recommendations simply emphasizing that osteogenic adaptation is load-dependent and site-specific [ 4 ]. The Center for Disease Control (CDC), the American College of Sports Medicine, and the Healthy People 2010 recommendations provide guidelines for aerobic activity for public health purposes [ 49 ]. Adults should engage in PA of moderate intensity for at least 150 min per week or engage in PA of vigorous intensity for at least 75 min per week. Bouts of exercise may be broken up into smaller increments lasting at least 10 min [ 49 ].
Should Anyone Not be Exercising?
Jim Fixx was a celebrity journalist who helped contribute to the running craze seen in the 1980s in the USA. His sudden death from cardiovascular disease, while jogging, raised questions as to the need for medical evaluation prior to engaging in a program of increasing PA. Moderately strenuous PA may trigger ischemic events, particularly among sedentary people. There is an increased incidence of primary heart attack in high-intensity exercise. In competitive athletes, 80 % of deaths are caused by coronary artery disease. Some subjects do need to have their health risks assessed prior to engaging in an aggressive program.
The degree to which a person is evaluated prior to exercise depends on the presence or absence of cardiovascular disease risk factors and whether the exercise will be moderate or vigorous in intensity [ 50 ]. Subjects at low risk would be those who are young in age (<45 years for male, <55 years for female), are asymptomatic, and have ≤1 cardiovascular risk disease factors. These patients do not need a medical evaluation or stress test for moderate or even vigorous exercise. Subjects at moderate risk are older (men >45 years, women >55 years), or have ≥2 risk factors for cardiovascular disease. For moderate exercise, no medical evaluation may be needed, but these subjects should undergo a stress test. If exercise of vigorous intensity is planned, both a medical evaluation and a stress test should be performed. For those patients at high risk, however, defined by ≥1 sign or symptom of cardiovascular, pulmonary, or metabolic disease, both a full medical evaluation and stress test should be performed before any program is undertaken [ 50 ].
Specifically, those subjects who should not be exercising are those experiencing an acute myocardial infarction, subjects with unstable angina, systolic blood pressure >180, diastolic pressure >110 ml/Hg, uncontrolled diabetes mellitus, poorly controlled congestive heart failure, or thrombophlebitis [ 50 ].
While formal studies have shown that physician counseling is time-intensive and only minimally effective in changing behavior, physicians should no longer avoid the subject of recommendations for exercise as part of the healthcare they deliver to their patients. Physicians can begin by suggesting lifestyle changes such as climbing stairs at work, parking further away from the door on errands, walking regularly, and doing chores at home and in the yard. Clinicians should write on a prescription pad for the patient, specifying the type of exercise, duration, frequency, and intensity. The physician upon discharge from an office visit should determine plans for support and follow up to encourage success, manage obstacles, and prevent relapses. Clinicians should encourage their outpatients to involve community services such as physical therapy, mall-walking programs, school tracks, safe neighborhoods, the YMCA, and walk-a-thon’s.
Physicians should counsel that exercise is not an option. The exercise does not have to be continuous to be effective, and any physical activity counts. Patients should sit less, stand more, and plan their exercise activity at the beginning of each week. Subjects should be encouraged to find activities which they enjoy and involve others to maintain compliance. As Edward Stanley, the Earl of Derby in 1873 said, “Those who think they have not time for bodily exercise will sooner or later have to find time for illness” [ 51 ].
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rezende LF, Rey Lopez JP, Matsudo VK, Luiz OD. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health. 2014;14(1):333.
Article PubMed PubMed Central Google Scholar
www.cdc.gov/nchs/fastats/exercise.htm . Accessed June 12, 2016. Center for Disease Control and Prevention statistics regarding current levels of PA in the United States and trends in physican recommendations to patients relevant to exercise.
Lee IM, Djoussé L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA. 2010;303(12):1173–9.
Article CAS PubMed PubMed Central Google Scholar
Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–9.
Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Morán M, et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015;18(1):57–89. Discussion regarding the exercise mediated attenuation of physical fitness and functional decline in aging.
"Working With Special Populations Sport Essay." UKessays.com. 11 2013. All Answers Ltd. 06 2016 < https://www.ukessays.com/essays/sports/working-with-special-populations-sport-essay.php?cref=1 >
Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–56.
Article CAS PubMed Google Scholar
Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: a review. J Psychosom Res. 1993;37(6):565–74.
Macera CA, Hootman JM, Sniezek JE. Major public health benefits of physical activity. Arthritis Rheum. 2003;49(1):122–8.
Article PubMed Google Scholar
Blair SN, Kohl 3rd HW, Paffenbarger Jr RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–401.
Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–703.
Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682–92.
Hambrecht R, Niebauer J, Marburger C, Grunze M, Kälberer B, Hauer K, et al. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22(2):468–77.
Sarzynski MA1, Burton J2, Rankinen T2, Blair SN3, Church TS2, Després JP4, et al. The effects of exercise on the lipoprotein subclass profile: a meta-analysis of 10 interventions. Atherosclerosis. 2015;243(2):364–72.
Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, et al. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961–7.
Helmrich SP, Ragland DR, Leung RW, Paffenbarger Jr RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325(3):147–52.
Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163(12):1440–7.
Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–27.
Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33(6 Suppl):S530–50. discussion S609-10.
Lee IM. Physical activity and cancer prevention—data from epidemiologic studies. Med Sci Sports Exerc. 2003;35(11):1823–7.
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86.
Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9(1):1–12.
Kujala UM, Kaprio J, Kannus P, Sarna S, Koskenvuo M. Physical activity and osteoporotic hip fracture risk in men. Arch Intern Med. 2000;160(5):705–8.
Liu-Ambrose TY, Khan KM, Eng JJ, Heinonen A, McKay HA. Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom. 2004;7(4):390–8.
Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288(18):2300–6.
Ranjbar E, Memari AH, Hafizi S, Shayestehfar M, Mirfazeli FS. Eshghi MA. J Sports Med. 2015;6(2):e24055.
Google Scholar
Cloud J. Why exercise won’t make you thin. Time. 2009;174(6):49–51.
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–7.
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71.
Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515.
Miller CT, Fraser SF, Levinger I, Straznicky NE, Dixon JB, Reynolds J, et al. The effects of exercise training in addition to energy restriction on functional capacities and body composition in obese adults during weight loss: a systematic review. PLoS One. 2013;8(11):e81692.
Hill JO, Thompson H, Wyatt H. Weight maintenance: what’s missing? J Am Diet Assoc. 2005;105(5 Suppl 1):S63–6.
Steffens D, Maher CG, Pereira LS, Stevens ML, Oliveira VC, Chapple M, et al. Prevention of low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(2):199–208.
Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117(12):912–8.
Warburton DE, Gledhill N, Quinney A. Musculoskeletal fitness and health. Can J Appl Physiol. 2001;26(2):217–37.
Buckley JP, Hedge A, Yates T, Copeland RJ, Loosemore M, Hamer M, et al. The sedentary office: an expert statement on the growing case for change towards better health and productivity. Br J Sports Med. 2015;49(21):1357–62. Expert consensus on techniques to combat increased periods of sitting and inactivity in the modern workplace.
Tremblay MS, Esliger DW, Tremblay A, Colley R. Incidental movement, lifestyle-embedded activity and sleep: new frontiers in physical activity assessment. Can J Public Health. 2007;98 Suppl 2:S208–17.
PubMed Google Scholar
McManus AM. Physical activity—a neat solution to an impending crisis. J Sports Sci Med. 2007;6(3):368–73.
PubMed PubMed Central Google Scholar
Levine JA. Non-exercise activity thermogenesis (NEAT). Nutr Rev. 2004;62(7 Pt 2):S82–97.
Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr. 1997;127(5 Suppl):943S–7S.
CAS PubMed Google Scholar
Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–5.
Campbell L, Wallman K, Green D. The effects of intermittent exercise on physiological outcomes in an obese population: continuous versus interval walking. J Sports Sci Med. 2010;9(1):24–30.
Fisher G, Brown AW, Bohan Brown MM, Alcorn A, Noles C, Winwood L, et al. High intensity interval- vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One. 2015;10(10):e0138853.
Tudor-Locke C, Bassett Jr DR. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8.
Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–43.
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–82.
Paffenbarger Jr RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–13.
Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen RD, Salonen R, et al. Moderately intense physical activities and high levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent diabetes mellitus in middle-aged men. Arch Intern Med. 1996;156(12):1307–14.
U.S. Department of Health and Human Services. Healthy People 2010: Understanding and improving health. 2nd ed. Washington DC: US Government Printing Office; 2000.
Balady GJ, Weiner DA. Exercise testing for sports and the exercise prescription. Cardiol Clin. 1987;5(2):183–96.
http://www.britannica.com/biography/Edward-Stanley-3rd-earl-of-Derby/article-supplemental-information.htm Accessed June 2016
Download references
Author information
Authors and affiliations.
Department of Surgery, University of Louisville School of Medicine, Louisville, KY, 40202, USA
Keith R. Miller
Department of Medicine, University of Louisville School of Medicine, Louisville, KY, USA
Stephen A. McClave, Kristine Krueger & Sarah Landes
National Board of Physician Nutrition Specialists, Valley Village, CA, 91607, USA
Melina B. Jampolis
Department of Medicine, Mayo Clinic, Rochester, MN, USA
Ryan T. Hurt
Department of Surgery, East Virginia University, Roanoke, VA, USA
Bryan Collier
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Keith R. Miller .
Ethics declarations
Conflict of interest.
Keith R. Miller has received compensation from Nestlé for serving as faculty in its fellowship program, from Abbott for serving on a surgical advisory board, and from Metagenics for serving on an advisory board.
Stephen A. McClave declares that he has no conflict of interest.
Melina B. Jampolis declares that she has no conflict of interest.
Ryan T. Hurt has received compensation from Nestlé Nutrition for service as a consultant.
Kristine Krueger declares that she has no conflict of interest.
Sarah Landes declares that she has no conflict of interest.
Bryan Collier declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gastroenterology, Critical Care, and Lifestyle Medicine
Rights and permissions
Reprints and permissions
About this article
Miller, K.R., McClave, S.A., Jampolis, M.B. et al. The Health Benefits of Exercise and Physical Activity. Curr Nutr Rep 5 , 204–212 (2016). https://doi.org/10.1007/s13668-016-0175-5
Download citation
Published : 14 July 2016
Issue Date : September 2016
DOI : https://doi.org/10.1007/s13668-016-0175-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Physical activity
- Energy expenditure
- Non-exercise activity thermogenesis
- Find a journal
- Publish with us
- Track your research
- Research article
- Open access
- Published: 16 November 2020
Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews
- Pawel Posadzki 1 , 2 ,
- Dawid Pieper ORCID: orcid.org/0000-0002-0715-5182 3 ,
- Ram Bajpai 4 ,
- Hubert Makaruk 5 ,
- Nadja Könsgen 3 ,
- Annika Lena Neuhaus 3 &
- Monika Semwal 6
BMC Public Health volume 20 , Article number: 1724 ( 2020 ) Cite this article
30k Accesses
128 Citations
132 Altmetric
Metrics details
Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity for various health outcomes.
Overview and meta-analysis. The Cochrane Library was searched from 01.01.2000 to issue 1, 2019. No language restrictions were imposed. Only CSRs of randomised controlled trials (RCTs) were included. Both healthy individuals, those at risk of a disease, and medically compromised patients of any age and gender were eligible. We evaluated any type of exercise or physical activity interventions; against any types of controls; and measuring any type of health-related outcome measures. The AMSTAR-2 tool for assessing the methodological quality of the included studies was utilised.
Hundred and fifty CSRs met the inclusion criteria. There were 54 different conditions. Majority of CSRs were of high methodological quality. Hundred and thirty CSRs employed meta-analytic techniques and 20 did not. Limitations for studies were the most common reasons for downgrading the quality of the evidence. Based on 10 CSRs and 187 RCTs with 27,671 participants, there was a 13% reduction in mortality rates risk ratio (RR) 0.87 [95% confidence intervals (CI) 0.78 to 0.96]; I 2 = 26.6%, [prediction interval (PI) 0.70, 1.07], median effect size (MES) = 0.93 [interquartile range (IQR) 0.81, 1.00]. Data from 15 CSRs and 408 RCTs with 32,984 participants showed a small improvement in quality of life (QOL) standardised mean difference (SMD) 0.18 [95% CI 0.08, 0.28]; I 2 = 74.3%; PI -0.18, 0.53], MES = 0.20 [IQR 0.07, 0.39]. Subgroup analyses by the type of condition showed that the magnitude of effect size was the largest among patients with mental health conditions.
There is a plethora of CSRs evaluating the effectiveness of physical activity/exercise. The evidence suggests that physical activity/exercise reduces mortality rates and improves QOL with minimal or no safety concerns.
Trial registration
Registered in PROSPERO ( CRD42019120295 ) on 10th January 2019.
Peer Review reports
The World Health Organization (WHO) defines physical activity “as any bodily movement produced by skeletal muscles that requires energy expenditure” [ 1 ]. Therefore, physical activity is not only limited to sports but also includes walking, running, swimming, gymnastics, dance, ball games, and martial arts, for example. In the last years, several organizations have published or updated their guidelines on physical activity. For example, the Physical Activity Guidelines for Americans, 2nd edition, provides information and guidance on the types and amounts of physical activity that provide substantial health benefits [ 2 ]. The evidence about the health benefits of regular physical activity is well established and so are the risks of sedentary behaviour [ 2 ]. Exercise is dose dependent, meaning that people who achieve cumulative levels several times higher than the current recommended minimum level have a significant reduction in the risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events [ 3 ]. Benefits of physical activity have been reported for numerous outcomes such as mortality [ 4 , 5 ], cognitive and physical decline [ 5 , 6 , 7 ], glycaemic control [ 8 , 9 ], pain and disability [ 10 , 11 ], muscle and bone strength [ 12 ], depressive symptoms [ 13 ], and functional mobility and well-being [ 14 , 15 ]. Overall benefits of exercise apply to all bodily systems including immunological [ 16 ], musculoskeletal [ 17 ], respiratory [ 18 ], and hormonal [ 19 ]. Specifically for the cardiovascular system, exercise increases fatty acid oxidation, cardiac output, vascular smooth muscle relaxation, endothelial nitric oxide synthase expression and nitric oxide availability, improves plasma lipid profiles [ 15 ] while at the same time reducing resting heart rate and blood pressure, aortic valve calcification, and vascular resistance [ 20 ].
However, the degree of all the above-highlighted benefits vary considerably depending on individual fitness levels, types of populations, age groups and the intensity of different physical activities/exercises [ 21 ]. The majority of guidelines in different countries recommend a goal of 150 min/week of moderate-intensity aerobic physical activity (or equivalent of 75 min of vigorous-intensity) [ 22 ] with differences for cardiovascular disease [ 23 ] or obesity prevention [ 24 ] or age groups [ 25 ].
There is a plethora of systematic reviews published by the Cochrane Library critically evaluating the effectiveness of physical activity/exercise for various health outcomes. Cochrane systematic reviews (CSRs) are known to be a source of high-quality evidence. Thus, it is not only timely but relevant to evaluate the current knowledge, and determine the quality of the evidence-base, and the magnitude of the effect sizes given the negative lifestyle changes and rising physical inactivity-related burden of diseases. This overview will identify the breadth and scope to which CSRs have appraised the evidence for exercise on health outcomes; and this will help in directing future guidelines and identifying current gaps in the literature.
The objectives of this research were to a. answer the following research questions: in children, adolescents and adults (both healthy and medically compromised) what are the effects (and adverse effects) of exercise/physical activity in improving various health outcomes (e.g., pain, function, quality of life) reported in CSRs; b. estimate the magnitude of the effects by pooling the results quantitatively; c. evaluate the strength and quality of the existing evidence; and d. create recommendations for future researchers, patients, and clinicians.
Our overview was registered with PROSPERO (CRD42019120295) on 10th January 2019. The Cochrane Handbook for Systematic Reviews of interventions and Preferred Reporting Items for Overviews of Reviews were adhered to while writing and reporting this overview [ 26 , 27 ].
Search strategy and selection criteria
We followed the practical guidance for conducting overviews of reviews of health care interventions [ 28 ] and searched the Cochrane Database of Systematic Reviews (CDSR), 2019, Issue 1, on the Cochrane Library for relevant papers using the search strategy: (health) and (exercise or activity or physical). The decision to seek CSRs only was based on three main aspects. First, high quality (CSRs are considered to be the ‘gold methodological standard’) [ 29 , 30 , 31 ]. Second, data saturation (enough high-quality evidence to reach meaningful conclusions based on CSRs only). Third, including non-CSRs would have heavily increased the issue of overlapping reviews (also affecting data robustness and credibility of conclusions). One reviewer carried out the searches. The study screening and selection process were performed independently by two reviewers. We imported all identified references into reference manager software EndNote (X8). Any disagreements were resolved by discussion between the authors with third overview author acting as an arbiter, if necessary.
We included CSRs of randomised controlled trials (RCTs) involving both healthy individuals and medically compromised patients of any age and gender. Only CSRs assessing exercise or physical activity as a stand-alone intervention were included. This included interventions that could initially be taught by a professional or involve ongoing supervision (the WHO definition). Complex interventions e.g., assessing both exercise/physical activity and behavioural changes were excluded if the health effects of the interventions could not have been attributed to exercise distinctly.
Any types of controls were admissible. Reviews evaluating any type of health-related outcome measures were deemed eligible. However, we excluded protocols or/and CSRs that have been withdrawn from the Cochrane Library as well as reviews with no included studies.
Data analysis
Three authors (HM, ALN, NK) independently extracted relevant information from all the included studies using a custom-made data collection form. The methodological quality of SRs included was independently evaluated by same reviewers using the AMSTAR-2 tool [ 32 ]. Any disagreements on data extraction or CSR quality were resolved by discussion. The entire dataset was validated by three authors (PP, MS, DP) and any discrepant opinions were settled through discussions.
The results of CSRs are presented in a narrative fashion using descriptive tables. Where feasible, we presented outcome measures across CSRs. Data from the subset of homogeneous outcomes were pooled quantitatively using the approach previously described by Bellou et al. and Posadzki et al. [ 33 , 34 ]. For mortality and quality of life (QOL) outcomes, the number of participants and RCTs involved in the meta-analysis, summary effect sizes [with 95% confidence intervals (CI)] using random-effects model were calculated. For binary outcomes, we considered relative risks (RRs) as surrogate measures of the corresponding odds ratio (OR) or risk ratio/hazard ratio (HR). To stabilise the variance and normalise the distributions, we transformed RRs into their natural logarithms before pooling the data (a variation was allowed, however, it did not change interpretation of results) [ 35 ]. The standard error (SE) of the natural logarithm of RR was derived from the corresponding CIs, which was either provided in the study or calculated with standard formulas [ 36 ]. Binary outcomes reported as risk difference (RD) were also meta-analysed if two more estimates were available. For continuous outcomes, we only meta-analysed estimates that were available as standardised mean difference (SMD), and estimates reported with mean differences (MD) for QOL were presented separately in a supplementary Table 9 . To estimate the overall effect size, each study was weighted by the reciprocal of its variance. Random-effects meta-analysis, using DerSimonian and Laird method [ 37 ] was applied to individual CSR estimates to obtain a pooled summary estimate for RR or SMD. The 95% prediction interval (PI) was also calculated (where ≥3 studies were available), which further accounts for between-study heterogeneity and estimates the uncertainty around the effect that would be anticipated in a new study evaluating that same association. I -squared statistic was used to measure between study heterogeneity; and its various thresholds (small, substantial and considerable) were interpreted considering the size and direction of effects and the p -value from Cochran’s Q test ( p < 0.1 considered as significance) [ 38 ]. Wherever possible, we calculated the median effect size (with interquartile range [IQR]) of each CSR to interpret the direction and magnitude of the effect size. Sub-group analyses are planned for type and intensity of the intervention; age group; gender; type and/or severity of the condition, risk of bias in RCTs, and the overall quality of the evidence (Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria). To assess overlap we calculated the corrected covered area (CCA) [ 39 ]. All statistical analyses were conducted on Stata statistical software version 15.2 (StataCorp LLC, College Station, Texas, USA).
The searches generated 280 potentially relevant CRSs. After removing of duplicates and screening, a total of 150 CSRs met our eligibility criteria [ 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 ] (Fig. 1 ). Reviews were published between September 2002 and December 2018. A total of 130 CSRs employed meta-analytic techniques and 20 did not. The total number of RCTs in the CSRs amounted to 2888; with 485,110 participants (mean = 3234, SD = 13,272). The age ranged from 3 to 87 and gender distribution was inestimable. The main characteristics of included reviews are summarised in supplementary Table 1 . Supplementary Table 2 summarises the effects of physical activity/exercise on health outcomes. Conclusions from CSRs are listed in supplementary Table 3 . Adverse effects are listed in supplementary Table 4 . Supplementary Table 5 presents summary of withdrawals/non-adherence. The methodological quality of CSRs is presented in supplementary Table 6 . Supplementary Table 7 summarises studies assessed at low risk of bias (by the authors of CSRs). GRADE-ings of the review’s main comparison are listed in supplementary Table 8 .
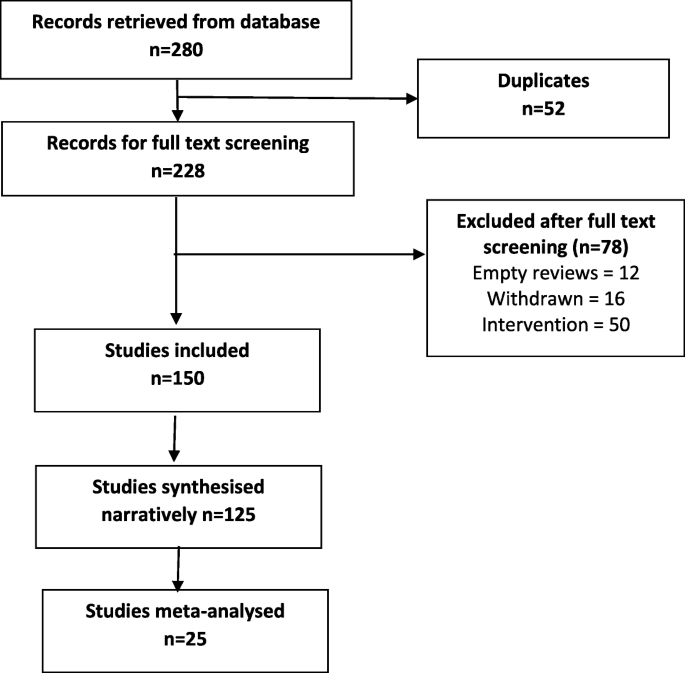
Study selection process
There were 54 separate populations/conditions, considerable range of interventions and comparators, co-interventions, and outcome measures. For detailed description of interventions, please refer to the supplementary tables . Most commonly measured outcomes were - function 112 (75%), QOL 83 (55%), AEs 70 (47%), pain 41 (27%), mortality 28 (19%), strength 30 (20%), costs 47 (31%), disability 14 (9%), and mental health in 35 (23%) CSRs.
There was a 13% reduction in mortality rates risk ratio (RR) 0.87 [95% CI 0.78 to 0.96]; I 2 = 26.6%, [PI 0.70, 1.07], median effect size (MES) = 0.93 [interquartile range (IQR) 0.81, 1.00]; 10 CSRs, 187 RCTs, 27,671 participants) following exercise when compared with various controls (Table 1 ). This reduction was smaller in ‘other groups’ of patients when compared to cardiovascular diseases (CVD) patients - RR 0.97 [95% CI 0.65, 1.45] versus 0.85 [0.76, 0.96] respectively. The effects of exercise were not intensity or frequency dependent. Sessions more than 3 times per week exerted a smaller reduction in mortality as compared with sessions of less than 3 times per week RR 0.87 [95% CI 0.78, 0.98] versus 0.63 [0.39, 1.00]. Subgroup analyses by risk of bias (ROB) in RCTs showed that RCTs at low ROB exerted smaller reductions in mortality when compared to RCTs at an unclear or high ROB, RR 0.90 [95% CI 0.78, 1.02] versus 0.72 [0.42, 1.22] versus 0.86 [0.69, 1.06] respectively. CSRs with moderate quality of evidence (GRADE), showed slightly smaller reductions in mortality when compared with CSRs that relied on very low to low quality evidence RR 0.88 [95% CI 0.79, 0.98] versus 0.70 [0.47, 1.04].
Exercise also showed an improvement in QOL, standardised mean difference (SMD) 0.18 [95% CI 0.08, 0.28]; I 2 = 74.3%; PI -0.18, 0.53], MES = 0.20 [IQR 0.07, 0.39]; 15 CSRs, 408 RCTs, 32,984 participants) when compared with various controls (Table 2 ). These improvements were greater observed for health related QOL when compared to overall QOL SMD 0.30 [95% CI 0.21, 0.39] vs 0.06 [− 0.08, 0.20] respectively. Again, the effects of exercise were duration and frequency dependent. For instance, sessions of more than 90 mins exerted a greater improvement in QOL as compared with sessions up to 90 min SMD 0.24 [95% CI 0.11, 0.37] versus 0.22 [− 0.30, 0.74]. Subgroup analyses by the type of condition showed that the magnitude of effect was the largest among patients with mental health conditions, followed by CVD and cancer. Physical activity exerted negative effects on QOL in patients with respiratory conditions (2 CSRs, 20 RCTs with 601 patients; SMD -0.97 [95% CI -1.43, 0.57]; I 2 = 87.8%; MES = -0.46 [IQR-0.97, 0.05]). Subgroup analyses by risk of bias (ROB) in RCTs showed that RCTs at low or unclear ROB exerted greater improvements in QOL when compared to RCTs at a high ROB SMD 0.21 [95% CI 0.10, 0.31] versus 0.17 [0.03, 0.31]. Analogically, CSRs with moderate to high quality of evidence showed slightly greater improvements in QOL when compared with CSRs that relied on very low to low quality evidence SMD 0.19 [95% CI 0.05, 0.33] versus 0.15 [− 0.02, 0.32]. Please also see supplementary Table 9 more studies reporting QOL outcomes as mean difference (not quantitatively synthesised herein).
Adverse events (AEs) were reported in 100 (66.6%) CSRs; and not reported in 50 (33.3%). The number of AEs ranged from 0 to 84 in the CSRs. The number was inestimable in 83 (55.3%) CSRs. Ten (6.6%) reported no occurrence of AEs. Mild AEs were reported in 28 (18.6%) CSRs, moderate in 9 (6%) and serious/severe in 20 (13.3%). There were 10 deaths and in majority of instances, the causality was not attributed to exercise. For this outcome, we were unable to pool the data as effect sizes were too heterogeneous (Table 3 ).
In 38 CSRs, the total number of trials reporting withdrawals/non-adherence was inestimable. There were different ways of reporting it such as adherence or attrition (high in 23.3% of CSRs) as well as various effect estimates including %, range, total numbers, MD, RD, RR, OR, mean and SD. The overall pooled estimates are reported in Table 3 .
Of all 16 domains of the AMSTAR-2 tool, 1876 (78.1%) scored ‘yes’, 76 (3.1%) ‘partial yes’; 375 (15.6%) ‘no’, and ‘not applicable’ in 25 (1%) CSRs. Ninety-six CSRs (64%) were scored as ‘no’ on reporting sources of funding for the studies followed by 88 (58.6%) failing to explain the selection of study designs for inclusion. One CSR (0.6%) each were judged as ‘no’ for reporting any potential sources of conflict of interest, including any funding for conducting the review as well for performing study selection in duplicate.
In 102 (68%) CSRs, there was predominantly a high risk of bias in RCTs. In 9 (6%) studies, this was reported as a range, e.g., low or unclear or low to high. Two CSRs used different terminology i.e., moderate methodological quality; and the risk of bias was inestimable in one CSR. Sixteen (10.6%) CSRs did not identify any studies (RCTs) at low risk of random sequence generation, 28 (18.6%) allocation concealment, 28 (18.6%) performance bias, 84 (54%) detection bias, 35 (23.3%) attrition bias, 18 (12%) reporting bias, and 29 (19.3%) other bias.
In 114 (76%) CSRs, limitation of studies was the main reason for downgrading the quality of the evidence followed by imprecision in 98 (65.3%) and inconsistency in 68 (45.3%). Publication bias was the least frequent reason for downgrading in 26 (17.3%) CSRs. Ninety-one (60.7%) CSRs reached equivocal conclusions, 49 (32.7%) reviews reached positive conclusions and 10 (6.7%) reached negative conclusions (as judged by the authors of CSRs).
In this systematic review of CSRs, we found a large body of evidence on the beneficial effects of physical activity/exercise on health outcomes in a wide range of heterogeneous populations. Our data shows a 13% reduction in mortality rates among 27,671 participants, and a small improvement in QOL and health-related QOL following various modes of physical activity/exercises. This means that both healthy individuals and medically compromised patients can significantly improve function, physical and mental health; or reduce pain and disability by exercising more [ 190 ]. In line with previous findings [ 191 , 192 , 193 , 194 ], where a dose-specific reduction in mortality has been found, our data shows a greater reduction in mortality in studies with longer follow-up (> 12 months) as compared to those with shorter follow-up (< 12 months). Interestingly, we found a consistent pattern in the findings, the higher the quality of evidence and the lower the risk of bias in primary studies, the smaller reductions in mortality. This pattern is observational in nature and cannot be over-generalised; however this might mean less certainty in the estimates measured. Furthermore, we found that the magnitude of the effect size was the largest among patients with mental health conditions. A possible mechanism of action may involve elevated levels of brain-derived neurotrophic factor or beta-endorphins [ 195 ].
We found the issue of poor reporting or underreporting of adherence/withdrawals in over a quarter of CSRs (25.3%). This is crucial both for improving the accuracy of the estimates at the RCT level as well as maintaining high levels of physical activity and associated health benefits at the population level.
Even the most promising interventions are not entirely risk-free; and some minor AEs such as post-exercise pain and soreness or discomfort related to physical activity/exercise have been reported. These were typically transient; resolved within a few days; and comparable between exercise and various control groups. However worryingly, the issue of poor reporting or underreporting of AEs has been observed in one third of the CSRs. Transparent reporting of AEs is crucial for identifying patients at risk and mitigating any potential negative or unintended consequences of the interventions.
High risk of bias of the RCTs evaluated was evident in more than two thirds of the CSRs. For example, more than half of reviews identified high risk of detection bias as a major source of bias suggesting that lack of blinding is still an issue in trials of behavioural interventions. Other shortcomings included insufficiently described randomisation and allocation concealment methods and often poor outcome reporting. This highlights the methodological challenges in RCTs of exercise and the need to counterbalance those with the underlying aim of strengthening internal and external validity of these trials.
Overall, high risk of bias in the primary trials was the main reason for downgrading the quality of the evidence using the GRADE criteria. Imprecision was frequently an issue, meaning the effective sample size was often small; studies were underpowered to detect the between-group differences. Pooling too heterogeneous results often resulted in inconsistent findings and inability to draw any meaningful conclusions. Indirectness and publication bias were lesser common reasons for downgrading. However, with regards to the latter, the generally accepted minimum number of 10 studies needed for quantitatively estimate the funnel plot asymmetry was not present in 69 (46%) CSRs.
Strengths of this research are the inclusion of large number of ‘gold standard’ systematic reviews, robust screening, data extractions and critical methodological appraisal. Nevertheless, some weaknesses need to be highlighted when interpreting findings of this overview. For instance, some of these CSRs analysed the same primary studies (RCTs) but, arrived at slightly different conclusions. Using, the Pieper et al. [ 39 ] formula, the amount of overlap ranged from 0.01% for AEs to 0.2% for adherence, which indicates slight overlap. All CSRs are vulnerable to publication bias [ 196 ] - hence the conclusions generated by them may be false-positive. Also, exercise was sometimes part of a complex intervention; and the effects of physical activity could not be distinguished from co-interventions. Often there were confounding effects of diet, educational, behavioural or lifestyle interventions; selection, and measurement bias were inevitably inherited in this overview too. Also, including CSRs only might lead to selection bias; and excluding reviews published before 2000 might limit the overall completeness and applicability of the evidence. A future update should consider these limitations, and in particular also including non-CSRs.
Conclusions
Trialists must improve the quality of primary studies. At the same time, strict compliance with the reporting standards should be enforced. Authors of CSRs should better explain eligibility criteria and report sources of funding for the primary studies. There are still insufficient physical activity trends worldwide amongst all age groups; and scalable interventions aimed at increasing physical activity levels should be prioritized [ 197 ]. Hence, policymakers and practitioners need to design and implement comprehensive and coordinated strategies aimed at targeting physical activity programs/interventions, health promotion and disease prevention campaigns at local, regional, national, and international levels [ 198 ].
Availability of data and materials
Data sharing is not applicable to this article as no raw data were analysed during the current study. All information in this article is based on published systematic reviews.

Abbreviations
Adverse events
Cardiovascular diseases
Cochrane Database of Systematic Reviews
Cochrane systematic reviews
Confidence interval
Grading of Recommendations Assessment, Development and Evaluation
Hazard ratio
Interquartile range
Mean difference
Prediction interval
Quality of life
Randomised controlled trials
Relative risk
Risk difference
Risk of bias
Standard error
Standardised mean difference
World Health Organization
https://www.who.int/dietphysicalactivity/pa/en/ . (Accessed 8 June 2020).
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for AmericansPhysical activity guidelines for AmericansPhysical activity guidelines for Americans. Jama. 2018;320(19):2020–8.
PubMed Google Scholar
Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. BMJ. 2016;354:i3857.
PubMed PubMed Central Google Scholar
Abell B, Glasziou P, Hoffmann T. The contribution of individual exercise training components to clinical outcomes in randomised controlled trials of cardiac rehabilitation: a systematic review and meta-regression. Sports Med Open. 2017;3(1):19.
Anderson D, Seib C, Rasmussen L. Can physical activity prevent physical and cognitive decline in postmenopausal women? A systematic review of the literature. Maturitas. 2014;79(1):14–33.
Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic review. Physiother Can. 2010;62(1):25–34.
Barlow PA, Otahal P, Schultz MG, Shing CM, Sharman JE. Low exercise blood pressure and risk of cardiovascular events and all-cause mortality: systematic review and meta-analysis. Atherosclerosis. 2014;237(1):13–22.
CAS PubMed Google Scholar
Aljawarneh YM, Wardell DW, Wood GL, Rozmus CL. A systematic review of physical activity and exercise on physiological and biochemical outcomes in children and adolescents with type 1 diabetes. J Nurs Scholarsh. 2019.
Chastin SFM, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M, Stamatakis E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53(6):370–6.
Abdulla SY, Southerst D, Cote P, Shearer HM, Sutton D, Randhawa K, Varatharajan S, Wong JJ, Yu H, Marchand AA, et al. Is exercise effective for the management of subacromial impingement syndrome and other soft tissue injuries of the shoulder? A systematic review by the Ontario protocol for traffic injury management (OPTIMa) collaboration. Man Ther. 2015;20(5):646–56.
Alanazi MH, Parent EC, Dennett E. Effect of stabilization exercise on back pain, disability and quality of life in adults with scoliosis: a systematic review. Eur J Phys Rehabil Med. 2018;54(5):647–53.
Adsett JA, Mudge AM, Morris N, Kuys S, Paratz JD. Aquatic exercise training and stable heart failure: a systematic review and meta-analysis. Int J Cardiol. 2015;186:22–8.
Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(7):1329–38.
Abdin S, Welch RK, Byron-Daniel J, Meyrick J. The effectiveness of physical activity interventions in improving well-being across office-based workplace settings: a systematic review. Public Health. 2018;160:70–6.
Albalawi H, Coulter E, Ghouri N, Paul L. The effectiveness of structured exercise in the south Asian population with type 2 diabetes: a systematic review. Phys Sportsmed. 2017;45(4):408–17.
Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, Bragazzi N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187.
Hagen KB, Dagfinrud H, Moe RH, Østerås N, Kjeken I, Grotle M, Smedslund G. Exercise therapy for bone and muscle health: an overview of systematic reviews. BMC Med. 2012;10(1):167.
Burton DA, Stokes K, Hall GM. Physiological effects of exercise. Contin Educ Anaesth Crit Care Pain. 2004;4(6):185–8.
Google Scholar
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–61.
Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. 2018;5:135.
CAS PubMed PubMed Central Google Scholar
Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12.
Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–56.
Excellence NIfHaC: Cardiovascular disease prevention public health guideline [PH25] Published date: June 2010. Available at: https://www.nice.org.uk/guidance/ph25/resources/cardiovascular-disease-prevention-pdf-1996238687173 .
Excellence NIfHaC: Obesity prevention clinical guideline [CG43] published date: December 2006 Last updated: March 2015. Available at: https://www.nice.org.uk/guidance/cg43/resources/obesity-prevention-pdf-975445344709 .
Excellence NIfHaC: Physical activity for children and young people public health guideline [PH17] Published date: January 2009. Available at: https://www.nice.org.uk/guidance/ph17/resources/physical-activity-for-children-and-young-people-pdf-1996181580229 .
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 6 [updated September 2018] edition. Available from www.cochrane-handbook.org : The Cochrane Collaboration, 2011. 2011.
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich A-B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24.
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18.
Handoll H, Madhok R. Quality of Cochrane reviews. Another study found that most Cochrane reviews are of a good standard. BMJ. 2002;324(7336):545.
Petticrew M, Wilson P, Wright K, Song F. Quality of Cochrane reviews. Quality of Cochrane reviews is better than that of non-Cochrane reviews. BMJ. 2002;324(7336):545.
Shea B, Moher D, Graham I, Pham B, Tugwell P. A comparison of the quality of Cochrane reviews and systematic reviews published in paper-based journals. Eval Health Prof. 2002;25(1):116–29.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Posadzki PP, Bajpai R, Kyaw BM, Roberts NJ, Brzezinski A, Christopoulos GI, Divakar U, Bajpai S, Soljak M, Dunleavy G, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018;16(1):18.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9.
Walter SD, Cook RJ. A comparison of several point estimators of the odds ratio in a single 2 x 2 contingency table. Biometrics. 1991;47(3):795–811.
Khan H, Sempos CT. Statistical methods in epidemiology. New York: Oxford University Press; 1989.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
CAS Google Scholar
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019): Cochrane; 2019. Available from www.training.cochrane.org/handbook .
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;7.
Al-Khudairy L, Loveman E, Colquitt JL, Mead E, Johnson RE, Fraser H, Olajide J, Murphy M, Velho RM, O'Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017;6.
Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013;7.
Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4.
Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;1.
Andriolo RB, El Dib RP, Ramos L, Atallah Á, da Silva EMK. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database Syst Rev. 2010;5.
Araujo DN, Ribeiro CTD, Maciel ACC, Bruno SS, Fregonezi GAF, Dias FAL. Physical exercise for the treatment of non-ulcerated chronic venous insufficiency. Cochrane Database Syst Rev. 2016;12.
Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;1.
Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsøe B, Dagfinrud H, Lund H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3.
Beggs S, Foong YC, Le HCT, Noor D, Wood-Baker R, Walters JAE. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev. 2013;4.
Bergenthal N, Will A, Streckmann F, Wolkewitz KD, Monsef I, Engert A, Elter T, Skoetz N. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014;11.
Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Góes SM, Boden C, Foulds HJA. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;6.
Bidonde J, Busch AJ, van der Spuy I, Tupper S, Kim SY, Boden C. Whole body vibration exercise training for fibromyalgia. Cochrane Database Syst Rev. 2017;9.
Bidonde J, Busch AJ, Webber SC, Schachter CL, Danyliw A, Overend TJ, Richards RS, Rader T. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;10.
Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJL. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3.
Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2015;1.
Broderick J, Crumlish N, Waugh A, Vancampfort D. Yoga versus non-standard care for schizophrenia. Cochrane Database Syst Rev. 2017;9.
Broderick J, Knowles A, Chadwick J, Vancampfort D. Yoga versus standard care for schizophrenia. Cochrane Database Syst Rev. 2015;10.
Broderick J, Vancampfort D. Yoga as part of a package of care versus standard care for schizophrenia. Cochrane Database Syst Rev. 2017;9.
Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. 2017;6.
Busch AJ, Barber KA, Overend TJ, Peloso PMJ, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;4.
Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, Danyliw A, Sawant A, Dal Bello-Haas V, Rader T, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;12.
Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9.
Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;9.
Carvalho APV, Vital FMR, Soares BGO. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev. 2012;4.
Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6.
Cavalheri V, Tahirah F, Nonoyama ML, Jenkins S, Hill K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev. 2013;7.
Ceysens G, Rouiller D, Boulvain M. Exercise for diabetic pregnant women. Cochrane Database Syst Rev. 2006;3.
Choi BKL, Verbeek JH, Tam WWS, Jiang JY. Exercises for prevention of recurrences of low-back pain. Cochrane Database Syst Rev. 2010;1.
Colquitt JL, Loveman E, O'Malley C, Azevedo LB, Mead E, Al-Khudairy L, Ells LJ, Metzendorf MI, Rees K. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database Syst Rev. 2016;3.
Connolly B, Salisbury L, O'Neill B, Geneen LJ, Douiri A, Grocott MPW, Hart N, Walsh TS, Blackwood B. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev. 2015;6.
Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE. Exercise for depression. Cochrane Database Syst Rev. 2013;9.
Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev. 2015;10.
Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1.
Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11.
Dal Bello-Haas V, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev. 2013;5.
Dale MT, McKeough ZJ, Troosters T, Bye P, Alison JA. Exercise training to improve exercise capacity and quality of life in people with non-malignant dust-related respiratory diseases. Cochrane Database Syst Rev. 2015;11.
Daley A, Stokes-Lampard H, Thomas A, MacArthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;11.
de Morton N, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007;1.
Dobbins M, Husson H, DeCorby K, LaRocca RL. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database Syst Rev. 2013;2.
Doiron KA, Hoffmann TC, Beller EM. Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev. 2018;3.
Ekeland E, Heian F, Hagen KB, Abbott JM, Nordheim L. Exercise to improve self-esteem in children and young people. Cochrane Database Syst Rev. 2004;1.
Elbers RG, Verhoef J, van Wegen EEH, Berendse HW, Kwakkel G. Interventions for fatigue in Parkinson's disease. Cochrane Database Syst Rev. 2015;10.
Felbel S, Meerpohl JJ, Monsef I, Engert A, Skoetz N. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev. 2014;6.
Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4.
Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1.
Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;4.
Freitas DA, Holloway EA, Bruno SS, Chaves GSS, Fregonezi GAF, Mendonça K. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2013;10.
Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9.
Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev. 2013;1.
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9.
Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Database Syst Rev. 2010;5.
Grande AJ, Keogh J, Hoffmann TC, Beller EM, Del Mar CB. Exercise versus no exercise for the occurrence, severity and duration of acute respiratory infections. Cochrane Database Syst Rev. 2015;6.
Grande AJ, Reid H, Thomas EE, Nunan D, Foster C. Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults. Cochrane Database Syst Rev. 2016;8.
Grande AJ, Silva V, Andriolo BNG, Riera R, Parra SA, Peccin MS. Water-based exercise for adults with asthma. Cochrane Database Syst Rev. 2014;7.
Gross A, Kay TM, Paquin JP, Blanchette S, Lalonde P, Christie T, Dupont G, Graham N, Burnie SJ, Gelley G, et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev. 2015;1.
Hageman D, Fokkenrood HJP, Gommans LNM, van den Houten MML, Teijink JAW. Supervised exercise therapy versus home-based exercise therapy versus walking advice for intermittent claudication. Cochrane Database Syst Rev. 2018;4.
Han A, Judd M, Welch V, Wu T, Tugwell P, Wells GA. Tai chi for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2004;3.
Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7.
Hartley L, Dyakova M, Holmes J, Clarke A, Lee MS, Ernst E, Rees K. Yoga for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;5.
Hartley L, Flowers N, Lee MS, Ernst E, Rees K. Tai chi for primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;4.
Hartley L, Lee MS, Kwong JSW, Flowers N, Todkill D, Ernst E, Rees K. Qigong for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;6.
Hassett L, Moseley AM, Harmer AR. Fitness training for cardiorespiratory conditioning after traumatic brain injury. Cochrane Database Syst Rev. 2017;12.
Hayden J, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;3.
Hay-Smith EJC, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2011;12.
Heine M, van de Port I, Rietberg MB, van Wegen EEH, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015;9.
Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;10.
Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué i Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12.
Herbert RD, de Noronha M, Kamper SJ. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst Rev. 2011;7.
Heymans MW, van Tulder MW, Esmail R, Bombardier C, Koes BW. Back schools for non-specific low-back pain. Cochrane Database Syst Rev. 2004;4.
Holland AE, Hill CJ, Jones AY, McDonald CF. Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;10.
Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7.
Hurkmans E, van der Giesen FJ, Vliet Vlieland TPM, Schoones J, Van den Ende E. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev. 2009;4.
Hurley M, Dickson K, Hallett R, Grant R, Hauari H, Walsh N, Stansfield C, Oliver S. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4.
Jones M, Harvey A, Marston L, O'Connell NE. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013;5.
Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev. 2015;10.
Kendrick D, Kumar A, Carpenter H, Zijlstra GAR, Skelton DA, Cook JR, Stevens Z, Belcher CM, Haworth D, Gawler SJ, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;11.
Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;3.
Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1.
Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2017;12.
Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2017;4.
Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst Rev. 2006;3.
Lauret GJ, Fakhry F, Fokkenrood HJP, Hunink MGM, Teijink JAW, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev. 2014;7.
Lawrence M, Celestino Junior FT, Matozinho HHS, Govan L, Booth J, Beecher J. Yoga for stroke rehabilitation. Cochrane Database Syst Rev. 2017;12.
Lin CWC, Donkers NAJ, Refshauge KM, Beckenkamp PR, Khera K, Moseley AM. Rehabilitation for ankle fractures in adults. Cochrane Database Syst Rev. 2012;11.
Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3.
Long L, Anderson L, Dewhirst AM, He J, Bridges C, Gandhi M, Taylor RS. Exercise-based cardiac rehabilitation for adults with stable angina. Cochrane Database Syst Rev. 2018;2.
Loughney LA, West MA, Kemp GJ, Grocott MPW, Jack S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst Rev. 2018;12.
Macedo LG, Saragiotto BT, Yamato TP, Costa LOP, Menezes Costa LC, Ostelo R, Maher CG. Motor control exercise for acute non-specific low back pain. Cochrane Database Syst Rev. 2016;2.
Macêdo TMF, Freitas DA, Chaves GSS, Holloway EA, Mendonça K. Breathing exercises for children with asthma. Cochrane Database Syst Rev. 2016;4.
Martin A, Booth JN, Laird Y, Sproule J, Reilly JJ, Saunders DH. Physical activity, diet and other behavioural interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. Cochrane Database Syst Rev. 2018;3.
McKeough ZJ, Velloso M, Lima VP, Alison JA. Upper limb exercise training for COPD. Cochrane Database Syst Rev. 2016;11.
McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA. Water-based exercise training for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;12.
McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;6.
Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, Olajide J, Mainardi GM, Corpeleijn E, O'Malley C, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6.
Meekums B, Karkou V, Nelson EA. Dance movement therapy for depression. Cochrane Database Syst Rev. 2015;2.
Meher S, Duley L. Exercise or other physical activity for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2006;2.
Mehrholz J, Kugler J, Pohl M. Water-based exercises for improving activities of daily living after stroke. Cochrane Database Syst Rev. 2011;1.
Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;11.
Mehrholz J, Thomas S, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev. 2017;8.
Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8.
Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8.
Montgomery P, Dennis JA. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002;4.
Morris NR, Kermeen FD, Holland AE. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev. 2017;1.
Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6.
Ngai SPC, Jones AYM, Tam WWS. Tai chi for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2016;6.
Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2012;7.
O'Brien K, Nixon S, Glazier R, Tynan AM. Progressive resistive exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2004;4.
O'Brien K, Nixon S, Tynan AM, Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. 2010;8.
Østerås N, Kjeken I, Smedslund G, Moe RH, Slatkowsky-Christensen B, Uhlig T, Hagen KB. Exercise for hand osteoarthritis. Cochrane Database Syst Rev. 2017;1.
Page MJ, Green S, Kramer S, Johnston RV, McBain B, Chau M, Buchbinder R. Manual therapy and exercise for adhesive capsulitis (frozen shoulder). Cochrane Database Syst Rev. 2014;8.
Page MJ, Green S, McBain B, Surace SJ, Deitch J, Lyttle N, Mrocki MA, Buchbinder R. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;6.
Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst Rev. 2012;6.
Panebianco M, Sridharan K, Ramaratnam S. Yoga for epilepsy. Cochrane Database Syst Rev. 2017;10.
Perry A, Lee SH, Cotton S, Kennedy C. Therapeutic exercises for affecting post-treatment swallowing in people treated for advanced-stage head and neck cancers. Cochrane Database Syst Rev. 2016;8.
Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev. 2017;11.
Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, Ravaud P. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;10.
Ren J, Xia J. Dance therapy for schizophrenia. Cochrane Database Syst Rev. 2013;10.
Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005;1.
Risom SS, Zwisler AD, Johansen PP, Sibilitz KL, Lindschou J, Gluud C, Taylor RS, Svendsen JH, Berg SK. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst Rev. 2017;2.
Romano M, Minozzi S, Bettany-Saltikov J, Zaina F, Chockalingam N, Kotwicki T, Maier-Hennes A, Negrini S. Exercises for adolescent idiopathic scoliosis. Cochrane Database Syst Rev. 2012;8.
Ryan JM, Cassidy EE, Noorduyn SG, O'Connell NE. Exercise interventions for cerebral palsy. Cochrane Database Syst Rev. 2017;6.
Saragiotto BT, Maher CG, Yamato TP, Costa LOP, Menezes Costa LC, Ostelo R, Macedo LG. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst Rev. 2016;1.
Saunders DH, Sanderson M, Hayes S, Kilrane M, Greig CA, Brazzelli M, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2016;3.
Schulzke SM, Kaempfen S, Trachsel D, Patole SK. Physical activity programs for promoting bone mineralization and growth in preterm infants. Cochrane Database Syst Rev. 2014;4.
Seron P, Lanas F, Pardo Hernandez H, Bonfill Cosp X. Exercise for people with high cardiovascular risk. Cochrane Database Syst Rev. 2014;8.
Shaw KA, Gennat HC, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4.
Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;11.
Sibilitz KL, Berg SK, Tang LH, Risom SS, Gluud C, Lindschou J, Kober L, Hassager C, Taylor RS, Zwisler AD. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev. 2016;3.
Silva IS, Fregonezi GAF, Dias FAL, Ribeiro CTD, Guerra RO, Ferreira GMH. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;9.
States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev. 2009;3.
Strike K, Mulder K, Michael R. Exercise for haemophilia. Cochrane Database Syst Rev. 2016;12.
Takken T, Van Brussel M, Engelbert RH, van der Net JJ, Kuis W, Helders P. Exercise therapy in juvenile idiopathic arthritis. Cochrane Database Syst Rev. 2008;2.
Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, Lough F, Rees K, Singh SJ, Mordi IR. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4.
Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3.
Ussher MH, Taylor AH, Faulkner GEJ. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8.
Valentín-Gudiol M, Mattern-Baxter K, Girabent-Farrés M, Bagur-Calafat C, Hadders-Algra M, Angulo-Barroso RM. Treadmill interventions in children under six years of age at risk of neuromotor delay. Cochrane Database Syst Rev. 2017;7.
van der Heijden RA, Lankhorst NE, van Linschoten R, Bierma-Zeinstra SMA, van Middelkoop M. Exercise for treating patellofemoral pain syndrome. Cochrane Database Syst Rev. 2015;1.
Vloothuis JDM, Mulder M, Veerbeek JM, Konijnenbelt M, Visser-Meily JMA, Ket JCF, Kwakkel G, van Wegen EEH. Caregiver-mediated exercises for improving outcomes after stroke. Cochrane Database Syst Rev. 2016;12.
Voet NBM, van der Kooi EL. Riphagen, II, Lindeman E, van Engelen BGM, Geurts ACH: strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2013;7.
White CM, Pritchard J, Turner-Stokes L. Exercise for people with peripheral neuropathy. Cochrane Database Syst Rev. 2004;4.
Wieland LS, Skoetz N, Pilkington K, Vempati R, D'Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;1.
Williams AD, Bird ML, Hardcastle SGK, Kirschbaum M, Ogden KJ, Walters JAE. Exercise for reducing falls in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;10.
Williams MA, Srikesavan C, Heine PJ, Bruce J, Brosseau L, Hoxey-Thomas N, Lamb SE. Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev. 2018;7.
Yamamoto S, Hotta K, Ota E, Matsunaga A, Mori R. Exercise-based cardiac rehabilitation for people with implantable ventricular assist devices. Cochrane Database Syst Rev. 2018;9.
Yamato TP, Maher CG, Saragiotto BT, Hancock MJ, Ostelo R, Cabral CMN, Menezes Costa LC, Costa LOP. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7.
Yang ZY, Zhong HB, Mao C, Yuan JQ, Huang YF, Wu XY, Gao YM, Tang JL. Yoga for asthma. Cochrane Database Syst Rev. 2016;4.
Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015;4.
Zainuldin R, Mackey MG, Alison JA. Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;11.
Mok A, Khaw K-T, Luben R, Wareham N, Brage S. Physical activity trajectories and mortality: population based cohort study. Bmj. 2019;365:l2323.
Ekelund U, Brown WJ, Steene-Johannessen J, Fagerland MW, Owen N, Powell KE, Bauman AE, Lee IM. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br J Sports Med. 2019;53(14):886–94. https://doi.org/10.1136/bjsports-2017-098963 . Epub 2018 Jul 10.
Article PubMed Google Scholar
Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A, Lee IM. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–10.
Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, et al. The effect of physical activity on mortality and cardiovascular disease in 130000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643–54.
Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–95.
Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51.
Horton R. Offline: the gravy train of systematic reviews. Lancet. 2019;394(10211):1790.
Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6(10):e1077–86.
Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC health promotion series. J Am Coll Cardiol. 2018;72(14):1622–39.
Download references
Acknowledgements
Not applicable.
There was no funding source for this study. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Kleijnen Systematic Reviews Ltd., York, UK
Pawel Posadzki
Nanyang Technological University, Singapore, Singapore
Institute for Research in Operative Medicine, Witten/Herdecke University, Witten, Germany
Dawid Pieper, Nadja Könsgen & Annika Lena Neuhaus
School of Medicine, Keele University, Staffordshire, UK
Jozef Pilsudski University of Physical Education in Warsaw, Faculty Physical Education and Health, Biala Podlaska, Poland
Hubert Makaruk
Health Outcomes Division, University of Texas at Austin College of Pharmacy, Austin, USA
Monika Semwal
You can also search for this author in PubMed Google Scholar
Contributions
PP wrote the protocol, ran the searches, validated, analysed and synthesised data, wrote and revised the drafts. HM, NK and ALN screened and extracted data. MS and DP validated and analysed the data. RB ran statistical analyses. All authors contributed to writing and reviewing the manuscript. PP is the guarantor. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Dawid Pieper .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Supplementary Table 1. Main characteristics of included Cochrane systematic reviews evaluating the effects of physical activity/exercise on health outcomes ( n = 150). Supplementary Table 2. Additional information from Cochrane systematic reviews of the effects of physical activity/exercise on health outcomes ( n = 150). Supplementary Table 3. Conclusions from Cochrane systematic reviews “quote”. Supplementary Table 4 . AEs reported in Cochrane systematic reviews. Supplementary Table 5. Summary of withdrawals/non-adherence. Supplementary Table 6. Methodological quality assessment of the included Cochrane reviews with AMSTAR-2. Supplementary Table 7. Number of studies assessed as low risk of bias per domain. Supplementary Table 8. GRADE for the review’s main comparison. Supplementary Table 9. Studies reporting quality of life outcomes as mean difference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Posadzki, P., Pieper, D., Bajpai, R. et al. Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health 20 , 1724 (2020). https://doi.org/10.1186/s12889-020-09855-3
Download citation
Received : 01 April 2020
Accepted : 08 November 2020
Published : 16 November 2020
DOI : https://doi.org/10.1186/s12889-020-09855-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Essay about Exercise – Benefits
Is exercise beneficial? How is it good for you? And what is exercise? Essays like the one below will help you discover the answers to these questions.
Introduction
- Benefits of Exercise
Works Cited
Do you want to live a good life feeling great with improved mental psyche and energy levels void of some chronic diseases coupled with sound sleep all in one package? Look no further; regular exercising will offer this all-inclusive package of benefits. Many people just know very little about goodness of exercise; regrettably, they do not know how good it can get over time.
The good news is that, exercise delivers results regardless of sex, occupation, physical ability, or age. Exercise results are yours for taking; once you put the input, the output is almost certain.
Unfortunately, people will always find excuses for not exercising and even some will quote myths associated with exercise for them to stay out. Many exercise activities are not strenuous and require very little efforts; for instance, dancing for fun. The truth is; exercise is good because it will help you have good moods, manage chronic diseases, and manage weight giving you good shape.
Goodness of Exercise
Exercise helps in improving one’s mood and mind status. Mood is a state of mind. Physical activity triggers the body to release chemicals known as endorphins. These chemicals enable one to be happy and peaceful. The contemporary society is set in a way that people can work without involving a lot of physical exercise.
Moreover, many people are being forced by circumstances to take jobs that they do not like. Chances that such people will slump into stress, depression, low self-esteem, and insomnia are high.
However, exercising improves all these by improving one’s mood. For instance, during exercise, individuals set goals and by beating the timeline to accomplish these set goals, make one feel good and this improves his or her self-confidence and self-esteem. It is logical that a confident and self-esteemed person will have good moods. Human body is made up of active cells and they need to be kept active; exercise offers these.
As Mayo Clinic Staff posit, “As you exercise, your body gets fitter and stronger, and thus, your mind starts seeing everything, including yourself in a better light” (Para 4). Have you ever heard of people claiming the only thing they do when stressed up is exercise? These few individuals have discovered the secret to let go of in-built pressure. Brain is made up of muscles and exercise is good for brain muscles just as food is good for the body.
Good news to those battling with chronic diseases as hypertension, diabetes and osteoporosis among others; exercise is the way out. Research indicates that regular exercise combats these diseases. According to Hawk, “exercise is the silver bullet for improved health” (Para. 1). In combating heart diseases, exercise strengthens heart muscles, increases High Density Lipoproteins (HDLs), and reduces Low Density Lipoproteins (LDLs). These lipoproteins are cholesterol derivatives and scientists term HDL as ‘good’ cholesterol and LDL as ‘bad’ cholesterol.
Reduction of LDL with subsequent increase of HDL promotes smooth blood flow and replenishes heart functions. In Type II diabetes, the body is insensitive to insulin probably due to weight gain amongst other causes. Exercise helps in shedding weight and this makes the body insulin sensitive. Consequently, this insulin breaks down sugar molecules in the body thus lowering blood sugar levels eventually combating diabetes type II.
Nowadays, poor feeding habits and little or no exercise makes people as young as fifteen to suffer from osteoporosis. However, exercise offers reprieve to this nightmare by strengthening bone tissue formation and maintenance. Finally, research indicates that exercise improves immune system response and this helps to keep minor infections at bay.
Finally, exercise helps in keeping body shape. No one likes obesity or out of shape body. Unfortunately, many people love talking how they hate their over weight bodies without doing anything about it; lip service. Exercise is the way to attaining that elusive figure you have always craved for, over the years.
It is natural that, during physical exercise, the body burns thousands of calories. Body weight results from excess energy, which is converted to fat and stored in different body parts. As one exercises, metabolism rate increases leading to breakdown of fats making your body slimmer and healthy.
Research indicates that, “To lose one pound of fat, you must burn approximately 3500 calories over and above what you already burn doing daily activities” (Buemann & Tremblay 193). These researchers make it clear that normal daily activities are not sufficient to burn the required calories in the body; therefore, exercise is the way out. Fortunately, you do not need to check into a gym to manage weight loss; far from it, 20 to 30 minute walk every day or cycling for fun is enough to keep weight gain under control.
Unfortunately, many people complain that exercise is not good because it is not fun. This is true and it does not apply to exercise alone; it applies to anything that someone does. If you do not enjoy what you are doing, it can never be fun. However, the claims that exercise is not fun are only excuses; not reasons. The fact is, there are many exercise programs, and out of them, every one can find a program that excites him or her. Exercise can be done in a group or individually.
Moreover, most of practices that people do for fun qualify as exercises. For instance, taking a stroll in the neighborhood in the evening is enough to relax one’s mind. Teenagers are fond of cycling for fun; however, even though they do not know it, cycling is a great lot of exercise.
So, what is the way out of these excuses? Identify an exercise that excites you; an exercise that you will do without much hustle. For instance, decide to engage in flexibility training exercises like yoga and sit-ups or even join a dancing group. By doing something that excites you, fun will be inevitable. However, remember to do whatever you are doing on purpose with discipline. Discipline is the key to any successful story you will ever hear.
Exercise is all-inclusive package that will enable you live almost a stress-free life, full of energy with improved self esteem and sound sleep not forgetting how you will be able to combat some diseases like hypertension and diabetes type II. During exercise, the body releases endorphins that restore peace and felicity.
Moreover, nothing equals the thrill that one gets by accomplishing set goals and exercise offers this opportunity to set both short and long-term goals and accomplish them. Again, exercise prompts the body to produce more ‘good’ cholesterol and eliminate the ‘bad’ one thus improving blood flow thus keeping hypertension at bay.
Any good doctor will tell you exercise is as essential as sleep or feeding. Finally, exercise enables you to maintain body shape by shedding those extra pounds. Unfortunately, many people complain that exercise is not fun; however, this is just an excuse, not a reason. There are many exercise programs to choose from, every one can get a program that excites him or her, and as the excitement sets in, fun follows. Anyway, who does not want to live a peaceful and happy life? Not even you, start exercising and start today.
Buemann, Baines & Tremblay, Albert. “Effects of Exercise Training On Abdominal Obesity and Related Metabolic Complications”. Sports Medicine. 2007, 21(1): 191-212.
Hawk, Patricia. “Here’s why Exercise is good for You.” 2009.
Mayo Clinic Staff. “ Exercise: 7 Benefits of Regular Physical Activity. ” 2010. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2023, October 29). Essay about Exercise - Benefits. https://ivypanda.com/essays/exercise-is-good/
"Essay about Exercise - Benefits." IvyPanda , 29 Oct. 2023, ivypanda.com/essays/exercise-is-good/.
IvyPanda . (2023) 'Essay about Exercise - Benefits'. 29 October.
IvyPanda . 2023. "Essay about Exercise - Benefits." October 29, 2023. https://ivypanda.com/essays/exercise-is-good/.
1. IvyPanda . "Essay about Exercise - Benefits." October 29, 2023. https://ivypanda.com/essays/exercise-is-good/.
Bibliography
IvyPanda . "Essay about Exercise - Benefits." October 29, 2023. https://ivypanda.com/essays/exercise-is-good/.
- The Novel “Birdsong”, Which Excites by Its Romantic and Realistic Combination
- Olympic Cycling Champion in the UAE
- Organic Photovoltaic Cells Technology Production
- Opposites From Opposites: The Conception
- Annotated Bibliography: Sport Cycling
- Cycling Culture in France
- The Abu Dhabi Cycling Tour: Strategic Risk Management Plan
- The Cycling Studio Project in the Fitness Industry
- The Cycling Process: Physical Issues
- Increasing Post-Pandemic Cycling Safety Through Improved Communication on the Road
- Terra Cycle: A Mogul of Re- and Up-Cycling Waste
- R-insulin: Article Critique
- Importance of Physical Fitness
- Cholesterol Screening Program and Health Promotion
- Science Principles in Exercising
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
How to Thrive as You Age
Women who do strength training live longer. how much is enough.

Allison Aubrey

Strength training is good for everyone, but women who train regularly get a significantly higher boost in longevity than men. Gary Yeowell/Getty Images hide caption
Strength training is good for everyone, but women who train regularly get a significantly higher boost in longevity than men.
Resistance training does more than help us build strong muscles.
A new study finds women who do strength training exercises two to three days a week are more likely to live longer and have a lower risk of death from heart disease, compared to women who do none.
"We were incredibly impressed by the finding," says study author Martha Gulati , who is also the director of preventive cardiology at Cedars Sinai in Los Angeles.
Of the 400,000 people included in the study, only 1 in 5 women did regular weight training. But those who did, saw tremendous benefits.
"What surprised us the most was the fact that women who do muscle strengthening had a reduction in their cardiovascular mortality by 30%," Gulati says. "We don't have many things that reduce mortality in that way."
Strength training is also good for bones, joints, mood and metabolic health. And at a time when many women focus on aerobic activity and hesitate to do weight training, the findings add to the evidence that a combination of both types of exercise is powerful medicine. "Both should be prescribed," Gulati says.

Shots - Health News
Millions of women are 'under-muscled.' these foods help build strength.
The findings are part of a larger study, published in The Journal of the American College of Cardiology, which evaluated the differences in the effects of exercise between men and women.
While the study finds that even small doses of exercise are beneficial for everyone, the data show that women need less exercise than men to get the same gains in longevity.
Women who did moderate intensity exercise, such as brisk walking, five times a week, reduced their risk of premature death by 24%, compared to 18% for men.
"The take home message is – let's start moving," says Eric Shiroma , a prevention-focused researcher at the National Heart, Lung, and Blood Institute, part of the National Institutes of Health, which provided grant support for the research..
It's not exactly clear what drives the variance between sexes, but there are physiological differences between men and women, and differences in heart disease risks , too.
People born female have less muscle and lower aerobic capacity in general. Also, women have more capillaries feeding part of their muscles, Shiroma says. The findings show women need to do less exercise to change their baseline of aerobic and muscular strength. "It might be that this relative increase in strength [in women compared to men] is what's driving this difference in benefit," he says.
The results show a little can go a long way. "The benefits start as soon as you start moving," Shiroma says.
It's increasingly common to see female weight lifters and body builders on social media, and many gyms and work-out studios now incorporate weight training into many of their classes and offerings. But, given that about 80% of women in the study said they don't participate in regular weight training, there's still a lot of hesitancy.

Ann Martin says her mood improves with resistance training. "It gets your blood flowing," she says. "It feels good." Deb Cutler/Ann Martin hide caption
"I was always the awkward one in gym class back in school days," says Ann Martin, 69, of Wilmington, Del. She shied away from gyms and weight-training machines. Martin has always been a walker, but she realized she needed to build more strength, so last year she started working out with a trainer to learn how to use the equipment. "It's fun now," she says. "I can feel my muscles getting stronger."
Strength training can be intimidating, Shiroma says. "But it's not all bodybuilders trying to lift super amounts of weight." He says there are many ways to incorporate resistance training into your life.
All activities that require your muscles to work against a weight or force count as strength training. This includes the use of weight machines, resistance bands or tubes, as well as all the many ways we can use our own body weight, as we do with push-ups and squats.
The findings of this new study fit with the Physical Activity Guidelines for Americans , which recommend that adults get a minimum of 2.5 hours of moderate-intensity exercise a week, that's about 30 minutes, most days of the week. The guidelines also call for doing strength-based activities at least two days a week.

This 22-Minute Workout Has Everything You Need
The increase in lifespan can likely be explained in part, by the well-being that comes from the other hidden benefits. Here are 5 ways building strength can boost good health.
1. Strength training helps protect joints.
Physical therapists often recommend resistance training for patients with knee and hip pain. "Strength training protects joints, resulting in less stress through the body," says Todd Wheeler, a physical therapist at MedStar Health Physical Therapy in Washington, D.C . "If joints could talk, they would say 'It's not my fault I'm irritated," Wheeler says. They'd blame it on weak muscles. He says strong muscles support the joints, which can help decrease joint pain. Wheeler recommends starting small and simply. For instance, try a few squats and table pushups. "Listen to your body and gradually increase intensity over time," he says.
2. Building muscle burns more calories
Aerobic exercise – such as running and cycling – typically burns more calories in real time compared to strength training. But people who weight train can get a boost in calorie burning over the long-term.
"When you're doing resistance training, you're building muscle. That muscle requires energy," says Bryant Johnson , a trainer who wrote The RBJ Workout . So, adding muscle mass can help people burn more calories.
Dr. Gulati also points to research that shows weight lifting and resistance training can help people lose more fat and improve body composition.
3. Resistance training protects against injuries and falls
As we've reported, millions of Americans, especially women, are under-muscled, and muscle mass is a predictor of longevity .
Since muscle mass peaks in our 30s and then starts a long, slow decline, we need to take steps to slow this down. If we don't do strength training exercise, we're more likely to become weak, increasing the risk of falls, which is the top cause of death among older adults in the U.S.
And since muscle loss - also known as sarcopenia - affects more than 45% of older adults in the U.S., "it's important to know about it and take steps to prevent it," says Richard Joseph , a wellness focused physician. He says strength training improves bone density which also protects against injuries and falls.
Joseph says people can get the biggest bang for their buck when they're starting out by focusing on lower body exercises that work big muscle groups in the legs.
4. Strength training helps control blood sugar
About 1 in 3 adults in the U.S. has prediabetes. Strength training can help control blood sugar by clearing glucose out of the bloodstream.
When we use our muscles during exercise, whether it's pushing, pulling, lifting or moving, they require more glucose for energy. This explains why exercise after meals can help control blood sugar.
And a recent study found strength training can be even more effective than aerobic activity in controlling blood sugar in people with diabetes.
5. Muscle building may help boost mood
A meta-analysis published in the medical journal JAMA Psychiatry in 2018, which included the results of more than 30 clinical trials, found a reduction in symptoms of depression among people who did weight training two times a week or more.
Strength training has also been shown to improve depressive symptoms in people at risk of metabolic disease. And, research shows strength training can tamp down anxiety, too.
Ann Martin says it makes sense that our moods improve when we move. "It gets your blood flowing," she says. "It feels good."

Scientists can tell how fast you're aging. Now, the trick is to slow it down
This story was edited by Jane Greenhalgh
- resistance training
- strength training
- weight training
- heart disease
Importance of Exercise Essay
500 words essay on exercise essay.
Exercise is basically any physical activity that we perform on a repetitive basis for relaxing our body and taking away all the mental stress. It is important to do regular exercise. When you do this on a daily basis, you become fit both physically and mentally. Moreover, not exercising daily can make a person susceptible to different diseases. Thus, just like eating food daily, we must also exercise daily. The importance of exercise essay will throw more light on it.

Importance of Exercise
Exercising is most essential for proper health and fitness. Moreover, it is essential for every sphere of life. Especially today’s youth need to exercise more than ever. It is because the junk food they consume every day can hamper their quality of life.
If you are not healthy, you cannot lead a happy life and won’t be able to contribute to the expansion of society. Thus, one needs to exercise to beat all these problems. But, it is not just about the youth but also about every member of the society.
These days, physical activities take places in colleges more than often. The professionals are called to the campus for organizing physical exercises. Thus, it is a great opportunity for everyone who wishes to do it.
Just like exercise is important for college kids, it is also essential for office workers. The desk job requires the person to sit at the desk for long hours without breaks. This gives rise to a very unhealthy lifestyle.
They get a limited amount of exercise as they just sit all day then come back home and sleep. Therefore, it is essential to exercise to adopt a healthy lifestyle that can also prevent any damaging diseases .
Benefits of Exercise
Exercise has a lot of benefits in today’s world. First of all, it helps in maintaining your weight. Moreover, it also helps you reduce weight if you are overweight. It is because you burn calories when you exercise.
Further, it helps in developing your muscles. Thus, the rate of your body will increases which helps to burn calories. Moreover, it also helps in improving the oxygen level and blood flow of the body.
When you exercise daily, your brain cells will release frequently. This helps in producing cells in the hippocampus. Moreover, it is the part of the brain which helps to learn and control memory.
The concentration level in your body will improve which will ultimately lower the danger of disease like Alzheimer’s. In addition, you can also reduce the strain on your heart through exercise. Finally, it controls the blood sugar levels of your body so it helps to prevent or delay diabetes.
Get the huge list of more than 500 Essay Topics and Ideas
Conclusion of Importance of Exercise Essay
In order to live life healthily, it is essential to exercise for mental and physical development. Thus, exercise is important for the overall growth of a person. It is essential to maintain a balance between work, rest and activities. So, make sure to exercise daily.
FAQ of Importance of Exercise Essay
Question 1: What is the importance of exercise?
Answer 1: Exercise helps people lose weight and lower the risk of some diseases. When you exercise daily, you lower the risk of developing some diseases like obesity, type 2 diabetes, high blood pressure and more. It also helps to keep your body at a healthy weight.
Question 2: Why is exercising important for students?
Answer 2: Exercising is important for students because it helps students to enhance their cardiorespiratory fitness and build strong bones and muscles. In addition, it also controls weight and reduces the symptoms of anxiety and depression. Further, it can also reduce the risk of health conditions like heart diseases and more.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App


Essay on Benefits of Exercise
Students are often asked to write an essay on Benefits of Exercise in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Benefits of Exercise
Introduction.
Exercise is a vital part of our daily routine. It helps in maintaining our health, improving our mood, and enhancing our overall well-being.
Physical Health
Exercise strengthens our heart and lungs, reducing the risk of diseases. It helps in maintaining a healthy weight and promotes better sleep.
Mental Health
Regular exercise releases endorphins, chemicals that make us feel happier and relaxed. It also boosts our self-esteem and improves concentration.
In conclusion, exercise benefits us in many ways. It’s an excellent tool to stay healthy, happy, and focused. Therefore, we should include it in our daily routine.
250 Words Essay on Benefits of Exercise
Exercise, often underrated, is a potent tool for enhancing physical and mental health. It is a universal remedy that offers numerous benefits, transcending age, gender, and physical ability.
Physical Health Benefits
Exercise primarily enhances physical wellbeing. Regular physical activity strengthens the cardiovascular system, reducing the risk of heart diseases. It aids in maintaining a healthy weight, thus preventing obesity-related illnesses. Moreover, exercise improves bone density, reducing the risk of osteoporosis, and enhances muscular strength and flexibility, thereby preventing injuries.
Mental Health Benefits
Beyond physical health, exercise significantly contributes to mental wellbeing. It stimulates the production of endorphins, the body’s natural mood elevators, leading to reduced stress levels and increased happiness. Regular exercise can also alleviate symptoms of depression and anxiety, enhancing overall mental health.
Cognitive Benefits
Exercise also plays a crucial role in cognitive function. It promotes better sleep, aids in maintaining focus, and improves memory. Studies suggest that regular physical activity can delay the onset of cognitive decline in later years, reinforcing its long-term benefits.
In conclusion, the benefits of exercise are manifold, spanning physical, mental, and cognitive domains. It is a cost-effective, accessible strategy to enhance overall health and wellbeing. As college students, embracing exercise as a regular habit can significantly contribute to academic success and lifelong health. The adage, “A healthy mind in a healthy body,” indeed holds.
500 Words Essay on Benefits of Exercise
Exercise, often regarded as a panacea for numerous health-related issues, has been a subject of extensive research over the years. It is a powerful tool that aids in the enhancement of both physical and mental well-being. This essay aims to explore the multifaceted benefits of exercise, ranging from improved physical health to enhanced cognitive abilities.
The first and most apparent advantage of exercise is its profound impact on physical health. Regular physical activity strengthens the cardiovascular system, reducing the risk of heart disease and stroke. Exercise aids in the regulation of blood pressure and cholesterol levels, two significant risk factors for these conditions.
In addition to cardiovascular health, exercise contributes to better respiratory health by enhancing lung capacity and efficiency. It also plays a crucial role in weight management, as it helps burn calories, preventing obesity and associated diseases like diabetes and certain types of cancer.
The benefits of exercise are not limited to physical health; they also extend to mental well-being. Regular physical activity has been shown to reduce symptoms of anxiety and depression. It stimulates the production of endorphins, often referred to as ‘feel-good’ hormones, which elevate mood and promote a sense of well-being.
Exercise also aids in stress management. Engaging in physical activity diverts the mind from stressors, providing a respite from negative thoughts. Furthermore, the accomplishment of fitness goals often boosts self-esteem and confidence.
Recent research has unveiled the cognitive benefits of regular exercise. It has been found to enhance memory and thinking skills. Exercise promotes the growth of new brain cells and improves connections between neurons, leading to better brain health. It can also slow down the cognitive decline associated with aging, thereby reducing the risk of diseases like Alzheimer’s and dementia.
Social Benefits
Exercise often serves as a social activity, providing opportunities to meet new people and strengthen relationships. Participating in group exercises or sports can foster a sense of community and belonging, which is crucial for emotional well-being. Moreover, it can also enhance teamwork and leadership skills, which are invaluable in various aspects of life.
In conclusion, the benefits of exercise are manifold, ranging from physical health improvements to mental and cognitive enhancements. It is a cost-effective and accessible method to maintain overall health and improve quality of life. As college students, integrating regular physical activity into our routine can provide us with the stamina to deal with academic pressures and equip us with skills that are beneficial in the long run. Therefore, exercise is not just about maintaining physical fitness; it is a comprehensive approach to holistic well-being.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Being a Child
- Essay on Being the Oldest Sibling
- Essay on Mountain Climbing
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
An exercise pill may soon offer the same benefits as a workout, scientists say

You know exercise is good for your health , but life can get in the way of your physical fitness. Perhaps you don’t have as much time to hit the gym as you’d like, or have a medical condition that prevents you from exercising at all. Maybe you hate working out. Wouldn’t it be easier if you could just take a pill to reap the rewards of exercise?
The idea may not be so far-fetched. After testing new drug compounds that appear to mimic the physical benefits of exercise in rodents, scientists say a pill may someday be able to do the same in humans. Bahaa Elgendy, Ph.D. , a medicinal chemist and associate professor of anesthesiology at the Washington University School of Medicine in St. Louis , was scheduled to present his team’s findings Monday at the spring meeting of the American Chemical Society (ACS) .
As you age, you gradually lose muscle mass, strength, and function—a type of atrophy called sarcopenia . Elgendy’s team hopes an exercise-mimicking pill could treat muscle atrophy and improve the physical fitness of people with serious ailments such as cancer, heart failure, and neurodegenerative diseases , according to an ACS news release .
“We’re not saying by any means or forms that people shouldn’t exercise,” Elgendy tells Fortune ahead of his presentation. “But [the drug] hopefully will help people who cannot exercise. And in other cases, it can complement exercise programs to give more benefits to patients as well.
“Or it can be combined with the new wave of drugs: antidiabetic drugs and drugs that are used for obesity and weight loss .”
Elgendy and his team spent about a decade developing a compound called SLU-PP-332 . It activates a group of proteins called estrogen-related receptors —ERRα, ERRβ, and ERRγ—which Elgendy says are “responsible for activating some of the most important metabolic pathways in tissues with high-energy demand.” The most elusive target, ERRα, regulates exercise-induced stress adaptation as well as physiological muscle processes. Mice treated with SLU-PP-332 showed improved endurance running on a rodent treadmill. The treatment also increased a fatigue-resistant muscle fiber in the animals, researchers found.
Next, the team created new, patentable molecules with the goal of making them more safe, potent, and efficacious than SLU-PP-332, Elgendy says. Further study of rat heart cells suggests the new compounds do more strongly mimic the effects of exercise.
“The new generations we developed that I’m going to talk about today, these are predicted to make it hopefully to the clinic one day in the next five years,” Elgendy tells Fortune . “The translation from animals to humans takes a long time. We need to do a lot more preclinical testing, which is vital to ensure safety.”
What are exercise mimetics?
Exercise mimetics are a proposed class of drugs that aim to do exactly what the name suggests: mimic the health benefits of exercise. SLU-PP-332 and the new compounds fall into this category.
“These compounds simulate some of the same adaptations that occur in muscle with exercise. So, your muscles think that they are exercising even though they may not be,” Thomas Burris, Ph.D. , chair of the Department of Pharmacodynamics at the University of Florida , tells Fortune . “They improve metabolic health, they cause weight loss, fat mass loss, improved insulin sensitivity, [and] improved exercise endurance.”
Burris is part of Elgendy’s research team. With the skyrocketing popularity of GLP-1 agonists —the class of drugs that treat obesity such as Wegovy and Zepbound—people are increasingly turning to pharmaceuticals to aid in weight loss, Burris says. But he stresses that GLP-1s cause the loss of muscle mass and function in addition to body fat. The exercise mimetics he and his colleagues are studying may temper those effects.
“There’s a great need for something to be used in combination so that people who are losing that weight are primarily losing fat mass and retaining good-quality muscle, because that can lead to future complications,” Burris tells Fortune . “Older people who go on [GLP-1s] who already have maybe some reduced muscle quality, losing that mass could lead to some disabilities.”
Jamie Alan, Pharm.D., Ph.D. , an associate professor in the Department of Pharmacology and Toxicology at Michigan State University , wasn’t involved in the research but tells Fortune the results are exciting. She’s intrigued by the team’s plan to focus on the brain in future study.
“These particular drugs that they used in this first go-around, they don’t cross into the brain at all,” Alan says, referring to SLU-PP-332. “[Researchers] said it could potentially have more far-reaching benefits if we design a drug that still hits these receptors but has the ability to get into the brain.” That way, they can study the compounds as possible treatments for neurodegenerative diseases. Previous research has highlighted ERRα as a potential therapeutic target for Alzheimer’s disease .
Ideally, an exercise-mimicking drug would be in the form of a pill people could take once a day, says Burris, who has collaborated with Elgendy for years.
“He’s making the compounds and I’m testing them, and we test them all the way from biochemical methods to the whole animal,” Burris says. “We continually optimize them, trying to make them more druglike so that they can go into humans hopefully in the not-too-far future.”
For more on the health benefits of exercise:
- Women may get more health benefits from regular exercise than men—even if they work out less
- Exercise and antidepressants may be the most effective combo for treating depression
- The new ‘lazy girl workout’? In a world of comfort-seeking, some find happy medium in ‘cozy cardio’
- Even 30-second micro-workouts can boost your energy and help you get fit. How to add them to your day
Subscribe to Well Adjusted, our newsletter full of simple strategies to work smarter and live better, from the Fortune Well team. Sign up for free today.
Most Popular


Women see greater health benefits from regular exercise than men do
Women generally exercise less than men do , but new research suggests they see greater health benefits from it.
A national study found that women who exercised regularly — at least 2½ hours of moderate exercise or 75 minutes of vigorous exercise per week — had a 24% lower risk of dying over the study period compared with women who didn’t exercise. By contrast, men who exercised regularly were 15% less likely to die than men who didn’t exercise.
Men also needed more exercise than women to achieve the same health benefits: Five hours of moderate or vigorous exercise per week reduced their risk of dying by 18% compared with men who didn’t exercise. But just 140 minutes of weekly exercise had the same effect among women.
“Women got the same benefit at lower levels of physical activity,” said a co-author of the study, Dr. Martha Gulati, the director of preventive cardiology at Cedars-Sinai’s Smidt Heart Institute in Los Angeles.
Women who exercised regularly also had a 36% lower risk of dying from a cardiovascular issue such as a heart attack or stroke, the study found, whereas men who exercised regularly had a 14% lower risk.
The findings were published Monday in the Journal of the American College of Cardiology. The researchers analyzed the self-reported exercise habits of more than 412,000 men and women who participated in the National Health Interview Survey over from 1997 to 2017.
Roughly one-third of the women regularly engaged in aerobic exercises — ones that elevate the heart rate, such as brisk walking, jumping rope or taking spin classes — compared with 43% of men in the study. Women were also less likely than men to do muscle-strengthening activities, such as lifting weights.
Nevertheless, regular muscle strengthening — roughly one session per week, on average — was associated with a 30% lower risk of women dying from cardiovascular problems and a 19% lower risk of dying overall. Among men, the same weekly exercises lowered the risk of dying from cardiovascular problems by 11% and of death by the same percentage.
Gulati said one major limitation of the study is that it didn't account for how active women were outside workout settings.
“Missing from our data are the things that we do every day — the other physical activity that’s not going to the gym but running after kids, doing gardening, doing household chores,” she said.
Should men and woman have different exercise recommendations?
The Department of Health and Human Services recommends that adults get 150 minutes of moderate physical activity per week, including two days of muscle-strengthening activities.
But Gulati said those guidelines “can be very overwhelming to someone who does zero.” Many of her female patients struggle to find time for exercise, she said.
“Women are busy. Women work. Women usually take the bulk of family responsibilities — whether that’s children, whether that’s elderly parents — and by the time the day finishes, there’s very little time,” Gulati said.
Data from the National Health Interview Survey suggests that women in 2022 were more likely than men to have been advised over the past year by doctors or other health professionals to increase their amounts of physical activity.
"Rather than talking about 150 minutes a week, the way that we should be saying it is: What can you fit in?" Gulati said.
Paul Arciero, a professor of sports, medicine and nutrition at the University of Pittsburgh, said it’s only logical to have separate exercise guidelines for men and women.
“There are clear, sex-based differences in response to exercise,” he said. “We have to move beyond thinking that men and women respond similarly."
What's causing the sex-based difference?
Many studies have shown that exercise doesn't affect men and women in the same way.
Arciero’s research in 2022 found that women had greater reductions in blood pressure when they exercised in the morning, whereas men had greater reductions at night. A 2020 review also found that women’s muscles are more resistant to fatigue from high-intensity exercise.
But scientists have been less certain about how those differences affect people’s long-term health.
The new study shows that “women are basically more efficient in responding to exercise, particularly when it comes to heart health and mortality,” said Arciero, who wasn’t involved in the paper.
Arciero said physiological differences may contribute to the advantage: Women have more capillaries (tiny blood vessels) in a given section of muscle than men do, which could allow more blood and oxygen to flow to the heart during exercise.
Women also have higher levels of the hormone estrogen, which enhances blood flow, said Lynda Ransdell, the chair of the kinesiology department at Boise State University.
Ransdell pointed to a third factor as, well: Women tend to be less physically active, so it may take less effort to improve their health relative to their baselines.
“I would call it the principle of diminishing returns,” Ransdell said. “Since women typically start at lower levels of fitness, they can see significant gains with taking up a little less physical activity.”
But scientists have more to learn, she added.
“While I love this study and I think it’s groundbreaking and landmark, I also believe that it’s one piece of a puzzle,” Ransdell said. "I would love to see them do more research with objective measures of physical activity like pedometers or Apple watches.”
This article was originally published on NBCNews.com

Exercise in a Pill? Breakthrough May Mimic Workout Benefits, Study Finds
But could it really replace your gym membership? Experts break it down.

- A pill may be able to mimic the effects of exercise, new research shows.
- Researchers found that a recently developed compound may be able to increase muscle fiber and improve endurance, similar to the effects of exercise.
- Experts explain the findings and what this could mean for your fitness routine.
What if you could get the same metabolic benefits of hitting the gym without breaking a sweat—simply by taking an “exercise pill?” Scientists from the Washington University School of Medicine in St. Louis spent 10 years creating a compound to mimic the effects of exercise on the body, in pill form.
The idea of a pharmaceutical therapy that can replicate the biological effects of exercise in the body is certainly an exciting one, says Michael Racke, M.D. , medical director of neurology for Quest Diagnostics. The health benefits of regular exercise are well documented. But, unfortunately, Dr. Racke explains that “many individuals cannot be physically active on a regular basis, sometimes due to medical reasons.” For instance, patients with neurological disorders, including advanced dementia and certain neuromuscular disorders, may not be able to be physically active to a meaningful degree, he notes. The idea that by taking a pill one can reap the physical benefits of working out could do wonders for these populations.
But, it’s not a magic pill. There are a few aspects to keep in mind—mainly that because this research is done on rodents and not on humans, we can’t say for certain whether or not people would see the same effects from taking this pill, says Gregory Katz, M.D. , a cardiologist at NYU Langone Health. In addition, as the abstract notes, there are a number of organ systems that can be affected by ERR activation where human studies may be very informative, says Dr. Racke.
And, keep in mind that this compound only activates one family of receptors and exercise has many other effects on the body, says Dr. Katz. At this point, this compound is only shown to have beneficial metabolic effects. So, we don’t yet know if this drug could benefit specific health conditions.
While studies in animals with this compound indicate that it could potentially be beneficial against conditions such as obesity, heart failure, or a decline in kidney function with age, this drug will not likely be used as a treatment for any health conditions any time soon, he explains. “There’s a lot of work to do before this would ever be considered for use in a human,” he notes.
The bottom line
It’s important to reiterate that this research is still in very early stages and the results should be taken with a grain of salt. There are people trying to figure out whether the benefits of exercise can be replicated with medications, but there’s a lot of hard work to do before we have any clue whether this is science or science fiction, says Dr. Katz.
“Significant additional research in human subjects is clearly needed before any of us should think an ‘exercise pill’ will enable us to skip the treadmill and barbells anytime soon,” adds Dr. Racke.
Madeleine, Prevention ’s assistant editor, has a history with health writing from her experience as an editorial assistant at WebMD, and from her personal research at university. She graduated from the University of Michigan with a degree in biopsychology, cognition, and neuroscience—and she helps strategize for success across Prevention ’s social media platforms.

Here’s How to Ease IBS Pain at Home

Avoid Allergies With These Easy Solutions

Can Working Out Before Bed Affect Your Sleep?

There's a Link Between Regular Exercise and Sleep

Halle Berry Misdiagnosed Perimenopause for Herpes

Does Mouth Taping Help You Sleep?

What to Know About Endo Belly

Prince William Has Gone Into ‘Protection Mode’

10 Best Acid Reflux Pillows, According to Experts

Gisele Bündchen Discusses ‘Severe‘ Panic Attacks

Do You Really Need to Wear Solar Eclipse Glasses?

IMAGES
VIDEO
COMMENTS
Overwhelming evidence exists that lifelong exercise is associated with a longer health span, delaying the onset of 40 chronic conditions/diseases. What is beginning to be learned is the molecular mechanisms by which exercise sustains and improves quality of life. The current review begins with two short considerations.
Since ancient times, the health benefits of regular physical activity/exercise have been recognized and the classic studies of Morris and Paffenbarger provided the epidemiological evidence in support of such an association. Cardiorespiratory fitness, often measured by maximal oxygen uptake, and habitual physical activity levels are inversely related to mortality. Thus, studies exploring the ...
Abstract. Exercise has long been recognized for its physical health benefits, but its impact on mental health has gained increasing attention in recent years. This paper provides a comprehensive ...
Potential Benefits of Exercise Training on Heart Failure. Increased exercise capacity: Reduced heart rate response to submaximal exercise: ... In May 2015, the average time from submission to first decision for all original research papers submitted to Circulation Research was 15.49 days. Correspondence to Carl J. Lavie, MD, Exercise ...
Physical inactivity is a modifiable risk factor (similar to dyslipidemia and hypertension) for a variety of chronic diseases, including cancer and cardiovascular disease. Exercise provides a clear health benefit, which serves in the primary and secondary prevention of these disease processes (the most important being a reduction in cardiovascular disease and premature death). The physiologic ...
benefits, but exercise has numerous benefits to an individual's psychology as well. Research has repeatedly suggested that regular exercise can improve mental health and lessen symptoms of depression, anxiety, and generalized stress significantly. (Mikkelsen et al., 2017). There have even been studies that suggest that physical activity can ...
Background The treatment of noncommunicable diseases (NCD), like coronary heart disease or type 2 diabetes mellitus, causes rising costs for the health system. Physical activity is supposed to reduce the risk for these diseases. Results of cross-sectional studies showed that physical activity is associated with better health, and that physical activity could prevent the development of these ...
Background Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity ...
Physical activity and exercise can reduce stress and anxiety, boost happy chemicals, improve self-confidence, increase the brain power, sharpen the memory and increase our muscles and bones ...
Benefits of Exercise Thursday, March 13, 2014 ... pave the way for research using human subjects and in 2012 led the effort to secure a $4.9 million federal grant to expand the capacity of Dimock's health center facility. Widely respected for her expertise and insight, Dr. Jordan has published articles in medical ...
Findings from these studies demonstrate that sport participants gain greater social and psychological benefits than individuals who exercise at gyms. Previous research has highlighted the social culture often associated with team sports (R. M. Eime et al., Citation 2013b, b) as well as a greater emphasis on maintaining existing rather than ...
Conclusion. Exercise is all-inclusive package that will enable you live almost a stress-free life, full of energy with improved self esteem and sound sleep not forgetting how you will be able to combat some diseases like hypertension and diabetes type II. During exercise, the body releases endorphins that restore peace and felicity.
Resistance training does more than help us build strong muscles. A new study finds women who do strength training exercises two to three days a week are more likely to live longer and have a lower ...
Answer 1: Exercise helps people lose weight and lower the risk of some diseases. When you exercise daily, you lower the risk of developing some diseases like obesity, type 2 diabetes, high blood pressure and more. It also helps to keep your body at a healthy weight.
Recent research has unveiled the cognitive benefits of regular exercise. It has been found to enhance memory and thinking skills. Exercise promotes the growth of new brain cells and improves connections between neurons, leading to better brain health. It can also slow down the cognitive decline associated with aging, thereby reducing the risk ...
This Type of Exercise May Have the Most Anti-Aging Benefits, According to New Research. Reviewed by Dietitian Jessica Ball, M.S., RD. There are many benefits of regular exercise, including ...
Exercise mimetics are a proposed class of drugs that aim to do exactly what the name suggests: mimic the health benefits of exercise. SLU-PP-332 and the new compounds fall into this category ...
Women generally exercise less than men do, but new research suggests they see greater health benefits from it.. A national study found that women who exercised regularly — at least 2½ hours of ...
Experts break it down. A pill may be able to mimic the effects of exercise, new research shows. Researchers found that a recently developed compound may be able to increase muscle fiber and ...