An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Nanomaterials (Basel)


Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications
Ashok kumar mandal.
1 Natural Product Research Laboratory, Thapathali, Kathmandu 44600, Nepal
Saurav Katuwal
2 Central Department of Chemistry, Tribhuvan University, Kirtipur 44618, Nepal
Felix Tettey
3 Department of Chemical, Biological, and Bioengineering, North Carolina A&T State University, Greensboro, NC 27411, USA
Aakash Gupta
4 Department of Chemistry and Biochemistry, University of Massachusetts Dartmouth, North Dartmouth, MA 02747, USA
Salyan Bhattarai
5 Paraza Pharma, Inc., 2525 Avenue Marie-Curie, Montreal, QC H4S 2E1, Canada
Shankar Jaisi
Devi prasad bhandari, ajay kumar shah.
6 Faculty of Health Sciences, School of Health and Allied Sciences, Pokhara University, Lekhnath 33700, Nepal
Narayan Bhattarai
Niranjan parajuli, associated data.
All data generated or analyzed during this study are available within the article.
Zinc oxide nanoparticles (ZnO-NPs) have piqued the curiosity of researchers all over the world due to their extensive biological activity. They are less toxic and biodegradable with the capacity to greatly boost pharmacophore bioactivity. ZnO-NPs are the most extensively used metal oxide nanoparticles in electronic and optoelectronics because of their distinctive optical and chemical properties which can be readily modified by altering the morphology and the wide bandgap. The biosynthesis of nanoparticles using extracts of therapeutic plants, fungi, bacteria, algae, etc., improves their stability and biocompatibility in many biological settings, and its biofabrication alters its physiochemical behavior, contributing to biological potency. As such, ZnO-NPs can be used as an effective nanocarrier for conventional drugs due to their cost-effectiveness and benefits of being biodegradable and biocompatible. This article covers a comprehensive review of different synthesis approaches of ZnO-NPs including physical, chemical, biochemical, and green synthesis techniques, and also emphasizes their biopotency through antibacterial, antifungal, anticancer, anti-inflammatory, antidiabetic, antioxidant, antiviral, wound healing, and cardioprotective activity. Green synthesis from plants, bacteria, and fungus is given special attention, with a particular emphasis on extraction techniques, precursors used for the synthesis and reaction conditions, characterization techniques, and surface morphology of the particles.
1. Introduction
A diverse application of nanomaterial-based technology has opened a new horizon in material science over the past decades because nanomaterials offer a high surface area and other very distinctive physical, chemical, and biological properties compared to their bulk counterparts [ 1 ]. Nanoparticle (NP) research has gained distinct interest due to the enhanced electrochemical reactivity, thermal conductivity, and nonlinear optical properties of nanoparticles which offer unique applications [ 2 ]. Zinc oxide nanoparticles (ZnO-NPs) are the most commonly used metal oxide nanoparticles because their distinctive optical and chemical properties can be easily modified by altering the morphology and the wide bandgap (3.37 eV) and high excitation binding energy (60 meV) to simulate the ZnO-NPs to be a potent photocatalytic and photo-oxidizing moiety against chemical and biological species [ 3 , 4 ]. They are less toxic to the human body and offer biocompatibility as the Zn ion (Zn 2+ ), a soluble form of ZnO, is a trace element found in the human physiological system. ZnO-based structures have been proven to exhibit biodegradability both in the bulk phase and in the form of nanoparticles [ 5 ]. Zn ions also act as the principal mediators of intracellular bacterial toxicity, disrupting their cell membranes [ 6 ].
Some potential applications where ZnO-NPs have been researched are: therapeutic carriers, biological sensing, gene transfer, nanomedicine discovery, biological labeling, medical implant coatings, electronic sensors, wastewater treatment, and communication [ 4 , 7 , 8 ]. The medical implant coating with zinc oxide and hydroxyapatite exhibited antibacterial and osteoconductive properties, emphasizing the potential of ZnO-NPs in therapeutic diagnostics. ZnO-NPs exhibited cytotoxicity in human cancer cells, resulting in cell death via the apoptotic pathway [ 9 ]. They also promoted antiproliferative activity in triple-negative breast cancer cells [ 10 ], nonautophagic cell death in human lung adenocarcinoma cells with an epidermal growth factor receptor (EGFR) mutation [ 11 ], and anticancer activity via apoptosis in chronic myeloid leukemia cells using a transcriptomic approach [ 12 ]. It has also been shown to induce cytotoxicity in the A549 epithelium and cancer cells [ 13 ]. Recent investigations on the ZnO-Au nanocomposite have developed an electrochemical DNA biosensor [ 14 ], ZnO-NPs for tracing studies in plants [ 15 ], and material in the development of electrochemical sensors in the detection of food additive aspartame [ 16 ]. ZnO-NPs have been shown to influence horizontal gene transfer where it impacts the transformation efficiency of Bacillus subtilis [ 17 ], and the ZnO-Ag NPs have decreased the rate of biofilm formation and gene expression in Staphylococcus aureus at a subminimum inhibitory concentration [ 18 ]. ZnO-NPs have been shown to reduce the parameters responsible for hepatic fibrosis (hydroxyproline) and nephrotoxicity (creatinine, urea, and uric acid) [ 19 ], also attenuating the gonadal toxicity which is induced by cyclophosphamide (an anticancer and immunosuppressant drug) through their antioxidant and antiapoptotic function [ 20 ], and cancer cell death through autophagy induction which supports the release of zinc ions and the generation of reactive oxygen species (ROS) [ 21 ].
In a critical study, zinc ions and ZnO-NPs both showed cytotoxic effects in the earthworm GI tract where it affected the gut epithelium and chlorogenic tissues [ 22 ]. However, ZnO-NPs dissolve slowly in human physiological conditions (pH 6–8), and the United States Food and Drug Administration (USFDA) safety datasheet indicates ZnO as a “Generally Recognized as Safe” (GRAS) substance and nonhemolytic against human red blood cells [ 23 ]. ZnO could be discovered to be a useful nanocarrier to facilitate the drug-delivering and release processes [ 24 , 25 ]. Much research endorses ZnO-NPs as the most beneficial metal nanoparticles, with minimal toxicity and excellent biocompatibility. The structural atom allocation mimics the most bioactive agent, emphasizing its pharmacological effectiveness against various ailments. With all this potential, the objective of this review article is to explore the various synthesis approaches and characterization techniques of ZnO-NPs with a comprehensive mechanistic approach to its biological activity. Although there is an increased number of studies revealing the mutually exclusive and exhaustive area of ZnO-NPs, this review is a comprehensive compilation of recent advances with clear illustrations for a better understanding of the importance of ZnO-NPs in biomedical research.
2. Biological Activities of ZnO-NPs
2.1. antibacterial action of zno-nps.
Bacteria portray a severe threat to human life as the world grapples with escalating antibiotic resistance and bacterial infection. ZnO-NPs have remarkable photo-oxidation and photocatalytic characteristics, and their exceptional antimicrobial properties have led to their recognition as potent agents against MDR [ 26 ]. Although the mechanism of antimicrobial action of ZnO-NPs is not well established, its properties, such as zinc ions and ROS generation, are widely assumed to result in oxidative stress and DNA damage, as well as photocatalytic activity, contributing to antibacterial efficacy ( Figure 1 ). According to Sirelkhatim et al., the oxygen annealing of ZnO increases the number of oxygen atoms on the surface, resulting in increased oxygen atom adsorption and the generation of more ROS, resulting in enhanced oxidation, and hence, a facilitated antimicrobial property [ 27 ]. Moreover, ZnO-NPs cause cytoplasmic shrinkage and the disruption of cell walls leading to cytoplasmic spillage ( Figure 2 ). ZnO-NPs act as an effective bactericidal agent against both Gram-positive as well as Gram-negative bacteria and are found to have direct interaction with the cell wall of bacteria leading to the disruption of its integrity [ 28 ].

Illustration of the antimicrobial property of ZnO-NPs against the bacterial cell wall. They act as potent antibacterial agents through these possible steps: (1) production of reactive oxygen species (ROS) causing oxidative stress, and membrane and DNA damage leading to bacterial death; (2) dissolution of ZnO-NPs into Zn 2+ and interference with bacterial enzymes, proteins, and amino acids; and (3) electrostatic interaction between ZnO-NPs and cell membrane, resulting in membrane plasma damage and intracellular content leakage. (Reprinted from [ 29 ]; open access under CC BY).

Image illustrating antibacterial efficacy against β-lactam-resistant K. pneumoniae obtained using transmission electron microscopy: ( a ) ZnO-NPs in the untreated state and ZnO-NPs in the treated state ( b – e ). Cytoplasmic shrinkage ( b ) disrupted cell wall and membrane ( c ), denatured protein shows as a dark electron-dense patch ( d ), and cytoplasmic spillage ( e , f ). The blue arrow represents an intact cell wall, the yellow arrow represents a disintegrating cell wall and cell membrane, and the violet arrow represents a denatured protein. (Reprinted from [ 30 ]; open access under CC BY).
2.2. Antifungal Action of ZnO-NPs
The antifungal properties of ZnO-NPs have been discovered in various studies in the literature. Their fungicidal activity varies depending on their structure, size, and concentration. The antifungal potency of biofabricated ZnO-NPs against Candida albicans isolates was investigated, and it was revealed that they were more effective against drug-resistant C. albicans isolates, demonstrating ZnO-NPs’ antifungal potency. Furthermore, it was shown that prophylactic treatment with lower concentrations of ZnO-NPs protects G. mellonella from the infection of C. albicans [ 31 , 32 ]. Similarly, the antifungal resistance of a 2% ZnO-NP-based cold cream exceeded the activity compared to a commercial antifungal cream at 2% on clinical isolates of Candida sp. [ 33 ]. ZnO-NPs have antifungal activity against both Aspergillus and Penicillium and have been investigated for their antidermatophytic activity on Trichophyton mentagrophytes and Trichophyton verrucosum [ 34 , 35 ]. Likewise, the bionanocomposite film of the soy protein isolate (SPI), cinnamaldehyde (CIN), and ZnO-NPs exhibited the highest antifungal activity among SPI, SPI-CIN, and SPI-ZnO-NPs films, where it was 1.56-fold stronger compared to the SPI-ZnO film and 1.24-fold stronger compared to the SPI-CIN film [ 36 ]. The antifungal activity studied against two pathogenic fungi— Botrytis cinerea and Penicillium expansum —revealed that activity is also dependent on nanoparticle concentrations, with the efficacy of the ZnO-NP treatment increasing as the concentration of ZnO-NPs rose from 3 to 12 mM. By affecting cellular functions, ZnO-NPs cause deformation in fungal hyphae, inhibiting the growth of B. cinerea . Similarly, P. expansum prevents the formation of conidiophores and conidia, resulting in the death of fungal hyphae, explaining the fact that P. expansum is found to be more sensitive than B. cinerea , i.e., microbe dependent. The activity detected in B. cinerea revealed the stronger the photo-activation, the greater the activity [ 37 , 38 , 39 ].
2.3. Cytotoxic Effect of ZnO-NPs
ZnO-NPs, compared to other metal oxide NPs, have a significant effect on cancer cells. The anticancer potential of ZnO-NPs is strongly influenced by their shape, size, and concentration. It has been discovered that the smaller the size and higher the concentration of NPs, the greater the anticancer activity [ 40 , 41 ]. They showed concentration-dependent anticancer activity against MCF7 human breast cancer cells, where 93% inhibition of proliferation of cells was noted at 100 µg/mL [ 40 ]. Similarly, fabricated ZnO-NPs exhibited concentration-dependent growth inhibition in human pancreatic cancer cell lines, PNAC-1, and AsPC-1, although they were shown to have a relatively smaller effect on the human normal fibroblast cell line (Hu02), which was found by an MTT assay [ 42 ]. The mechanistic approach ( Figure 3 ) underlying its anticancerous activity includes the production of sufficient ROS to cause substantial oxidative stress and DNA damage, disturbances on lipids and proteins in cells, and other cellular components due to their large semiconductor band gap [ 43 ]. Moreover, the establishment of a redox reaction system and the pro-inflammatory response of cells against ZnO-NPs induce cellular apoptosis. Discrimination between cancerous and normal cells has been a major challenge for a drug to be categorized as anticancerous. Failure to achieve selectivity results in systemic toxic effects. Several studies have revealed the selectivity of ZnO-NPs toward cancerous cells. ZnO-NPs have been demonstrated to be selective to Jurkat cancer cells with minimal toxicity toward normal CD4 + T cells [ 44 ]. Similarly, Hanley and the group proposed that ZnO-NPs had 28–35 times the specific cytotoxicity against cancer carcinoma cells compared to normal cells [ 45 ]. Selective localization by enhanced permeability and retention (EPR) time via extravasation toward tumor cells assists in selective activities affecting tumor cells rather than the normal cells. The electrostatic property of ZnO-NPs facilitates the targeting of tumor sites [ 46 ]. Thus, there is ample evidence that ZnO-NPs can exhibit anticancer effects in specific types of tumor cells in the body, which is depicted in Figure 3 .

A schematic representation of cytotoxicity potency of ZnO-NPs leading to the death of cancer cells. ZnO-NPs induce ROS production sequentially, leading to oxidative stress, DNA damage, p53 activation, and apoptosis of cancerous cells.
Despite various biomedical applications such as anticancer therapy, drug delivery, gene therapy, and tumor imaging, ZnO-NPs might have deleterious effects on several key organs including the lungs, kidneys, liver, CNS, reproductive system, and fetal development in animal models. However, the ZnO-NP-induced toxicity is multifactorial, and it is yet unknown just how toxic ZnO-NPs are for these organs [ 47 ].
2.4. Wound Healing Activity of ZnO-NPs
Wound healing is the phenomenon of cell injury responses, involving the activation of fibroblasts, endothelial cells, and macrophages where fibroblasts proliferate; an important step in wound healing for tissue regeneration [ 48 ]. It has been predicted that the delivery of ZnO via poly (lactide-co-glycolic acid) (PLGA)/silk fibroin (SF) nanofibers retains the bioavailability of NPs on the wound area and integrates with the unique structural features of electrospun nanofibers, which stimulate wound closure, re-epithelialization, collagen deposition, cellular migration, and angiogenesis [ 49 ]. Besides this, the ZnO-NPs loaded on bromelain-immobilized silk fibroin (SF-Br) reduced inflammation and promoted wound healing on a second-degree burn dressing [ 50 ]. During the healing process, the low doses of ZnO-NPs favored attachment and proliferation of fibroblasts, but the trend reversed at high doses. Metallic particles in nanocrystalline forms reduce wound infection along with promoting wound healing, as observed in adult male albino Wistar rats [ 51 ] and albino rats [ 52 ]. It was found that the functionalization of ZnO-NPs into triethoxysilane poly(amidoamine) dendrimer to generate a cross-linked collagen scaffold enhances re-epithelization and speedier collagen deposition than other scaffolds, which resulted in instantaneous wound healing [ 53 ]. In addition, the biodegradable thiolated bandage with implanted ZnO-NPs demonstrated an enhanced therapeutic agent for treating surgical site infections, satisfying the criteria for the optimal surgical dressing [ 54 ].
Similarly, the functionalization of bacterial nanocellulose (BNC) grafted with aminoalkyl silane and doped with Pullan-ZnO-NPs electrospun nanofibers (A-g-BNC/Pul-ZnO) exhibited superior performance in blood clotting and antibacterial activity that had a 5 log value higher than BNC, and was found to be safe in terms of cytotoxicity as tested in L929 fibroblast cells. It offers growth and proliferation, which was corroborated by the rat model where the scaffolds revealed rapid wound healing due to re-epithelization, and blood vessel and collagen formation [ 55 ]. An in vitro study reported that the bionanocomposite-based 3D chitosan/pectin/ZnO-NP porous films demonstrated no cytotoxicity (biocompatibility) and cell growth and migration (proliferation) for primary human dermal fibroblast cells (HFCs), suggesting a benign biomaterial for promoting wound healing [ 56 ].
Moreover, 3D-printed alginate-ZnO-NP hydrogels exhibited enhanced pore sizes, stiffness, and no detrimental effect on STO-fibroblasts or cell viability, making them a suitable scaffold for wound healing [ 57 ]. Generally, hydrogels are preferred with ZnO-NPs because they have a slow release of nanoparticles from the preparation, which reduces the cytotoxicity from ROS formation and improves wound healing. The above analyses support the findings of Saddik et al., where it was demonstrated that azithromycin-ZnO-NPs impregnated into an HPMC gel enhanced bacterial clearance and epidermal regeneration, which eventually stimulated tissue formation, leading to the rapid healing of the infected wound [ 58 , 59 ]. Another bioscaffold made from sodium alginate gum acacia ZnO-NP hydrogels showed a similar potential in expediting healing in terms of reducing inflammation and produced no scar at the excision wound on rabbit skin [ 60 ]. Thus, topical zinc application has been shown to improve the process of re-epithelialization, reduce inflammation, and inhibit the growth of bacteria in the case of foot ulcers and other topical wounds [ 61 ].
2.5. Anti-Inflammatory Activity of ZnO-NPs
The inflammatory response in the human body is a complicated process that involves immune system activation and the release of pro-inflammatory cytokines such as interleukin (IL)-1, -6, -12, -18, TNF-α, INFγ, and granulocyte-macrophage colony-stimulating factor (GMS-CF) [ 62 ] ( Figure 4 ). Nuclear factor-kappa b (NF-κβ) is a key transcription factor that regulates the expression of many genes that encode pro-inflammatory mediators, such as COX-2 and iNOS, which increase the synthesis of pro-inflammatory mediators such as PGE2 and nitric oxide [ 63 ]. The ZnO-NPs act as anti-inflammatory agents as they have been shown to inhibit the release of pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS) expression, myeloperoxidase, the NF-κβ pathway, and mast cell degranulation [ 64 ]. The mRNA expression of pro-inflammatory cytokines was suppressed by the ZnO-NPs synthesized using Polygala tenuifolia in a dose-dependent manner [ 65 ]. In addition, ZnO-NPs, when doped with aluminum, have been shown to reduce the production of thymic stromal lymphopoietin (TSLP) and caspase-1 activation in mast cells, leading to lowering the expression of pro-inflammatory cytokines, IL-1, IL-6, and TNF-α [ 66 ]. In a comparative study of ZnO-NPs and the ZnO standard form, it was revealed that ZnO-NPs relatively lowered the carrageenan-induced paw edema and amplified the anti-inflammatory activity of the nonsteroidal anti-inflammatory drug, ketoprofen, when administered intraperitoneally [ 67 ]. However, both forms were ineffective when administered per os (po) and guarded the gastric mucosa against the gastric ulcer induced by the administration of ketoprofen. ZnO-NPs have been discovered to have an excellent capping of flavones such as isoorientin, orientin, isovitexin, and vitexin, which have a potent anti-inflammatory response in a variety of ways, including the inhibition of cyclooxygenase, phospholipase A2, and lipoxygenases (enzymes that produce eicosanoids), resulting in a decline in leukotrienes and prostanoids [ 68 ].

Mechanism of anti-inflammatory potency of ZnO-NPs.
2.6. Orthopedic Implants and Bone Healing Activity of ZnO-NPs
Diseases such as osteoporosis, arthritis, and fibrous dysplasia can cause bone abnormalities and lasting disability. The implantation of orthopedic implants and scaffolds has significantly aided in the treatment of these bone diseases and abnormalities since they consist of materials with positive effects on the bone regeneration process [ 69 ]. Orthopedic implants are usually made of metals and alloys such as titanium, nitinol, stainless steel, and Co-Cr alloys [ 70 ]. Over the last several decades, these metals have been excessively utilized for deformity correction, joint replacements, fracture fixation, soft tissue anchorage, and most importantly, for accelerating bone growth [ 71 ]. Unfortunately, orthopedic implants are not free from side effects once placed in the body, leading to infections, limited corrosion resistance, low cell proliferation, excessive inflammation, and poor osseointegration [ 72 , 73 ]. If infection occurs, the implant loosens, bones take longer to heal, and sometimes prolonged suffering leads to death [ 74 ]. If corrosion occurs, toxicity incites, weakening the implant [ 70 ]. Metal oxide nanoparticles such as ZnO, magnesium oxide (MgO), iron oxide, zirconium oxide, titanium oxide, and silver oxide, when used with orthopedic implants, provide a wide range of solutions for the issues mentioned earlier. Figure 5 highlights how the ZnO coating on the implant helps in osteointegration, the prevention of biofilm formation, and the prevention of premature corrosion of the implant.

A diagram showing the effects of metal oxide (e.g., ZnO) coating on the orthopedic implant and bone.
Biodegradable metals (BMs) such as Zn, Mg, Ca, and Fe have additional desirable properties for their applications in orthopedics [ 75 , 76 ]. During biodegradation, these metals release metal ions, metal oxides, and hydroxides. The close interaction between the degraded by-product and the stem-progenitor cells at the interface is what gives bone tissue implants their bioactivity [ 77 ]. Therefore, altering the implant’s chemical composition can have a significant impact on the treatment’s effectiveness [ 77 ]. The integration of growth factors into bone tissue scaffolds and implants is a prominent area of interest in the research. Protein growth factors such as insulin-like growth factors and bone morphogenetic proteins can activate cellular signaling cascades to stimulate active healing [ 78 ], including angiogenesis, a crucial step in bone tissue regeneration [ 79 ].
Zn and ZnO have emerged as a recent alternative among these BMs and are commonly employed in combination with other biomaterials to gain diverse qualities in antibacterial ability, cytocompatibility, and corrosion resistance [ 80 , 81 ] due to their customizable size manipulation from micro to nano [ 82 ]. Bone is the principal repository for Zn since it stores about 30% [ 83 ], and Zn helps in the maintenance of bone mass [ 84 ]. It maintains the shape of cell membranes [ 83 ] and is crucial for bone quality. In osteoblastic cells, Zn can directly activate aminoacyl-tRNA synthetase, a rate-limiting enzyme during protein translation [ 85 ], accelerate cellular protein synthesis [ 86 ] and increase the gene expression of the transcription factor Runx2, which is connected to osteoblast differentiation. Zn also prevents the production of osteoclast-like cells from marrow cells, which minimizes osteoclastic bone resorption [ 87 ]. Bone mineralization is aided by the enzyme alkaline phosphatase, which employs zinc as a co-factor [ 88 , 89 , 90 ]. In an in vitro experiment, Zn doses between 7 and 20 nM enhanced alkaline phosphatase activity, but Zn concentrations over 5 µM decreased alkaline phosphatase activity [ 88 , 91 , 92 ]. These findings imply that a Zn shortage may affect bone growth by impairing osteoid mineralization or calcified cartilage production linked to endochondral ossification. Many distinct types of skeletal defects in prenatal and postnatal development are linked to Zn deficiency, and a study demonstrated that osteoporotic patients had lower skeletal Zn levels than the control [ 93 ]. By promoting collagen production, alkaline phosphatase (ALP) activity, and mineralization of bone nodules, Zn can improve osteogenesis ( Figure 6 ).

The diagram shows the functions of Zn in stimulating osteoblastic bone formation and mineralization. Zinc stimulates gene expression of various proteins including type I collagen, alkaline phosphatase, and osteocalcin in the cells. Zn is also known to increase the production of growth factors such as IGF-I and TGF-β1 in osteoblastic cells.
Yusa et al. showed that eluted Zn ions from Ti surfaces promoted osteoblast activities in human bone marrow-derived mesenchymal stem cells (hBMSCs) and dental pulp stem cells (hDPSCs) [ 94 ]. In both cell types, the eluted Zn ions stimulated the expression of osteoblast marker genes (collagen type I, ALP, and osteocalcin) and calcium deposition. In hDPSCs, Zn ions further stimulated the expression of Runx2, vascular endothelial growth factor A, and transforming growth factor-beta. Additionally, apoptosis rates in MC3T3-E1 cells increased from 7% in normal media to 75% and 90% when the cells were grown in Zn-deficient or Zn-free media, respectively [ 95 ]. Numerous studies have shown that increasing ZnO content improved antibacterial capacity [ 96 , 97 , 98 ], and nanocoating with ZnO may minimize S. epidermidis adherence, thus enhancing the efficacy of orthopedic implants [ 99 ]. Lin, M.-H. et al. detected that the chitosan/ZnO-NP coating showed 1.2-fold stronger antibacterial activity against E. coli than the chitosan coating alone and actively prevented the formation of biofilm [ 100 ].
Similar to Zn and ZnO, another degradable metal such as Mg provides similar benefits for tissue healing [ 101 ]. Adhikari, U. et al. mimicked the nanostructured architecture and chemical makeup of natural bone tissue matrices with a 3D scaffold made from chitosan, carboxymethyl chitosan, calcium phosphate monobasic, and magnesium oxide. This scaffold also served as a source for soluble metal ions that are beneficial to osteoblast cells and offers a favorable background to promote biomineralization [ 102 ]. Pure Mg corrodes too quickly in physiological pH and produces excessive hydrogen gas, which is its biggest drawback; thus, efforts to use the metal oxide coating in orthopedic applications have been limited [ 101 ]. In addition, the inclusion of biodegradable ZnO-NPs in polycaprolactone enables the gradual release of zinc, which has the potential to improve mesenchymal stem cell (MSC) differentiation as an added advantage. Although osteogenic differentiation was improved on scaffolds with an increased concentration of ZnO, MSC chondrogenic differentiation was boosted on scaffolds with a reduced proportion of ZnO [ 103 ].
2.7. Antidiabetic Action of ZnO-NPs
Diabetes is a metabolic disorder characterized by persistent hyperglycemia. Zinc has been discovered to have an important role in the production, storage, and secretion of insulin [ 104 ]. Furthermore, it improves insulin signaling through pathways, such as elevated PI3K activity, insulin receptor tyrosine phosphorylation, and the inhibition of glycogen synthase kinase [ 105 ]. It has been reported that zinc’s insulin-mimicking activity leads to enhanced lipogenesis and decreased nonesterified fatty acid release from adipocytes [ 106 ]. ZnO-NPs are more frequently chosen for antidiabetic effects over other metal nanoparticles because they increase the expression of GLUT-4 and INS genes due to the confluence of factors such as the enhanced cellular permeation of biosynthesized ZnO-NPs, the promotion of glycolysis via hepatic glycogenesis, and the elevation of insulin levels. Moreover, it imposes synergistic effects on the expression and activity of increased glucokinase and the expression levels of IRA and GLUT-2 [ 107 ].
A study revealed that zinc combined with insulin acts as an autocrine molecule, increasing GSIS from rat-isolated pancreatic islets [ 108 ], and interacts with several components of the insulin transduction system, facilitating glucose metabolism and insulin mRNA expression in hepatic tissue of diabetic rats [ 109 ]. In an alloxan-induced diabetic model, rats administered with 96 mg/dL of ZnO-NPs synthesized from the seed extract of Silybum marianum L. had considerably lower fasting blood sugar (FBS) levels than rats fed with 117 mg/dL of insulin, 110 mg/dL of zinc oxide, and 120 mg/dL of crude extract, implying the potent antidiabetic activity of ZnO-NPs. Antidiabetic medicinal plants have also been used to synthesize ZnO-NPs and studied for antidiabetic effects, such as Rheum ribes [ 110 ] and Cosus igneus [ 111 ]. Similarly, the antidiabetic effect of ZnO-NPs synthesized from the flower extract of Senna auriculata [ 112 ] and leaf extract of Andrographis paniculata was studied in terms of α-amylase inhibitory activity, where it showed a lower IC 50 value (121.42 µg/mL) than the leaf extract of A. paniculata (149.65 µg/mL) and ZnNO 3 (178.84 µg/mL) [ 113 ]. Moreover, the antidiabetic activity of ZnO-NPs synthesized from Withania somnifera was monitored in terms of inhibition of α-amylase and α-glucosidase, showing 90% and 95% inhibition, respectively, at 100 µg/mL [ 114 ]. According to the findings of these studies, ZnO-NPs have a substantial antidiabetic effect in terms of glucose and insulin levels, glucose tolerance, and diabetic dyslipidemia.
2.8. Antioxidant Activity of ZnO-NPs
In the modern world, the ingestion of some oxidized meals is associated with numerous serious ailments, such as hepatomegaly or necrosis of epithelial tissues, because they are capable of producing lipid peroxides and other toxic-free radicals [ 115 , 116 , 117 ]. Various natural and synthetic antioxidants are utilized to neutralize these damaging free radicals, but they have drawbacks such as high reactivity and toxicity when compared to the nanoparticles synthesized these days [ 118 , 119 ]. Das et al. investigated the antioxidant potential of ZnO-NPs and revealed that the antioxidant activity of ZnO-NPs is due to the transfer of electron density from oxygen to the odd electron located at the nitrogen atom in DPPH (2,2-diphenyl-1-picrylhydrazyl), resulting in a reduction in the intensity of the n→π* transition at the 517 nm wavelength [ 120 ].
The previous finding showed that the percentage of inhibition of free radicals by ZnO-NPs on DPPH increases along with that of the concentration, explaining the ZnO-NPs’ promising antioxidant potential [ 121 ]. Similarly, the antioxidant activity of ZnO-NPs synthesized using the Aquilegia pubiflora leaf extract was monitored through four different assays (total antioxidant capacity—TAC, total reducing power—TRP, free radical scavenging assay—FRSA (DPPH), and Trolox antioxidant assay—ABTS) for a better evaluation, and the obtained results in terms of ascorbic acid equivalent per milligram (µg AAE/mg) were directly proportional to the concentration of ZnO-NPs in each assay [ 68 ]. In addition to that, similar studies were carried out using ABTS, DPPH, hydrogen peroxide, and super peroxide scavenging assays, where the DPPH assay exhibited direct dose-dependent behavior and the order of antioxidant activity was as follows: ABTS > DPPH > SOR > H 2 O 2 [ 122 ]. Furthermore, several plant sources such as Salvia hispanica [ 123 ], Borassus flabellifer [ 124 ], and Punica granatum [ 125 ] have been utilized for evaluation of the antioxidant activity of ZnO-NPs. Generally, the antioxidant behavior of ZnO-NPs is due to the reducing ability of NPs and the phytochemicals adsorbed/capped on the surface of ZnO-NPs [ 126 ]. This reveals the unparalleled antioxidant capacity of ZnO-NPs.
2.9. Antiviral Action of ZnO-NPs
ZnO-NPs have been reported to exhibit significant antiviral activities against a plethora of viruses, such as herpes simplex virus (HSV), human papillomavirus (HPV), human immunodeficiency virus (HIV), hepatitis C and E virus (HCV, HEV), and severe acute respiratory syndrome coronavirus (SARS-CoV) [ 127 ]. The mechanism of action underlying the antiviral potency of ZnO-NPs is the stimulation of the innate and adaptive immune response via toll-like receptor signaling pathways and proteins down streaming, which results in the production of pro-inflammatory cytokines that inhibit the virus. Zn 2+ ions exhibit antiviral properties by preventing infection, inactivating virus adsorption/entry, blocking coating, impeding replication, assembly, and release during the virus’s life cycle, and producing reactive oxygen species [ 128 , 129 , 130 , 131 , 132 ]. Zinc inhibits the entry of viruses and viral polyprotein translation, as well as inhibiting viral RNA-dependent RNA polymerase activity, and has been shown to modulate the host immune response to limit viral replication. It is a mediator in the LPS (bacterial lipopolysaccharide)-induced TLR4 (toll-like receptor 4)-dependent MyD88 (myeloid differentiation primary response protein 88) signaling cascade, which results in early NF-κβ activation (nuclear factor-kappa b). This triggers the production of pro-inflammatory cytokines such as TNF-α (tumor necrosis factor-α), IL-1 (interleukin-1), and IL-6 to increase (interleukin-6), which plays a crucial role in the control of viral pathogens [ 133 , 134 ]. Moreover, ZnO-NPs can absorb UV–Vis light, dissociate water molecules, and release Zn 2+ ions, generating ROS such as hydrogen peroxide, hydroxyl radicals, and superoxide that disrupt the lipids, proteins, carbohydrates, and DNA of the virus, leading to its death [ 135 ]. According to Jana et al., polysaccharide-encapsulated ZnO-NPs showed exceptional antiviral action against human cytomegalovirus (HCMV), with cell survival rates of 93.6% and 92.4% at 400 µg/mL [ 136 ]. A survey reported that ZnO-NPs and PEGylated ZnO-NPs have inhibitory effects on the H1N1 influenza virus, with PEGylated ZnO-NPs showing higher anti-influenza activity with less cytotoxicity on MDCK-SIAT1 cells than ZnO-NPs, indicating that PEGylation on the surface of ZnO-NPs enhanced antiviral activity while reducing cytotoxicity [ 137 ]. A recent study on ZnO-NPs demonstrated compelling antiviral activity against SARS-CoV-2 at a very low concentration (IC 50 526 ng/mL), and it was found that ZnO-NPs can produce a large number of free radicals which ultimately induce significant damage to the membrane proteins of SARS-CoV-2. However, ZnO-NPs displayed cytotoxic levels (CC 50 292.2 ng/mL) against VERO-E6 cells [ 138 ]. Similarly, they exhibit excellent antiviral activity against the Chikungunya virus [ 139 ], and these findings suggest that ZnO-NPs might be good antiviral agents.
2.10. Cardioprotective Action of ZnO-NPs
As ZnO-NPs possess potent antioxidant activity, this gives us an idea about their use in the scavenging O 2 • — free radicals, which on the other side, possibly have cardioprotective effects. The O 2 • — free radicals are produced from lipid peroxides obtained from today’s fast foods and are made up of several flavoring/bleaching agents such as monosodium glutamate (MSG), which have several adverse effects on the heart, liver, kidney, testis, pancreas, brain, and other various tissues and organs with signs of inflammation [ 140 , 141 , 142 ]. These free radicals must be scavenged using ZnO-NPs to reduce the adverse effects of oxidative stress produced from the heart failure marker, lipid peroxidation (LPO), and lactoperoxidase-like reactive oxygen species free radicals. A study on the alleviation effect of the ZnO-NP/GTE complex on rats, through feeding two dosages of MSG and a dose of ZnO-NP/GTE (10 mg/kg) by oral gavages daily for 30 days, revealed that there was a reduction in LPO markers such as O 2 • — free radicals with a significant improvement in the level of endogenous antioxidants such as SOD, CAT, GSH, and GPx in cardiac tissue, indicating the protection against oxidative stress [ 143 ]. Thus, ZnO-NPs are believed to restore abnormal cardiac myofiber, implying their cardioprotective potential.
2.11. Anthelminthic Action of ZnO-NPs
ZnO-NPs have a strong anthelminthic effect, which is achieved by inducing oxidative stress by producing hydroxyl ions and ROS, which induces helminth membrane damage by electrostatic binding [ 144 , 145 ]. An in vitro study of ZnO-NPs on Gigantocotyle explanatum [ 146 ] revealed that they possess effective anthelminthic properties in higher concentrations. Flukes survive at lower quantities by increasing the activity of their intracellular antioxidant enzymes, SOD and GST, which scavenge reactive oxygen species [ 147 ], whereas with higher concentrations, SOD and GST possibly become saturated due to overproduction of ROS and hydroxyl ions, which leads to detoxification in flukes. These findings demonstrate sufficient evidence for the anthelminthic potential of ZnO-NPs.
3. Approaches for Synthesizing ZnO-NPs
ZnO-NPs are typically synthesized by utilizing physical, chemical, and biological processes that utilize either top-down or bottom-up approaches ( Figure 7 ). The cutting, grinding, or attrition of larger particles, followed by the formation of smaller particles at the nanoscale level, is referred to as a top-down technique. This method is commonly used for nanoparticle synthesis on a small scale [ 148 ]. The bottom-up approach is the process of synthesis of nanoparticles by gathering already miniaturized atoms/molecules through the application of chemical and physical methods. It is a cheaper method and faster than the top-down approach [ 149 ].

Synthesis approaches for ZnO-NPs.
3.1. Physical Methods
Physical methods are used to synthesize ZnO-NPs by attracting smaller molecules and atoms to produce nanoscale-sized particles that employ physical forces. Physical methods comprise ball milling, sputtering, physical vapor deposition, laser ablation, ion implantation, and electric arc deposition. Ball milling is a nonequilibrium phenomenon in which materials of a larger size are crushed with a ball mill due to collision with high-energy balls. The ball milling process has efficient production rates and is easier and more cost-effective. Salah et al. suggested that 15 spherical balls with a circumference of 20 mm concealed in a 500 mL bowl be used to form nanostructures of ZnO in a study on the antibacterial effectiveness of ZnO-NPs [ 149 ]. Laser ablation methods refer to the process of the removal of particles from the solid and liquid interface using a laser beam as an energy source. A study conducted by Mintcheva et al. provides a piece of evidence that the millisecond-pulsed laser ablation technique produced rod-shaped ZnO-NPs with lengths ranging from 40 to 110 nm and an average diameter of 30 nm [ 150 ]. Physical vapor depositions are a frequently used method in which the deposition of metals coating the surface involves two phenomena, such as evaporation and sputtering. Sputtering is the process of expelling particles from the surface by impacting high-energy particles with plasma ions [ 151 ]. Thermal evaporation is another physical approach in which powdered or condensed products are heated to a higher temperature, evaporation occurs, and the resulting vapors condense to form desirable nanoparticles under controlled conditions such as pressure, temperature, humidity, substrate, and so on [ 152 ].
3.2. Chemical Methods
The chemical methods for synthesizing ZnO-NPs are categorized based on their physical state, which includes solid-phase, liquid-phase, and gas-phase synthesis. Liquid-phase synthesis is a widespread method and a viable alternative to gaseous-phase synthesis. For liquid-phase synthesis, the sol-gel process, colloidal methods, precipitation and co-precipitation methods, microemulsion method, hydrothermal synthesis, and solvothermal and sonothermal methods can be utilized, whereas inert gas condensation methods and pyrolysis can be used for vapor-phase synthesis [ 153 ].
3.2.1. Liquid-Phase Synthesis
The sol-gel process is the process of conversion of prepared colloidal solution (sol) into gel through hydrolyzation, condensation, and polymerization reactions. Zinc acetate hydrate in alcohol is the most used precursor for the synthesis of ZnO-NPs [ 154 ]. Khan and companions synthesized pure and uniform thorn-like ZnO-NPs of a size < 50 nm for the first time by the sol-gel method [ 155 ]. Similarly, precipitation and co-precipitation methods involve the formation of a precipitate when inorganic alkalis act as a reducing agent combined with zinc salt. Sodium hydroxide and zinc sulfate heptahydrate are used as precursors, and by adjusting reaction conditions, these precipitates were washed and calcined at the requisite temperature to produce nanoparticles with the desired shape, size, and characteristics [ 156 ].
Solvothermal synthesis is a technique for facilitating a precursor interaction during synthesis by utilizing a solvent at moderate to high pressure (1–10,000 atm) and temperature (100–1000 °C) [ 157 ]. Hydrothermal synthesis, on the other hand, employs water and is normally performed below the supercritical temperature of the water, i.e., 374 °C. The microemulsion is another technique of synthesizing the thermodynamically stable dispersion of two immiscible liquids, namely, water and hydrocarbons. In general, two forms of microemulsions are utilized, such as oil-in-water (O/W) and water-in-oil (W/O), with the latter being predominantly used for the preparation of NPs by dispersing the metal salt (Zinc salt) precursor in the aqueous phase. Surfactant- and co-surfactant-charged hydrophilic groups aid to minimize interfacial tension between two phases and enhancing colloidal stability [ 158 ].
3.2.2. Gas-Phase Synthesis
The aerosol pyrolysis method is the most commonly used gas-phase synthesis method, in which aerosol droplets dispersed in the gas phase generate aerosol droplets of the precursor zinc salts when heated in a flame. The flame heating causes dehydration, which helps to reduce the size of particles in the nanoscale. The required material decomposes and sinters as a result of the heating over the flame [ 159 ]. Inert gas condensation is another major gas-phase synthesis technique. It involves evaporating zinc inside a heat-resistant compartment using a variety of heat sources, such as electron and laser beams or radio frequencies, and then condensing the vapors by migrating them to cooler chambers containing inert gas. Based on the catalyst, this approach is divided into two categories: physical vapor deposition intrigued without catalytic contact and chemical vapor deposition fascinated with catalytic interaction. It may cause agglomeration and coalescence of nanoparticles, which is a typical demerit of this process. Uhm and coworkers synthesized ZnO-NPs of a better shape and size with a 30 nm diameter by the levitational gas condensation method [ 160 ].
3.3. Green Synthesis
The terms “biological synthesis” and “green synthesis” are often used interchangeably. However, for a biological synthesis to be green, it should comply with the basic principles of green chemistry such as being environmentally friendly, no use of toxic chemicals, reduced derivatization, energy consumption, waste, and so on [ 161 ]. Here, green synthesis is the process of synthesizing nanoparticles by incorporating mainly cell extracts (microbial, plant, fungus, algae, etc.) into the substrate involving biofabrication, i.e., the capping of nanoparticles from natural products such as phytochemicals from plants and proteinous extracts from microorganisms and fungus without using any toxic chemicals. Green synthesis is to be nonhazardous, aligning with the principles of green chemistry. These methods provide merits of biocompatibility, cost-effectiveness, large-scale productivity, ecofriendliness, and being devoid of hazardous chemicals and adverse reaction conditions and are, therefore, an attractive alternative to traditional physical and chemical methods [ 162 ]. As such, microbial and plant extracts release phytochemicals that act as reducing agents as well as fabricating or stabilizing agents; this eliminates the dependence on industrial chemicals. On the contrary, if synthetic chemicals/solvents are employed to assist the reduction-stabilization process or to maintain pH in a green synthesis, such synthesis is better described as biochemical synthesis.
3.3.1. Plant-Mediated Synthesis of ZnO-NPs
A multitude of research supports the synthesis of crystalline ZnO-NPs by chelating a zinc complex with plant extracts. The aerial parts of plants, such as leaves and flowers, are commonly used in green synthesis. To optimize ZnO-NP synthesis, usually, reaction parameters such as temperature, pH, concentration, and time are adjusted. The appearance of a yellow coloration generally indicates the formation of ZnO-NPs, which is further confirmed by qualitative investigations such as UV–visible spectroscopy, SEM, and TEM [ 163 ].
The synthesis of ZnO-NPs with regulated shapes and sizes was accomplished by varying the concentration of plant extracts. Madan et al. synthesized NPs of varied sizes ranging from 9–40 nm and different shapes such as bud, cone, closed pine cone, bullet, and hexagonal disk by altering the concentrations of a plant extract from the leaves of Azadirachta indica [ 164 ]. The possible mechanism of the green synthesis has been explained by several researchers and the result is that the secondary metabolites and proteins present in the plant extracts act as capping and reducing agents which promote nanoparticle synthesis, whereas some studies have proposed that the nanoparticles of metal ions are formed due to the electrostatic interaction of plant proteins and metal ions. Proteins would reduce the metal ions, resulting in a change in the protein secondary structure, as well as in the formation of metal oxide nanoparticle seeds [ 163 , 165 ]. Plant components, from leaf to root, are extensively utilized in metal oxide nanoparticle synthesis because phytochemicals such as polyphenolic compounds, vitamins, polysaccharides, amino acids, alkaloids, terpenoids, etc. extracted from plants aid in the efficient bioreduction of metal ions for the synthesis of NPs that are stable and variable in structure and dimension. Bioreduction is the process of reducing metal ions or metal oxides to zero-valence metal NPs, fascinating in maintaining their stability. These techniques yield a large quantity of very pure nanoparticles that are free of contaminants [ 166 , 167 ]. Table 1 summarizes the key findings of extensive research on several plants employed in the synthesis of ZnO NPs.
Summary of the plant-mediated synthesis of zinc oxide nanoparticles.
3.3.2. Green Synthesis Using Bacterial Extracts
The nanoparticle synthesis using bacterial extracts is a complex and time-consuming technique of green synthesis. It is vital to ensure vigilant monitoring of the culture media throughout the process to avoid contamination. Otherwise, synthesized NPs could be less optimized and ineffective [ 2 ]. A study reported that the synthesis of ZnO-NPs can be carried out using Rhodococcus pyridinivorans and zinc sulfate as the substrate. The synthesized NPs were spherically shaped with a 100–130 nm size range confirmed through FE-SEM and XRD analysis [ 181 ]. The synthesis of nanoflowers (40 nm width and 400 nm height) with potent photocatalytic potency was also performed with B. licheniformis using the green synthesis technique [ 182 ]. The excellent antioxidant activity of NPs synthesized using Pseudomonas aeruginosa was also revealed, indicating that enhanced NP stability was attained due to the rhamnolipid of bacteria used. Thus, it is significant to consider that bacteria can be used as a better capping agent with outstanding stability and potency [ 183 ]. Green synthesis using a bacterial strain is well illustrated in Table 2 .
Summary of the bacteria-mediated synthesis of zinc oxide nanoparticles.
3.3.3. Green Synthesis Using Fungal Extracts
Due to the efficient and large-scale productivity, lower cost, and convenient processing, numerous fungal strains are being used for the green synthesis of ZnO-NPs over bacteria [ 2 ]. Fungi are more tolerable and have better metal bioaccumulative properties than bacterial strains, making them a stronger candidate for nanoparticle synthesis [ 191 ]. A study found that fungal strains such as Candida albicans could be employed to synthesize quasispherical-shaped ZnO-NPs [ 192 ]. Similarly, the mycelia of Aspergillus fumigatus were used to make spherical aggregate-shaped NPs, which agglomerate into a larger size after a few days, indicating the stability and potent capping activity of fungus as a substrate [ 193 ]. Some examples of fungal-mediated synthesis are included in Table 3 .
Summary of the fungal-mediated synthesis of zinc oxide nanoparticles.
3.3.4. Green Synthesis Using Microalgae and Macroalgae
Algae are photosynthetic organisms that are made up of single or multiple cells and lack essential components such as roots, stems, and leaves. Algae are classified into two types, macroalgae, and microalgae, as well as three groups, Rhodophyta (red pigmented), Phaeophyta (brown pigmented), and Chlorophyta (green pigmented). Algae have a limited significance in the synthesis of ZnO-NPs and are better suited for the production of other metal nanoparticles such as silver and gold nanoparticles. Microalgae are commonly employed for the green synthesis of NPs because they have a greater potential to minimize metal toxicity through the biodegradation process [ 198 ]. ZnO-NPs are typically synthesized using algae from the Sargassaceae family. Sargassum muticum was employed to make hexagonal wurtzite-shaped ZnO-NPs [ 199 ]. Similarly, nanoparticles of spherical, radial, triangular, hexagonal, and rod shapes were synthesized from S. myriocystum [ 200 ]. Furthermore, Chlamydomonas reinhardtii , a species of the Chlamydomonaceae family, was used to synthesize various-shaped NPs, such as nanorods, nanoflowers, and porous nanosheets [ 201 ]. Table 4 summarizes the ZnO-NPs synthesized by some of the algae.
Summary of the algal-mediated synthesis of zinc oxide nanoparticles.
4. Characterization of ZnO-NPs
A plethora of studies suggests that the morphology and surface chemistry of nanoparticles influence their biodistribution, safety, and effectiveness in biological systems ( Figure 8 ). Characterization is the core tool for successful applications and the understanding of nanoparticles. Nanoparticle size characterization is complicated by the polydispersity of materials, yet it is important to determine the morphology since the nanoparticle size’s resemblance to biological moieties is assumed to impart many of their distinct nanomedicine capabilities. Optical microscopy cannot resolve nanostructures; therefore, electron microscopy is used to characterize the nanoparticles. SEM and TEM are used to characterize the shapes and sizes, but TEM is used more often because it uses more powerful electrons and presents high resolution and informative image details regarding the atomic scale-like morphology, aggregation state, and distribution, and observes the functionality of capping agents/phytochemicals in enclosing NPs. Some biological molecules such as liposomes and proteins do not deflect the electron beam sufficiently and are invisible to electromagnetic radiation; therefore, dynamic light scattering (DLS), a nondestructive approach that uses a monochromatic laser and is also known as photon correlation spectroscopy, is used to characterize these compounds in suspensions and solutions. Here, small changes in the intensity of scattered laser light in the nanoparticle solution are regulated with a photon detector to analyze the hydrodynamic diameter and morphology of NPs [ 204 ].

Morphology of ZnO nanostructures: ( A ) needles, rods, and wires; ( B ) helixes and springs; ( C ) nanopellets/nanocapsules; ( D ) flower, snowflake, and dandelion; ( E ) peanut-like; ( F ) interwoven particle hierarchy; ( G ) raspberry, nanosheet/nanoplate; ( H ) circular/round or sphere-shaped. (Reprinted from [ 209 ]; open access under CC BY).
The characterization of nanoparticles in animal tissue is accomplished by energy dispersion X-ray analysis (EDX), which assists in identifying the elemental composition and linkage of metabolites and also facilitates the interpretation of biodistribution of synthesized nanoparticles. Furthermore, atomic force microscopy (AFM) helps in determining the 3D geography (height and volume) of NPs; Fourier transform infrared spectroscopy (FTIR)-attenuated total reflectance (ATR) is an easy and nondestructive technique that contributes metabolites, chemicals, etc. through the synthesis and capping of NPs; UV–visible-diffuse reflectance spectroscopy (UV-DRS) is used to study the optical property of colored samples where the reflectance measurements are utilized to investigate the surface plasmon resonance of metals and hypersensitive biological analysis [ 205 ]; thermal gravimetric-differential thermal analysis (TG-DTA) provides information about the thermal stability, phase transition, and effect of the oxidative as well as reductive environment; photoluminescence (PL) analysis is utilized to determine the band gap, and crystalline purity and impurities; and x-ray photoelectron spectroscopy (XPS) can be used to characterize the morphology, and bioactive surface and material surface chemistry of NPs [ 206 , 207 , 208 ].
ZnO is one of the most significant II-VI compound semiconductor materials in scientific research and technological applications with noncentrosymmetric structures and multiple shape-induced functions. By adjusting the hydrothermal reaction parameters (such as precursor concentration, reaction duration, and pH), several morphologies of ZnO, including microrods, hexagonal pyramid-like rods, and flower-like rod aggregates, have been synthesized, respectively, on glass substrates. The production of ZnO microrods is significantly influenced by the precursor concentration. With longer reaction times, ZnO crystals can change from hexagonal pyramids to rod-like laths. ZnO rod aggregates that resemble flowers are produced at higher pH levels. The findings could provide a strategy for producing ZnO crystals in a certain desirable form [ 210 ]. Similarly, in a recent study, Doustkhah et al. hydrothermally transformed zinc-based metal-organic frameworks into ZnO nanostructures with temperature-dependent tunable structures and catalytic activity, which at an elevated temperature displayed high crystallinity and better dye degradation efficiency than at a lower temperature [ 211 ].
Most of the group II-VI binary compound semiconductors crystallize as hexagonal wurtzite or cubic zinc-blende, with each anion surrounded by four cations at the corners of a tetrahedron. The iconicity of the II-VI compound semiconductor ZnO lies at the interface between covalent and ionic semiconductors. Wurtzite, blende, and rocksalt are potential ZnO crystal formations. Wurtzite is the most thermodynamically stable of these crystal forms at room temperature, but blende is stable when developed on a cubic substrate and rocksalt is stable when synthesized at very high temperatures [ 212 ]. In contrast to the zinc-blende structure, which has two interpenetrating face-centered-cubic (fcc) sublattices that are displaced along the body diagonal by one-quarter of a body diagonal, the wurtzite structure is made up of two interpenetrating hexagonal-closed-packed (hcp) sublattices. Due to the decrease in lattice dimensions, which favors iconicity over a covalent nature, and the structure’s six-fold coordination, wurtzite can undergo the same transformation as other II-VI semiconductors to become rocksalt [ 212 ].
5. Conclusions
This review aimed to explore the synthesis, characterization, and biological activities of ZnO-NPs, illustrating their mechanism of action. Extensive discussion was centered on the green synthesis approach and its biomedical applications. The pathways of different bioactivity were explained, with special emphasis on ZnO-NPs’ biopotency with regard to antibacterial, antifungal, anticancer, anti-inflammatory, antidiabetic, antioxidant, antiviral, wound healing, orthopedic implants, bone healing, and cardioprotective activity, along with the concise interpretation of the green synthesis of nanoparticles using biological sources. The importance and significance of ZnO-NPs in pharmaceutical and biological sectors have attracted scientists to perform an extensive study of their applications in multiple ailments. Green synthesis is an eco-friendly approach that reduces costs, increases production, and improves biocompatibility in humans. Biofabrication with natural compounds helps to stabilize the nanoparticles with reduced toxicity and higher reduction potential. ZnO-NPs possess several compelling pharmacological activities. Special focus should be given to ZnO-NP generation through plant-mediated synthesis, bearing tremendous applications in the fields of pharmaceuticals, food, and cosmetics. The advancement of nanotechnology in the formulation of metal oxide nanoparticles can contribute to the reduction in the dosage used with optimum desired effects and low toxicity.
Acknowledgments
We are thankful to Arpita Roy, Sharda University, India, for her feedback on the manuscript.
Abbreviations
ZnO-NPs: zinc oxide nanoparticles; ROS: reactive oxygen species; SOD: superoxide dismutase; GSTs: glutathione S-transferases; NPs: nanoparticles; SEM: scanning electron microscopy; TEM: transmission electron microscopy; XRD: X-ray diffractometer; DLS: dynamic light scattering; EM: electron microscopy; HRTEM: high-resolution transmission electron microscopy; HRSEM: high-resolution scanning electron microscopy; FE-SEM: filed emission scanning electron microscopy; AFM: atomic force microscopy; GSH: glutathione; GPx: glutathione peroxidase; MSG: monosodium glutamate; DPPH: 2,2-diphenyl-1-picrylhydrazyl; NEFA: nonesterified fatty acid; iNOS: inducible nitric oxide synthase; PGE2: prostaglandin E2; NF-κβ: nuclear factor-kappa b; COX 2: cyclooxygenases 2; IL-1: interleukin-1; TNF: tumor necrosis factor; IL-6: interleukin-6; IL-12: interleukin-12; IL-18: interleukin-18; ATR: attenuated total reflection; EDAX: energy dispersion analysis of X-ray; PL: photoluminescence; XPS: X-ray photoelectron microscopy; TG-DTA: thermal gravimetric-differential thermal analysis; UV-DRS: UV–visible reflectance spectroscopy; BMs: biodegradable metals; ALP: alkaline phosphatase; hBMSCs: human bone marrow-derived mesenchymal stem cells; hDPSCs: human dental pulp stem cells; MDR: multidrug-resistant; HPMC: hydroxypropyl methylcellulose; FBS: fasting blood sugar; CAT: catalase.
Funding Statement
Tettey and Bhattarai acknowledge funding support in part from National Science Foundation (EiR-2100861) for their contribution to this review work.
Author Contributions
Conceptualization, N.P. and N.B.; methodology, A.K.M.; writing—original draft preparation, A.K.M., S.B., A.G., F.T., N.B., A.K.S. and N.P.; writing—review and editing, S.K., S.J. and D.P.B.; editing images, S.B. and F.T.; supervision and project administration, N.P. and N.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- Reference Manager
- Simple TEXT file
People also looked at
Mini review article, zinc oxide nanoparticles: a review on its applications in dentistry.

- 1 Department of Pedodontics and Preventive Dentistry, Faculty of Dental Sciences, M.S. Ramaiah University of Applied Sciences, Bangalore, India
- 2 Department of Oral Pathology & Microbiology, Faculty of Dental Sciences, M.S. Ramaiah University of Applied Sciences, Bangalore, India
- 3 Oral Biology Department, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia
- 4 Restorative Dentistry Department, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia
- 5 Department of Restorative Dental Science, College of Dentistry, Shwajra Campus, Jazan University, Jazan, Saudi Arabia
- 6 Department of Maxillofacial Surgery and Diagnostic Sciences, Division of Oral Pathology, College of Dentistry, Shwajra Campus, Jazan University, Jazan, Saudi Arabia
Nanotechnology in modern material science is a research hot spot due to its ability to provide novel applications in the field of dentistry. Zinc Oxide Nanoparticles (ZnO NPs) are metal oxide nanoparticles that open new opportunities for biomedical applications that range from diagnosis to treatment. The domains of these nanoparticles are wide and diverse and include the effects brought about due to the anti-microbial, regenerative, and mechanical properties. The applications include enhancing the anti-bacterial properties of existing restorative materials, as an anti-sensitivity agent in toothpastes, as an anti-microbial and anti-fungal agent against pathogenic oral microflora, as a dental implant coating, to improve the anti-fungal effect of denture bases in rehabilitative dentistry, remineralizing cervical dentinal lesions, increasing the stability of local drug delivery agents and other applications.
1 Introduction
Nanotechnology, wherein matter is manipulated on a molecular scale, has revolutionized modern dentistry. “Nanodentistry” is the amalgamation of nanotechnology and dentistry and provides the scope for the formulation of innovative materials that can have many potential applications in clinical practice. The nano size confers a larger surface area, allows the controlled synthesis and is also capable of altering the desired physical and chemical properties that enables them for unique interactions with biomolecules. They also have a higher percentage of surface atoms, which maximized their ability due to an increase in surface reactivity ( Rasmussen et al., 2010 ).
Zinc is an essential trace element which is found in the muscle, bone, skin and also in the hard tissues of the tooth. Zinc Oxide Nanoparticle (ZnO NP) is a white colored odorless powder and has a molecular weight of 81.38 g/mol. FDA considers it as a generally recognized as safe (GRAS) substance. Its extensive applications in dentistry are credited to the unique optical, magnetic, morphological, electrical, catalytic, mechanical, and photochemical properties which can be easily altered as per the requirements: by modifying the size, doping with supplementary compounds, or adjusting the conditions of synthesis. As the size of the particles decrease, the desirable characteristics improve ( Baek et al., 2012 ).
In the present, ZnO NPs are being investigated as associates of anti-microbial agents which are one of the most important reasons for its use. A recent theory that explains this is the “Trojan Horse effect”, which states that the acidic lysosomal environment promotes nanoparticle degradation, that in turn brings about conversion of core metals to ions and the release of substances that are toxic and in turn interrupt cell reproduction. Other mechanisms of their anti-microbial action are by locally changing the microenvironments near the microbes and by producing reactive oxygen species (ROS) or by increasing solubility of these nanoparticles. This can induce interplay with -SH group of the enzymes in the microbes and cause malfunction of organelles causing denaturation of the proteins and resulting in damage to DNA. This in turn alters the DNA replication of the microorganisms. Another possible anti-microbial mechanismis by the release of H 2 O 2 ( Şuhani et al., 2018 ) and by the displacement of Magnesium ions which interferes with the metabolism of the bacteria. The enhanced effect against microbes is attributed to the increased ratio of surface/volume. Hence, the incorporation of ZnO NPs in dental restorative materials, luting materials, tissue conditioners, intracanal medicaments, irrigants, adhesives and other materials can have beneficial anti-microbial effects.
Further research is also being done on this nanoparticle, due to the unlimited fields of application such as regarding its anti-inflammatory activity in response to pathogens, its anti-demineralizing and remineralizing effect on the hard tissues of the tooth, its potential as an anti-cancer agent and many others ( Carrouel et al., 2020 ; Wiesmann et al., 2020 ). ZnO NPs hence have widespread applications in the field of restorative dentistry, endodontics, regenerative endodontics, prosthetic dentistry, orthodontics, preventive dentistry, implantology and periodontology ( Moradpoor et al., 2021 ). Although ZnO NPs are considered to be a biologically safe material that does not exhibit cell toxicity, however, further research into the regulatory and safety concerns in oral care products on long term use must be discussed, questioned and further researched upon. Majority of the research regarding these NPs are limited to in-vitro studies and few animal studies. Therefore, further investigations and clinical trials must be carried out in order to utilize it to its full potential.
2 Applications of Zinc Oxide Nanoparticles in Dentistry
Zinc Oxide Nanoparticles have a wide range of applications in the various branches of dentistry, such as in the field of restorative dentistry, endodontics, regenerative endodontics, periodontics, prosthodontics, orthodontics, oral medicine, cancer diagnosis, dental implantology, preventive dentistry and biomedical waste management. The research performed using these nanoparticles are summarized in Table1 and Figure 1 .
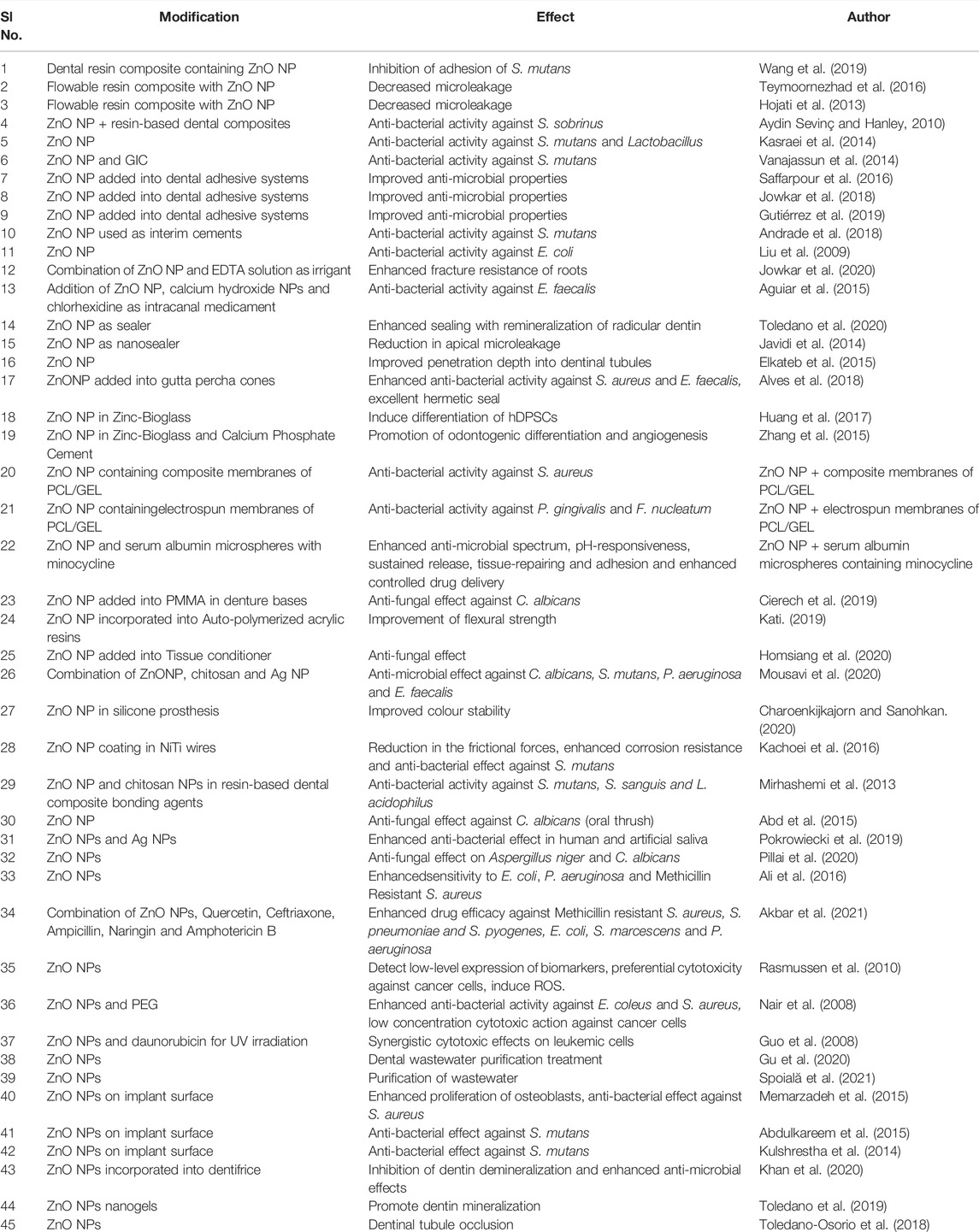
TABLE 1 . Studies focussing on the Applications of ZnO NPs in dentistry.
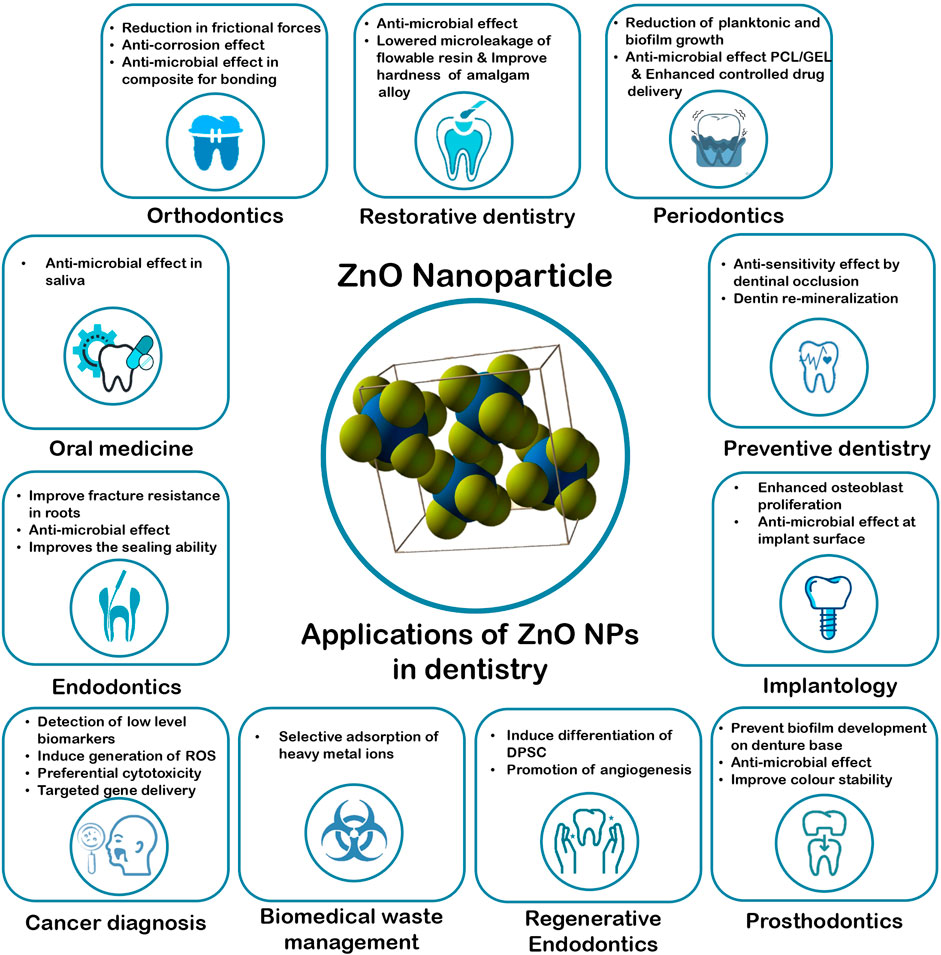
FIGURE 1 . Applications of ZnO NPs in Dentistry.
2.1 Restorative Dentistry
ZnO NPs have been found to improve the mechanical and anti-bacterial properties of dental restorative materials. According to a study by Wang et al., it was reported that when ZnO NPs were incorporated in dental resin composites, there was inhibition in the growth and adhesion of S. mutans , and in small amounts did not affect the mechanical properties. This is extremely beneficial in not only in the prevention of secondary caries but also in the interception of bulk fracture of the material ( Wang et al., 2019 ). Similarly, in a study done by Teymoornezhad et al., it was reported that incorporation of 3% ZnO NPs on flowable resin composite lowered the microleakage ( Teymoornezhad et al., 2016 ). A comparable outcome was reported in the study by Hojati et al. (2013), on flowable resin composite ( Tavassoli Hojati et al., 2013 ). When 10% ZnO NPs was added to resin-based dental composites, it showed anti-bacterial effectiveness against S. Sobrinus ( Aydin Sevinç and Hanley 2010 ). These NPs were also found to exhibit anti-bacterial activity against S. mutans and Lactobacillus ( Kasraei et al., 2014 ). ZnO NPs when incorporated in Glass Ionomer Cement (GIC) was also found to significantly improve the anti-bacterial properties against S. mutans without altering the mechanical properties ( Vanajassun et al., 2014 ).
In various studies, it has been reported that incorporation of ZnO NPs into dental adhesive systems significantly improved the anti-microbial properties without affecting the bond strength adversely ( Saffarpour et al., 2016 ; Jowkar et al., 2018 ; Gutiérrez et al., 2019 ). Zinc particles have proven to produce a strong bond at the interface of the dentin and resin by bringing about a decrease in the degeneration of collagen. The hardness of amalgam alloy was found to increase in proportion with the percentage loading of ZnO NPs ( Yahya et al., 2013 ).
ZnO NPs when added to interim cements also exhibited anti-bacterial activity against S. mutans ( Andrade et al., 2018 ). ZnO NPs were found to alter the lipid and protein contents of the cell membranes of E. coli , which caused distortion leading to leakage of cellular components, ultimately resulting in death ( Liu et al., 2009 ). These properties are extremely beneficial in preventing the occurrence of secondary caries.
However, it was reported that the addition of 1% and 2% by weight of ZnO NPs into GIC did not exhibit anti-microbial activity against strains of S. mutans . This might be attributed to the inherent anti-bacterial property of the cement ( Garcia et al., 2017 ). The incorporation of nano-spherical and nano-flower ZnO NPs to GIC was found to decrease the surface hardness, without affecting the flexural strength while incorporation of nano-rod ZnO NPs had no effect on the mechanical properties ( Panahandeh et al., 2018 ). In another study done by Wang et al., it was reported that with the increase in the quantity of ZnO NPs, there was a decrease in the mechanical properties of dental composite resins, with the exception of flexural strength, which may be attributed to the agglomeration of the nanoparticles ( Wang et al., 2019 ). In a systematic review by Arun et al., on the anti-bacterial properties of composite material incorporated with ZnO NPs, it was concluded that the material is unlikely to present a clinical advantage due to the short lifetime of anti-bacterial properties and the poor results against multi-species biofilms ( Arun et al., 2021 ).
2.2 Endodontics
The applications of ZnO NPs in endodontics are diverse. In a study by Jowkar et al., When incorporated in EDTA solution for irrigation, the fracture resistance of the roots was enhanced ( Jowkar et al., 2020a ). In a study done by Aguiar et al., it was reported that these NPs promoted alkalinization and action against E. faecalis when used as an intracanal medicament along with calcium hydroxide NPs and chlorhexidine ( Aguiar et al., 2015 ). ZnO NPs when used as an sealer after endodontic therapy was found to exhibit excellent sealing efficacy along with remineralization of the radicular dentin thereby strengthening the tooth ( Toledano et al., 2020 ). It was also reported that ZnO NPs brought about a reduction in the apical microleakage when used as a nano-sealer in endodontics ( Javidi et al., 2014 ). It also significantly improved the penetration depth into the dentinal tubules ( Elkateb et al., 2015 ). Pristine gutta percha cones that were pre-treated argon plasma treatment and coated with ZnO NPs were found to exhibit antibacterial activity against S. aureus and E. fecalis which provides an excellent hermetic seal thereby reducing chances of reinfection and subsequent endodontic failure ( Alves et al., 2018 ).
However, a study done by Jowkar et al., showed that push-out bond strength of the fiber posts did not improve on the addition of ZnO NPs ( Jowkar et al., 2020b ). When incorporated into Portland cement (PC) along with ZrO 2 , it was found not to impede with the anti-biofilm activity and to provide radiopacity to the cement. Also, the presence of ZnO NPs significantly reduced the compressive strength of the material ( Guerreiro-Tanomaru et al., 2014 ).
2.3 Regenerative Endodontics
Incorporation of these NPs along with SiO 2 , Na 2 O, CaO and P 2 O 5 to formulate Zinc-Bioglass, was reported to induce the differentiation of human Dental Pulp Stem Cells (hDPSCs) by bringing about an increase in the ALP activity ( Huang et al., 2017 ). Similarly, it was reported that Zinc-Bioglass when incorporated with Calcium Phosphate Cement brought about odontogenic differentiation and also promoted angiogenesis by activating the Wnt, integrin, NF-kB, and MAPK pathways ( Zhang et al., 2015 ). These play a pivotal role in the regeneration of the dentin-pulp tissues.
2.4 Periodontics
In the field of periodontal regeneration using guided tissue regeneration, the loading of ZnO NPs into composite membranes of polycaprolactone (PCL) and gelatin (GEL) which were electrospun, brought about reduction in the planktonic and the biofilm growth of the S. aureus significantly. These local anti-bacterial properties brought about enhancement in the clinical prognosis of treatments ( Prado-Prone et al., 2020 ). Similarly, when ZnO NPs were incorporated in electrospun membranes made of PCL and PCL/GEL, it showed anti-bacterial activity against P. gingivalis and F. nucleatum species which in turn brought about an enhanced and better predictable periodontal regeneration ( Münchow et al., 2015 ). ZnO NPs and serum albumin microspheres containing minocycline when incorporated in a Carbopol hydrogel exhibited enhancement of properties such as the anti-microbial spectrum, pH-responsiveness, sustained release, tissue-repairing and adhesion, and also enhanced controlled drug delivery that can increase stability of the drug ( Mou et al., 2019 ).
2.5 Prosthodontics
The incorporation of ZnO NPs into the PMMA in denture bases was found to prevent biofilm development by C. albicans without exerting a cytotoxic effect on the host cells. Further research can advocate its application as a novel denture base material ( Cierech et al., 2019 ). ZnO NPs in concentrations of 1wt% and 2wt% when incorporated in auto-polymerized acrylic resins was found to improve the flexural strength significantly ( Kati 2019 ). In a study wherein 15 wt% ZnO NPs were incorporated into the tissue conditioner was also found to exhibit an anti-fungal effect ( Homsiang et al., 2020 ). In another study, it was assessed that ZnO NPs along with chitosan and Silver NPs in the concentration of 2.5% inhibited the growth of C. albicans , and at a concentration of 5% inhibited the growth of S. mutans, P. aeruginosa and E. faecalis ( Mousavi et al., 2020 ). The incorporation of 1.5% of ZnO NPs was found to improve the colour stability of silicone prosthesis ( Charoenkijkajorn and Sanohkan 2020 ).
2.6 Orthodontics
Nanoparticles have been used in orthodontics to improve the quality of orthodontic treatment either in the form of nano-coated archwires, orthodontic adhesives, and orthodontic brackets ( Tahmasbi et al., 2019 ; Moradpoor et al., 2021 ). The zinc oxide nanoparticles coated orthodontic appliances minimise bacterial adhesion and enamel demineralization due to its antimicrobial and remineralization potential. Even attempts are made to add ZNO NPs into both orthodontic attachments and bonding materials since they provide a platform for bacterial attachment ( Jatania and Shivalinga 2014 ; Riad et al., 2015 ; Reddy et al., 2016 ; Tahmasbi et al., 2019 ).
It was reported that coating of the NiTi wires with ZnO NPs brought about reduction in the frictional forces by 21% and exhibited anti-bacterial activity against S. mutans . It was also reported that ZnO NPs exhibited anti-corrosion effect that enhanced the corrosion resistance propertiesin the orthodontic wires ( Kachoei et al., 2016 ). When a mixture of 10% weight each of ZnO NPs and chitosan NPs was incorporated into are resin-based dental composite bonding agents for the placement of brackets, it exhibited anti-bacterial activity against S. mutans, S. sanguis and L. acidophilus . This can significantly bring about reduction in the incidence of white-spot lesions during orthodontic therapy ( Mirhashemi et al., 2013 ). Another study investigated that ZNO and CuO NPs coated orthodontic brackets showed better antibacterial activity against S. mutans , thus reducing the incidence of dental caries ( Ramazanzadeh et al., 2015 ). It has been reported that when both orthodontic wires and brackets were coated with ZnO NPs the antibacterial potential against S. mutans was enhanced and reduced the frictional forces of coated wires ( Behroozian et al., 2016 ). Similarly the stainless steel wires and orthodontic brackets coated with chitosan NPs or ZnO NPs reduced the friction between orthodontic brackets and a Stainless steel wire thus enhances the anchorage control and root resorption risk ( Elhelbawy and Ellaithy 2021 ). Europium ions doped ZnO NPs were incorporated has orthodontic nanoadhesive enhanced the visibility of material for thorough removal of orthodontic adhesive after completion of treatment ( Yamagata et al., 2012 ). It has been reported that orthodontic adhesive with less titanium dioxide, zinc oxide, and silver NPs causes bracket failure because the combination reduces shear bond strength ( Reddy et al., 2016 ). The addition of ZnO to a light cure resin modified GIC as an orthodontic bonding agent improved the original compound’s antimicrobial, physical, and flexural properties ( Nuri Sari et al., 2015 ). Hence ZnO NPs have the potential to be widely used in orthodontic applications to improve treatment outcomes, including increased strength of materials and reduced bacterial count around the orthodontic appliance.
2.7 Oral Medicine
ZnO NPs have an inhibitory effect on C. albicans in saliva and hence can be used in the treatment of oral thrush, starting from a concentration of 0.05 mg/ml. It was also reported that ZnO NPs along with Silver NPs exhibited enhanced anti-bacterial effect in human and artificial saliva, which can have widespread applications in clinical scenarios ( Pokrowiecki et al., 2019 ). ZnO NPs which are biosynthesized from Beta vulgaris was found to exhibit anti-fungal effect on pathogens such as Aspergillus niger and C. albicans ( Pillai et al., 2020 ). ZnO NPs that are synthesized from Aloe vera leaf extract have been demonstrated to exhibit pronounced sensitivity to E. coli , P. aeruginosa and Methicillin Resistant S. aureus, and hence can be considered as a promising candidate for nano-antibiotics, which deals with the enhancement of the effect against the bacterial strains that are resilient to conventional antibiotics ( Ali et al., 2016 ). These NPs when conjugated with drugs such as Quercetin, Ceftriaxone, Ampicillin, Naringin and Amphotericin B showed enhanced drug efficacy against Methicillin resistant S. aureus, S. pneumoniae, S. pyogenes, E. coli, Serratia marcescens and P. aeruginosa. Hence, they provide a propitious approach in the combat against disease resistant pathogens ( Akbar et al., 2021 ).
2.8 Cancer Diagnosis
ZnO NPs can be implicated in the diagnosis of cancers as it is proven to detect low-level expression of biomarkers which are used for early cancer detection. In vitro , it exhibits an inherent preferential cytotoxicity against cancer cells. It also possesses the ability to induce the generation of Reactive Oxygen Species (ROS) due to its semiconductor properties, as the electrons within the NPs can react with O 2 or hydroxyl ions or water after migrating to the surface to form superoxide and hydroxyl radicals ( Manthe et al., 2010 ). These can set about cell death when the anti-oxidative capacity of the cell is exceeded. Research is being carried out on the utilization of ZnO NPs for gene silencing and targeted gene delivery, which can be utilized to combat cancer ( Rasmussen et al., 2010 ). ZnO NPs that were coated with polyethylene glycol (PEG) were found to exhibit enhanced anti-bacterial activity against E. coleus and S. aureus by bringing about damage to the cell membrane. They were also found to exhibit a low concentration threshold for cytotoxic action, with a which is due to the upregulation of the Fas ligand on the cell membrane which brings about apoptosis of the cancer cells ( Nair et al., 2008 ). ZnO NPs also exhibits an efficient role in non-surgical tumor ablation method used in cancer therapy. It was demonstrated that ZNO NPs when combined with anti-cancer drug daunorubicin, along with Ultra Violet irradiation, exhibited synergistic cytotoxic effects on the leukemic cells ( Guo et al., 2008 ).
2.9 Biomedical Waste Management
The ZnO NPs are found to selectively remove heavy metal ions such as Chromium by adsorption by virtue of their hydroxyl ions. It can therefore be used in dental waste water purification treatment as a green pollutant-diminishing strategy ( Gu et al., 2020 ). Other studies have proven the efficacy of ZnO NPs which can be used in nano-composite membranes used for the purification of water. This is due to its antibacterial activity against S. aureus and the favourable photocatalytic activity, which enhances the adsorption of organic pollutants, pesticides and microbes that are found in the wastewaterrendering it safe ( Spoială et al., 2021 ).
2.10 Implantology
Chemical modifications of dental implant surfaces with ZNO NPs, which are effective antimicrobial agents, have been carried out in order to reduce the risk of dental implant failure and improve osteointegration. On coating the implant surface with ZnO NPs, the underlying osteoblast cells exhibited an enhanced proliferation after 5 and 10 days. They also exhibited anti-microbial properties against S. aureus . These properties are useful to promote bone growth and in the inhibition of infection at the implant site ( Memarzadeh et al., 2015 ). Similar results were reported in studies by Abdulkareem et al. and Kulshrestha et al., on the effect of ZnO NPs against S. mutans biofilm on dental implant surfaces ( Kulshrestha et al., 2014 ; Abdulkareem et al., 2015 ). According to the findings, ZNO bio-functionalized thin films containing DMP1 peptides can improve the physicochemical, osteogenic, apatite nucleation and corrosion resistance properties of this material suggesting promising applications in dental implant ( Trino et al., 2018 ).
Titania (Ti)-zinc (Zn)-oxide nanocomposite-(nC) thin films were co-sputtered to strengthen the cohesiveness of metallic fixtures with bone. The developed thin film also exhibited strong antibacterial activity against S. aureus and E. coli ( Goel et al., 2019 ). Modified titanium implant materials developed using N-halamine and ZnO nanoparticles demonstrated remarkable antibacterial activity against P. aeruginosa , E. coli , and S. aureus without using antibiotics ( Li et al., 2017 ). Titanium implants with coatings of Poly (lactic-co-glycolic acid)/Silver/ZnO nanorods demonstrated long-lasting antibacterial activity against S. aureus and E. coli , as well as excellent cytocompatibility and biocompatibility ( Xiang et al., 2017 ).
2.11 Preventive Dentistry
ZnO NPs incorporated in a dentifrice was found to cause dentinal tubule occlusion. These can also be incorporated as preservatives in dentifrices as it not only brings about inhibition of dentin demineralization but also exhibits enhanced anti-microbial effects ( Khan et al., 2020 ). Its incorporation and in nanogels and application on eroded cervical dentin, was found to promote dentin mineralization ( Toledano et al., 2019 ). These can be utilized in achieving an anti-sensitivity effect. Studies have shown that dentin which is treated with ZnO NPs exhibited greater ability to produce dentinal tubule occlusion which makes it an effective agent in the treatment of dentinal hypersensitivity 67]. The ZnO NPs treated dentin was found to have higher levels of proteoglycans that act as bonding agents between the HAp crystals and collagen network. Further, they enhance the release small integrin-binding ligand N-linked glycoproteins and small leucine-rich proteoglycans from dentin through Matrix Metalloproteinase-3 activity. These proteins take part in the mineralization of dentin, and the immobilized phosphorylated proteins induce formation of mineral. Zinc NPs also reduces the collagen degradation which is mediated by Matrix Metalloproteinase-3 in dentin that is partially demineralized and hence promotes dentin re-mineralization ( Toledano-Osorio et al., 2018 ; Toledano et al., 2019 ).

3 Conclusion
Nano-dentistry has opened a new standpoint for revolution in oral care and portrays a growing field with the capability to address the new and improved applications in dentistry. ZnO NPs have a broad spectrum of applications the various fields of dentistry such as restorative dentistry, endodontics, regenerative endodontics, periodontology, prosthodontics, orthodontics, implantology, preventive dentistry among other fields. The use of ZnO NPs represents a broadening horizon for the diagnosis, treatment, and prevention of various oral conditions, and in enhancing the characteristics of existing dental materials. It is hence crucial to strengthen the symbiosis between cliniciansand materials scientists asnano-dentistry is still technologydriven, with many roadblocks ahead. However, most of the research is still in the development pipeline and for realizing the complete in vivo potential in dentistry, further research that focus on its clinical implications should be carried out.
Author Contributions
PC: Conceptualization, Resources, Data Curation, Original Draft Preparation, Supervision. GS: Conceptualization, Resources, Data Curation, Original Draft Preparation. SV: Original Draft Preparation, Review & Editing, Visualization. DA: Original Draft Preparation, Review & Editing, Visualization. AA: Review & Editing, Visualization, Supervision. BZ: Review & Editing, Visualization, Supervision. NA: Review & Editing, Visualization, Supervision. SP: Original Draft Preparation, Review & Editing, Visualization, Supervision. All authors agree to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank MS Ramaiah University of Applied Sciences for the support.
Abdulkareem, E. H., Memarzadeh, K., Allaker, R. P., Huang, J., Pratten, J., and Spratt, D. (2015). Anti-biofilm Activity of Zinc Oxide and Hydroxyapatite Nanoparticles as Dental Implant Coating Materials. J. Dent. 43, 1462–1469. doi:10.1016/j.jdent.2015.10.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Aguiar, A. S., Guerreiro-Tanomaru, J. M., Faria, G., Leonardo, R. T., and Tanomaru-Filho, M. (2015). J. Contemp. Dent. P. R. 16, 624–629.
PubMed Abstract | CrossRef Full Text
Akbar, N., Aslam, Z., Siddiqui, R., Shah, M. R., and Khan, N. A. (2021). Zinc Oxide Nanoparticles Conjugated with Clinically-Approved Medicines as Potential Antibacterial Molecules. Amb. Expr. 11, 104. doi:10.1186/s13568-021-01261-1
CrossRef Full Text | Google Scholar
Ali, K., Dwivedi, S., Azam, A., Saquib, Q., Al-Said, M. S., Alkhedhairy, A. A., et al. (2016). Aloe Vera Extract Functionalized Zinc Oxide Nanoparticles as Nanoantibiotics against Multi-Drug Resistant Clinical Bacterial Isolates. J. Colloid Interface Sci. 472, 145–156. doi:10.1016/j.jcis.2016.03.021
Alves, M. J., Grenho, L., Lopes, C., Borges, J., Vaz, F., Vaz, I. P., et al. (2018). Antibacterial Effect and Biocompatibility of a Novel Nanostructured ZnO-Coated Gutta-Percha Cone for Improved Endodontic Treatment. Mater. Sci. Eng. C 92, 840–848. doi:10.1016/j.msec.2018.07.045
Andrade, V., Martínez, A., Rojas, N., Bello-Toledo, H., Flores, P., Sánchez-Sanhueza, G., et al. (2018). J. Prosthet. Dent. 119, 862–e1. doi:10.1016/j.prosdent.2017.09.015
Arun, D., Adikari Mudiyanselage, D., Gulam Mohamed, R., Liddell, M., Monsur Hassan, N. M., and Sharma, D. (2021). Materials 14, 40.
Aydin Sevinç, B., and Hanley, L. (2010). J. Biomed. Mat. Res. B Appl. Biomater. 94, 22–31.
Baek, M., Chung, H.-E., Yu, J., Lee, J.-A., Kim, T.-H., Oh, J.-M., et al. (2012). Int. J. Nanomedicine 7, 3081.
PubMed Abstract |
Behroozian, A., Kachoei, M., Khatamian, M., and Divband, B. (2016). The Effect of ZnO Nanoparticle Coating on the Frictionalresistance between Orthodontic Wires and Ceramic Brackets. J. Dent. Res. Dent. Clin. Dent. Prospects 10 (2), 106–111. doi:10.15171/joddd.2016.017
Carrouel, F., Viennot, S., Ottolenghi, L., Gaillard, C., and Bourgeois, D. (2020). Nanoparticles as Anti-microbial, Anti-inflammatory, and Remineralizing Agents in Oral Care Cosmetics: a Review of the Current Situation. Nanomaterials 10 (1), 140. doi:10.3390/nano10010140
Charoenkijkajorn, D., and Sanohkan, S. (2020). The Effect of Nano Zinc Oxide Particles on Color Stability of MDX4-4210 Silicone Prostheses. Eur. J. Dent. 14, 525–532. doi:10.1055/s-0040-1713058
Cierech, M., Wojnarowicz, J., Kolenda, A., Krawczyk-Balska, A., Prochwicz, E., Woźniak, B., et al. (2019). Zinc Oxide Nanoparticles Cytotoxicity and Release from Newly Formed PMMA-ZnO Nanocomposites Designed for Denture Bases. Nanomaterials 9, 1318. doi:10.3390/nano9091318
Elhelbawy, N., and Ellaithy, M. (2021). Comparative Evaluation of Stainless-Steel Wires and Brackets Coated with Nanoparticles of Chitosan or Zinc Oxide upon Friction: An In Vitro Study. Int. Orthod. 19 (2), 274–280. doi:10.1016/j.ortho.2021.01.009
Elkateb, W., Massoud, A., and Mokhless, N. (2015). Trans. Shalaby , 11.
Garcia, P. P. N. S., Cardia, M. F. B., Francisconi, R. S., Dovigo, L. N., Spolidório, D. M. P., de Souza Rastelli, A. N., et al. (2017). Antibacterial Activity of Glass Ionomer Cement Modified by Zinc Oxide Nanoparticles. Microsc. Res. Tech. 80, 456–461. doi:10.1002/jemt.22814
Goel, S., Dubey, P., Ray, S., Jayaganthan, R., Pant, A. B., and Chandra, R. (2019). Co-sputtered Antibacterial and Biocompatible Nanocomposite Titania-Zinc Oxide Thin Films on Si Substrates for Dental Implant Applications. Mater. Technol. 34 (1), 32–42. doi:10.1080/10667857.2018.1488924
Gu, M., Hao, L., Wang, Y., Li, X., Chen, Y., Li, W., et al. (2020). The Selective Heavy Metal Ions Adsorption of Zinc Oxide Nanoparticles from Dental Wastewater. Chem. Phys. 534, 110750. doi:10.1016/j.chemphys.2020.110750
Guerreiro-Tanomaru, J. M., Trindade-Junior, A., Cesar Costa, B., da Silva, G. F., DrullisCifali, L., Basso Bernardi, M. I., et al. (2014). Sci. World J. 2014. doi:10.1155/2014/975213
CrossRef Full Text
Guo, D., Wu, C., Jiang, H., Li, Q., Wang, X., and Chen, B. (2008). Synergistic Cytotoxic Effect of Different Sized ZnO Nanoparticles and Daunorubicin against Leukemia Cancer Cells under UV Irradiation. J. Photochem. Photobiol. B Biol. 93, 119–126. doi:10.1016/j.jphotobiol.2008.07.009
Gutiérrez, M. F., Alegría-Acevedo, L. F., Méndez-Bauer, L., Bermudez, J., Dávila-Sánchez, A., Buvinic, S., et al. (2019). J. Dent. 82, 45–55.
Homsiang, W., Kamonkhantikul, K., Arksornnukit, M., and Takahashi, H. (2020). Dent. Mat. J.
Huang, M., Hill, R. G., and Rawlinson, S. C. F. (2017). Zinc Bioglasses Regulate Mineralization in Human Dental Pulp Stem Cells. Dent. Mater. 33, 543–552. doi:10.1016/j.dental.2017.03.011
Jatania, A., and Shivalinga, B. M. (2014). An In Vitro Study to Evaluate the Effects of Addition of Zinc Oxide to an Orthodontic Bonding Agent. Eur. J. Dent. 08 (01), 112–117. doi:10.4103/1305-7456.126262
Javidi, M., Zarei, M., Naghavi, N., Mortazavi, M., and Nejat, A. H. (2014). Contemp. Clin. Dent. 5, 20.
Jowkar, Z., Farpour, N., Koohpeima, F., Mokhtari, M. J., and Shafiei, F. (2018). J. Contemp. Dent. P. R. 19, 1404–1411.
Jowkar, Z., Hamidi, S. A., Shafiei, F., and Ghahramani, Y. (2020). The Effect of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Final Irrigation Solutions on the Fracture Resistance of Root-Filled Teeth. Ccide Vol. 12, 141–148. doi:10.2147/ccide.s253251
Jowkar, Z., Omidi, Y., and Shafiei, F. (2020). J. Clin. Exp. Dent. 12, e249.
Kachoei, M., Nourian, A., Divband, B., Kachoei, Z., and Shirazi, S. (2016). Zinc-oxide Nanocoating for Improvement of the Antibacterial and Frictional Behavior of Nickel-Titanium Alloy. Nanomedicine 11, 2511–2527. doi:10.2217/nnm-2016-0171
Kasraei, S., Sami, L., Hendi, S., AliKhani, M.-Y., Rezaei-Soufi, L., and Khamverdi, Z. (2014). Antibacterial Properties of Composite Resins Incorporating Silver and Zinc Oxide Nanoparticles onStreptococcus mutansandLactobacillus. Restor. Dent. Endod. 39, 109–114. doi:10.5395/rde.2014.39.2.109
Kati, F. A. (2019). Effect of the Incorporation of Zinc Oxide Nanoparticles on the Flexural Strength of Auto- Polymerized Acrylic Resins. J. Oral Res. 8, 37–41. doi:10.17126/joralres.2019.010
Khan, A. S., Farooq, I., Alakrawi, K. M., Khalid, H., Saadi, O. W., and Hakeem, A. S. (2020). Dentin Tubule Occlusion Potential of Novel Dentifrices Having Fluoride Containing Bioactive Glass and Zinc Oxide Nanoparticles. Med. Princ. Pract. 29, 338–346. doi:10.1159/000503706
Kulshrestha, S., Khan, S., Meena, R., Singh, B. R., and Khan, A. U. (2014). A Graphene/zinc Oxide Nanocomposite Film Protects Dental Implant Surfaces against cariogenicStreptococcus Mutans. Biofouling 30, 1281–1294. doi:10.1080/08927014.2014.983093
Li, Y., Liu, X., Tan, L., Cui, Z., Yang, X., Yeung, K. W. K., et al. (2017). Construction of N-Halamine Labeled Silica/zinc Oxide Hybrid Nanoparticles for Enhancing Antibacterial Ability of Ti Implants. Mater. Sci. Eng. C 76, 50–58. doi:10.1016/j.msec.2017.02.160
Liu, Y., He, L., Mustapha, A., Li, H., Hu, Z. Q., and Lin, M. (2009). Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 107, 1193–1201. doi:10.1111/j.1365-2672.2009.04303.x
Manthe, R. L., Foy, S. P., Krishnamurthy, N., Sharma, B., and Labhasetwar, V. (2010). Tumor Ablation and Nanotechnology. Mol. Pharm. 7, 1880–1898. doi:10.1021/mp1001944
Memarzadeh, K., Sharili, A. S., Huang, J., Rawlinson, S. C. F., and Allaker, R. P. (2015). Nanoparticulate Zinc Oxide as a Coating Material for Orthopedic and Dental Implants. J. Biomed. Mat. Res. 103, 981–989. doi:10.1002/jbm.a.35241
Mirhashemi, A., Bahador, A., Kassaee, M., Daryakenari, G., Ahmad-Akhoundi, M., and Sodagar, A. (2013). J. Med. Bacteriol. 2, 1–10.
Moradpoor, H., Safaei, M., Mozaffari, H. R., Sharifi, R., Imani, M. M., Golshah, A., et al. (2021). An Overview of Recent Progress in Dental Applications of Zinc Oxide Nanoparticles. RSC Adv. 11 (34), 21189–21206. doi:10.1039/d0ra10789a
Mou, J., Liu, Z., Liu, J., Lu, J., Zhu, W., and Pei, D. (2019). Hydrogel Containing Minocycline and Zinc Oxide-Loaded Serum Albumin Nanopartical for Periodontitis Application: Preparation, Characterization and Evaluation. Drug Deliv. 26, 179–187. doi:10.1080/10717544.2019.1571121
Mousavi, S. A., Ghotaslou, R., Khorramdel, A., Akbarzadeh, A., and Aeinfar, A. (2020). Ir. J. Med. Sci.
Münchow, E. A., Albuquerque, M. T. P., Zero, B., Kamocki, K., Piva, E., Gregory, R. L., et al. (2015). Dent. Mat. 31, 1038–1051.
Nair, S., Sasidharan, A., Divya Rani, V. V., Menon, D., Nair, S., Manzoor, K., et al. (2008). Role of Size Scale of ZnO Nanoparticles and Microparticles on Toxicity toward Bacteria and Osteoblast Cancer Cells. J. Mater Sci. Mater Med. 20, 235–241. doi:10.1007/s10856-008-3548-5
Nuri Sari, M., Rahmani, N., Araghbidi Kashani, M., Eslami Amirabadi, G., Akbari Sari, A., and Seyedtabaii, E. (2015). Effect of Incorporation of Nano-Hydroxyapatite and Nano-Zinc Oxide in Resin Modified Glass Ionomer Cement on Metal Bracket Debonding. J. Islamic Dent. Assoc. Iran 27 (2), 70–76.
Google Scholar
Panahandeh, N., Torabzadeh, H., Aghaee, M., Hasani, E., and Safa, S. (2018). J. Conserv. Dent. JCD 21, 130.
Pillai, A. M., Sivasankarapillai, V. S., Rahdar, A., Joseph, J., Sadeghfar, F., Anuf A, R., et al. (2020). Green Synthesis and Characterization of Zinc Oxide Nanoparticles with Antibacterial and Antifungal Activity. J. Mol. Struct. 1211, 128107. doi:10.1016/j.molstruc.2020.128107
Pokrowiecki, R., Wojnarowicz, J., Zareba, T., Koltsov, I., Lojkowski, W., Tyski, S., et al. (2019). Nanoparticles and Human Saliva: A Step towards Drug Delivery Systems for Dental and Craniofacial Biomaterials. Ijn 14, 9235–9257. doi:10.2147/ijn.s221608
Prado-Prone, G., Silva-Bermudez, P., Bazzar, M., Focarete, M. L., Rodil, S. E., Vidal-Gutiérrez, X., et al. (2020). Antibacterial Composite Membranes of Polycaprolactone/gelatin Loaded with Zinc Oxide Nanoparticles for Guided Tissue Regeneration. Biomed. Mat. 15, 035006. doi:10.1088/1748-605x/ab70ef
Ramazanzadeh, B., Jahanbin, A., Yaghoubi, M., Shahtahmassbi, N., Ghazvini, K., Shakeri, M., et al. (2015). Comparison of Antibacterial Effects of ZnO and CuO Nanoparticles Coated Brackets against Streptococcus Mutans. J. Dent. (Shiraz) 16 (3), 200–205.
PubMed Abstract | Google Scholar
Rasmussen, J. W., Martinez, E., Louka, P., and Wingett, D. G. (2010). Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 7, 1063–1077. doi:10.1517/17425247.2010.502560
Reddy, A. K., Kambalyal, P. B., Patil, S. R., Vankhre, M., Khan, M. Y., and Kumar, T. R. (2016). Comparative Evaluation and Influence on Shear Bond Strength of Incorporating Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles in Orthodontic Adhesive. J. Orthod. Sci. 5 (4), 127–131. doi:10.4103/2278-0203.192115
Riad, M., Harhash, A. Y., Elhiny, O. A., and Salem, G. A. (2015). Evaluation of the Shear Bond Strength of Orthodontic Adhesive System Containing Antimicrobial Silver Nano Particles on Bonding of Metal Brackets to Enamel. Life Sci. J. 12 (12), 27–34.
Saffarpour, M., Rahmani, M., Tahriri, M., and Peymani, A. (2016). Antimicrobial and Bond Strength Properties of a Dental Adhesive Containing Zinc Oxide Nanoparticles. Braz. J. Oral Sci. 15, 66–69. doi:10.20396/bjos.v15i1.8647127
Spoială, A., Ilie, C.-I., Trușcă, R.-D., Oprea, O.-C., Surdu, V.-A., ȘtefanVasile, B., et al. (2021). Mat. Basel Switz. 14, 4747.
Şuhani, M. F., Băciuţ, G., Băciuţ, M., Şuhani, R., and Bran, S. (2018). Clujul Med. 91, 274.
Tahmasbi, S., Mohamadian, F., Hosseini, S., and Eftekhar, L. (2019). A Review on the Applications of Nanotechnology in Orthodontics. Nanomedicine J. 6 (1), 11–18.
Tavassoli Hojati, S., Alaghemand, H., Hamze, F., Ahmadian Babaki, F., Rajab-Nia, R., Rezvani, M. B., et al. (2013). Antibacterial, Physical and Mechanical Properties of Flowable Resin Composites Containing Zinc Oxide Nanoparticles. Dent. Mater. 29, 495–505. doi:10.1016/j.dental.2013.03.011
Teymoornezhad, K., Alaghehmand, H., Daryakenari, G., Khafri, S., and Tabari, M. (2016). Evaluating the Microshear Bond Strength and Microleakage of Flowable Composites Containing Zinc Oxide Nano-Particles. Electron. Physician 8, 3289–3295. doi:10.19082/3289
Toledano, M., Cabello, I., Osorio, E., Aguilera, F. S., Medina-Castillo, A. L., Toledano-Osorio, M., et al. (2019). Zn-containing Polymer Nanogels Promote Cervical Dentin Remineralization. Clin. Oral Invest. 23, 1197–1208. doi:10.1007/s00784-018-2548-1
Toledano, M., Osorio, E., Aguilera, F. S., Muñoz-Soto, E., Toledano-Osorio, M., López-López, M. T., et al. (2020). Polymeric Nanoparticles for Endodontic Therapy. J. Mech. Behav. Biomed. Mater. 103, 103606. doi:10.1016/j.jmbbm.2019.103606
Toledano-Osorio, M., Osorio, E., Aguilera, F. S., Luis Medina-Castillo, A., Toledano, M., and Osorio, R. (2018). Improved Reactive Nanoparticles to Treat Dentin Hypersensitivity. Acta Biomater. 72, 371–380. doi:10.1016/j.actbio.2018.03.033
Trino, L. D., Albano, L. G. S., Bronze-Uhle, E. S., George, A., Mathew, M. T., and Lisboa-Filho, P. N. (2018). Physicochemical, Osteogenic and Corrosion Properties of Bio-Functionalized ZnO Thin Films: Potential Material for Biomedical Applications. Ceram. Int. 44 (17), 21004–21014. doi:10.1016/j.ceramint.2018.08.136
Vanajassun, P. P., Nivedhitha, M. S., Nishad, N. T., and Soman, D. (2014). Adv. Hum. Biol. 4, 31.
Wang, Y., Hua, H., Li, W., Wang, R., Jiang, X., and Zhu, M. (2019). Strong Antibacterial Dental Resin Composites Containing Cellulose Nanocrystal/zinc Oxide Nanohybrids. J. Dent. 80, 23–29. doi:10.1016/j.jdent.2018.11.002
Wiesmann, N., Tremel, W., and Brieger, J. (2020). Zinc Oxide Nanoparticles for Therapeutic Purposes in Cancer Medicine. J. Mat. Chem. B 8 (23), 4973–4989. doi:10.1039/d0tb00739k
Xiang, Y., Li, J., Liu, X., Cui, Z., Yang, X., Yeung, K. W. K., et al. (2017). Construction of Poly(lactic-Co-Glycolic acid)/ZnO nanorods/Ag Nanoparticles Hybrid Coating on Ti Implants for Enhanced Antibacterial Activity and Biocompatibility. Mater. Sci. Eng. C 79, 629–637. doi:10.1016/j.msec.2017.05.115
Yahya, N., Puspitasari, P., and Latiff, N. R. A. (2013). Hardness Improvement of Dental Amalgam Using Zinc Oxide and Aluminum Oxide Nanoparticles. Charact. Dev. Biosyst. Biomater. , 9–32. doi:10.1007/978-3-642-31470-4_2
Yamagata, S., Hamba, Y., Nakanishi, K., Abe, S., Akasaka, T., Ushijima, N., et al. (2012). Introduction of Rare-Earth-Element-Containing ZnO Nanoparticles into Orthodontic Adhesives. Nano Biomed. 4 (1), 11–17.
Zhang, J., Park, Y.-D., Bae, W.-J., El-Fiqi, A., Shin, S.-H., Lee, E.-J., et al. (2015). Effects of Bioactive Cements Incorporating Zinc-Bioglass Nanoparticles on Odontogenic and Angiogenic Potential of Human Dental Pulp Cells. J. Biomater. Appl. 29, 954–964. doi:10.1177/0885328214550896
Keywords: zinc oxide nanoparticles, biomedical application, nanodentistry, dental applications, restorative material
Citation: Pushpalatha C, Suresh J, Gayathri V, Sowmya S, Augustine D, Alamoudi A, Zidane B, Mohammad Albar NH and Patil S (2022) Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 10:917990. doi: 10.3389/fbioe.2022.917990
Received: 11 April 2022; Accepted: 28 April 2022; Published: 19 May 2022.
Reviewed by:
Copyright © 2022 Pushpalatha, Suresh, Gayathri, Sowmya, Augustine, Alamoudi, Zidane, Mohammad Albar and Patil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shankargouda Patil, [email protected]
This article is part of the Research Topic
Recent Advancements in the Dental Biomaterials Applied in Various Diagnostic, Restorative, Regenerative and Therapeutic Procedures
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 June 2020
Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential
- Minha Naseer 1 ,
- Usman Aslam ORCID: orcid.org/0000-0001-8145-8360 2 ,
- Bushra Khalid 3 , 4 &
- Bin Chen ORCID: orcid.org/0000-0002-0925-9209 5 , 6
Scientific Reports volume 10 , Article number: 9055 ( 2020 ) Cite this article
59k Accesses
322 Citations
Metrics details
- Antimicrobial resistance
- Nanoparticles
Development of plant based nanoparticles has many advantages over conventional physico-chemical methods and has various applications in medicine and biology. In present study, zinc oxide (ZnO) nanoparticles (NPs) were synthesized using leaf extracts of two medicinal plants Cassia fistula and Melia azadarach . 0.01 M zinc acetate dihydrate was used as a precursor in leaf extracts of respective plants for NPs synthesis. The structural and optical properties of NPs were investigated by X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, scanning electron microscope (SEM), ultraviolet-visible spectrophotometer (UV-Vis) and dynamic light scattering (DLS). The antibacterial potential of ZnO NPs was examined by paper disc diffusion method against two clinical strains of Escherichia coli ( E. coli ) and Staphylococcus aureus ( S. aureus ) based on the zone of inhibition and minimal inhibitory indices (MIC). Change in color of the reaction mixture from brown to white indicated the formation of ZnO NPs. UV peaks at 320 nm and 324 nm, and XRD pattern matching that of JCPDS card for ZnO confirmed the presence of pure ZnO NPs. FTIR further confirmed the presence of bioactive functional groups involved in the reduction of bulk zinc acetate to ZnO NPs. SEM analysis displayed the shape of NPs to be spherical whereas DLS showed their size range from 3 to 68 nm. The C. fistula and M. azadarach mediated ZnO NPs showed strong antimicrobial activity against clinical pathogens compared to standard drugs, suggesting that plant based synthesis of NPs can be an excellent strategy to develop versatile and eco-friendly biomedical products.
Similar content being viewed by others
Biosynthesis of copper nanoparticles using Alstonia scholaris leaves and its antimicrobial studies
Ahmad Nasir Labaran, Zakariyya Uba Zango, … Osamah A. Aldaghri
Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract
Manogar Priya, Raja Venkatesan, … Seong-Cheol Kim

Comparative evaluation of biomedical and phytochemical applications of zinc nanoparticles by using Fagonia cretica extracts
Bushra Hafeez Kiani, Fizza Ikram, … Iffat Naz
Introduction
Plant mediated synthesis of nanoparticles (NPs) is a revolutionary technique that has wide range of applications in agriculture, food industry and medicine. NPs synthesized via conventional methods have limited uses in clinical domain due to their toxicity. Due to the physio-chemical properties of plant based NPs, this method also offer an added advantage of increased life span of NPs that overcome the limitations of conventional chemical and physical methods of NPs synthesis 1 , 2 , 3 . Plants possess rich genetic variability with respect to number of biomolecules and metabolites like proteins, vitamins, coenzymes based intermediates, phenols, flavonoids and carbohydrates. These plant metabolites contain hydroxyl, carbonyl, and amine functional groups that react with metal ions and reduce their size into nano range. More specifically, flavonoids contain several functional groups and it is believed that -OH group of flavonoids is mainly considered responsible for the reduction of metal ions into NPs 4 . These molecules not only help in bioreduction of the ions to the nano scale size, but they also play a pivotal role in the capping of the nanoparticles which is important for stability and biocompatibility 5 . Reducing agents such as phenolic compounds, sterols and alkaloids can reduce metal ions into NPs in a single reaction 6 .
The type and nature of the metal used for NPs biosynthesis mainly determines the NPs end use industry. Several metals such as silver (Ag), copper (Cu), gold (Au) and many others have been widely used for the biosynthesis of NPs using plant extracts of various plant species 7 , 8 , 9 . However their higher toxicity to animals and humans pose a serious limitation for use in medical industry. ZnO is an inorganic compound which occurs rarely in nature. It is generally found in crystalline form. Naturally occurring ZnO has manganese impurities that give it a typical red or orange color appearance 10 . When purified, ZnO appears as white crystalline powder which is nearly insoluble in water. Due to their low toxicity and size dependent properties, ZnO NPs have been widely used for various applications in textiles, cosmetics, diagnostics and even in micro-electronics. Because ZnO is generally recognized as safe (GRAS) and exhibits antimicrobial properties, ZnO NPs hold greater potential to treat infectious diseases in humans and animals 11 .
ZnO has been found to be potentially useful and efficient than other metals for biosynthesis of NPs for clinical purposes. Several studies have demonstrated the synthesis of ZnO NPs using different plant extracts. For example, flower extract of the medicinal plant Cassia auriculata 12 and leaf extract of Hibiscus rosasinensi 13 were used as reducing agents for zinc nitrate to synthesize ZnO NPs.
Plant type or source species from which plant extract used for NPs synthesis also affects the size of NPs. For example, when Olea europea leaf extract was used to synthesize ZnO nano sheets, it ranged from 18–30 nm in size 14 . However, when Aloe barbadensis 15 and Ocimum tenuiflorum 11 were used as reducing agent for the green synthesis of ZnO nanoparticles, the average nanoparticle sizes were 25–40 nm and 13.86 nm respectively. Recently various reports have also demonstrated the antimicrobial activity of ZnO NPs. For example, ZnO NPs synthesized by using leaf extracts of Passiflora caerulea , Scadoxus multiflorus and Camellia sinensis showed strong antimicrobial efficacy against Klebsiella pneumonia , Aspergillus spp., and Staphylococcus aureus and Pseudomonas aeruginosa respectively 16 , 17 , 18 , suggesting that medicinal plant extract mediated synthesis of ZnO NPs can be very useful for medical industry.
Cassia fistula commonly known as Golden Shower or Amaltas is a deciduous tree with medicinal importance, native to Pakistan and India and found as an exotic species in Egypt, Australia, Ghana, Mexico, and Zimbabwe. It belongs to the family Fabaceae . It produces shiny green leaves which are about 30–40 cm long, pinnate in shape and arranged in alternate fashion on the terminal branches 19 . Leaves of C. fistula contain a wide variety of antioxidants for example; terpenoids, flavonoids, alkaloids, phenolic compounds, tannins, saponins, anthocyanosides, carbohydrates, proteins, steroids, cardiac glycosides and phlobatannins 20 .
Similarly, Melia azadarach commonly known as Cape Lilac and locally as Bakain belongs to the family Meliaceae . It is native to Southeast Asia and found naturally in most of the tropical and subtropical countries. This plant is locally famous for its anti-microbial, anti-inflammatory and anti-cancer activities and often used to treat stomach pains and parasitic infections. It produces dense array of dark green leaves which are short stalked and arranged in alternate pattern on terminal branches. Fruits are yellow colored, smooth and fleshy berries. M. azadarach is naturally enriched in phytochemicals. It is endowed with alkaloids, sterols, glycosides, flavonoids, limonoids, fixed oil and fats, phenolic compounds, tannins, saponins, gum and mucilages, triterpenes, azadirachitin, nimbin, melianoninol, melianol, meliandiol, vanillin, meliacin, quercertin and rutin 21 . Due to the presence of diverse array of these phytochemicals and medicinal properties, C. fistula and M. azedarach hold greater potential for efficient biosynthesis of NPs that can be useful to treat clinical pathogens.
Here, we report a simple and eco-friendly method of ZnO NPs synthesis from the plant extracts of C. fistula and M. azedarach as reducing agents and zinc acetate as precursor for their comparative analysis of antimicrobial potential. This research will increase the potential of usage of plant based NPs in biomedical industry.
Results and Discussion
Optical analysis of zno nps formation.
Adding zinc acetate dihydrate in leaf extracts of C. fistula and M. azedarach leads to physio-chemical changes in the aqueous solution. The most prominent of which is change in the colour of the reaction mixture that can be observed within few minutes. This was considered as an initial signature to formation of NPs. In present study, change of color from yellow to light brown and red to off-white indicated the formation of ZnO NPs in leaf extracts of C. fistula and M. azedarach , respectively. Flavonoides and phenolic compounds are thought to be responsible for Zn ions to ZnO NPs. In a period of few hours, the colour of the solution stopped changing further suggesting the complete bioreduction of ZnO salt into NPs. A clear illustration of change in color of the reaction mixtures due to formation of ZnO NPs has been shown in Fig. 1(A,B) . These results were consistent with the previous reports of color changes in plant based synthesis of ZnO NPs 22 . Temperature is considered an important contributing factor in synthesis of good sized nanoparticles. It is also well established that higher the temperature of reaction process of NPs synthesis, the smaller the size of the NPs 23 , 24 . Therefore, we use a relatively higher temperature of 70 °C for incubating the reactants that leads to the production of very small sized ZnO NPs.
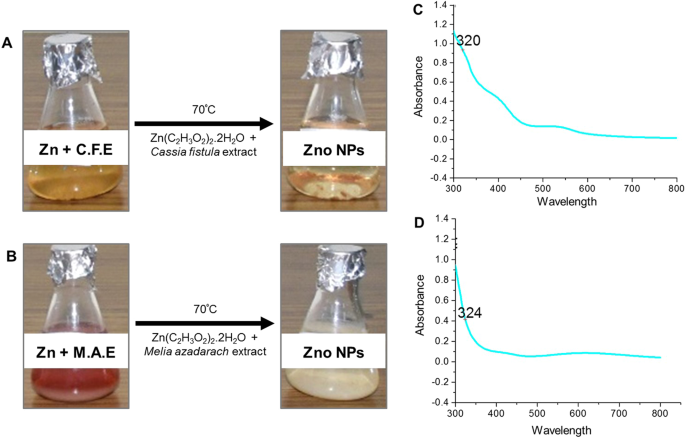
Optical analysis of ZnO NPs. ( A,B ) Color changes indicating formation of ZnO NPs. A) Cassia fistula mediated ZnO NPs. ( B ) Melia azadarach mediated ZnO NPs. ( C,D ) UV-visible absorption spectrum confirming presence of ZnO NPs. ( A ) Cassia fistula mediated ZnO NPs. ( B ) Melia azadarach mediated ZnO NPs.
The synthesis of ZnO NPs was further examined by UV spectrophotometry. Figure 1(C,D) shows the UV peaks recorded by the spectrophotometer. The maximum absorption peak for ZnO NPs synthesized via C. fistula was recorded at 320 nm and with that of M. azadarach at 324 nm that further verified the formation of ZnO NPs. Firstly, these results satisfy standard ZnO absorption pattern because all oxide materials have wide band gaps and tend to have shorter wavelengths. Moreover, if the material is of nanoscale, it tends to have further shorter wavelengths. This notion support the results observed for ZnO NPs here 25 .
Surface morphology of ZnO NPs
The presence of nanoparticles and examination of their structural properties were confirmed by X-ray diffractrometer. C. fistula and M. azedarach associated ZnO NPs showed peaks with 2θ values identified at 31.841°, 34.507°, 36.324°, 47.592°, 56.634°, 66.426°, 67.983°, 69.091°, and 76.987° which are indexed as (100), (002), (101), (102), (110), (103), (112), (201) and (202) planes (Fig. 2A,B ). These peaks were in accordance with those of data card (JCPDS-36-1451). Average crystal size calculated using the Scherrer’s equation ( \(Dp\,of\,ZnO\,NPs=(0.9(1.5406)/0.63(\cos \,36)\) came out to be around 2.72 nm for both C. fistula and M. azedarach associated ZnO NPs that is comparable with the size of good quality NPs in existing reports 26 .
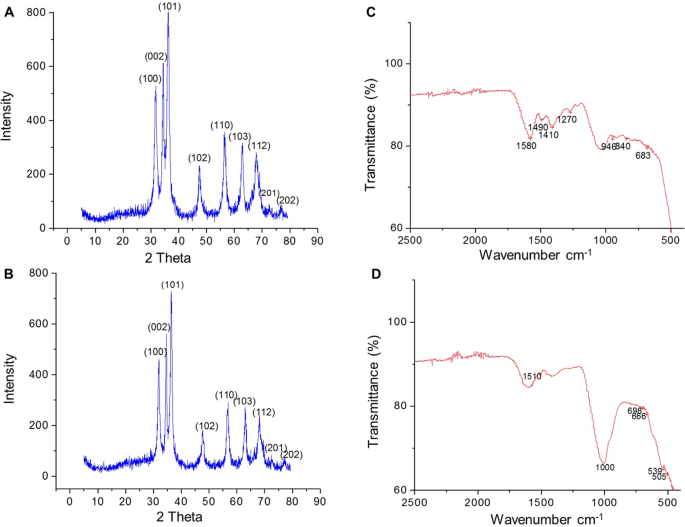
( A,B ) XRD pattern indicating presence of ZnO peaks. ( A ) Cassia fistula mediated ZnO NPs. ( B ) Melia azadarach mediated ZnO NPs. ( C,D ) FTIR pattern indicating the functional groups involved in ZnO NPs synthesis. ( C ) Cassia fistula mediated ZnO NPs. ( D ) Melia azadarach mediated ZnO NPs.
To identify the functional groups associated with the ZnO NPs formation, FTIR spectrometry was performed. Spectral peaks at 683–500 cm −1 and 698–505 cm −1 proposed the formation of ZnO nanoparticles in C. fistula and M. azedarach extracts, respectively (Fig. 2C,D ). Absence of peaks in the region of 3500 and 2500 cm −1 indicated no characteristic OH and N-H stretching of aldehydes. The bands at 1600–1510 cm −1 correspond to amide I and amide II regions arising due to carbonyl stretching in proteins and that of 1400 to 1000 cm −1 correspond to methylene from the proteins in the solution and C-N stretching vibrations of amine. Peaks from 1460–1410 cm −1 suggested C-C stretching vibration of alcohol, carboxylic acid, ether and ester and bands at 946–769 cm −1 demonstrated presence of carboxylic acid and aromatic C-H bending. Although, many changes were not observed at these frequencies but all peaks showed a shift to lower frequency and a decrease in intensity on binding with the nanoparticles. This trend of free carbonyl and NH 2 groups from proteins and amino acid residues indicates that they have ability to bind to a metal and that the proteins could possibly form a layer around the metal for preventing agglomeration and thereby stabilizing the nanoparticles. It is revealed from the FTIR spectra that in fact, the protein molecules present in the leaf extract possibly cause the reduction of metal ions which is in agreement with the previous reports 27 . These findings suggest that not only the OH group of flavonoids but also the protein molecules and their functional groups play important role in bioreduction of salts and capping of NPs.
Dynamic Light Scattering (DLS) measurements showed the average diameters of C. fistula and M. azadarach mediated ZnO NPs (Fig. 3 ). Average diameters of ZnO NPs synthesized from C. fistula and M. azadarach were 68.1 nm and 3.62 nm, respectively. The results demonstrated that the particles synthesized were ultrafine i.e. less than 100 nm in diameter. It clearly depicts that M. azadarach extract was more efficient than C. fistula for synthesizing smaller NPs. It may be attributed to the presence of more variety of phytochemicals in M. azadarach when compared to C. fistula . As it has already been mentioned in the introduction section that M. azadarech possesses complete set of phytochemicals that can be the reason behind higher efficacy of this plant as a reducing agent when compared to C. fistula . In addition, DLS analysis demonstrated that the NPs formed had fairly well-defined dimensions 28 . Smaller the size of the NPs, higher the surface area, thus higher the antimicrobial activity. Generally, bacterial cellular membranes have nanometer size. If the nanoparticles are smaller in size than cell membrane pores, there is more possibility of crossing the cell membrane barrier and thus inhibiting the bacterial growth 29 .
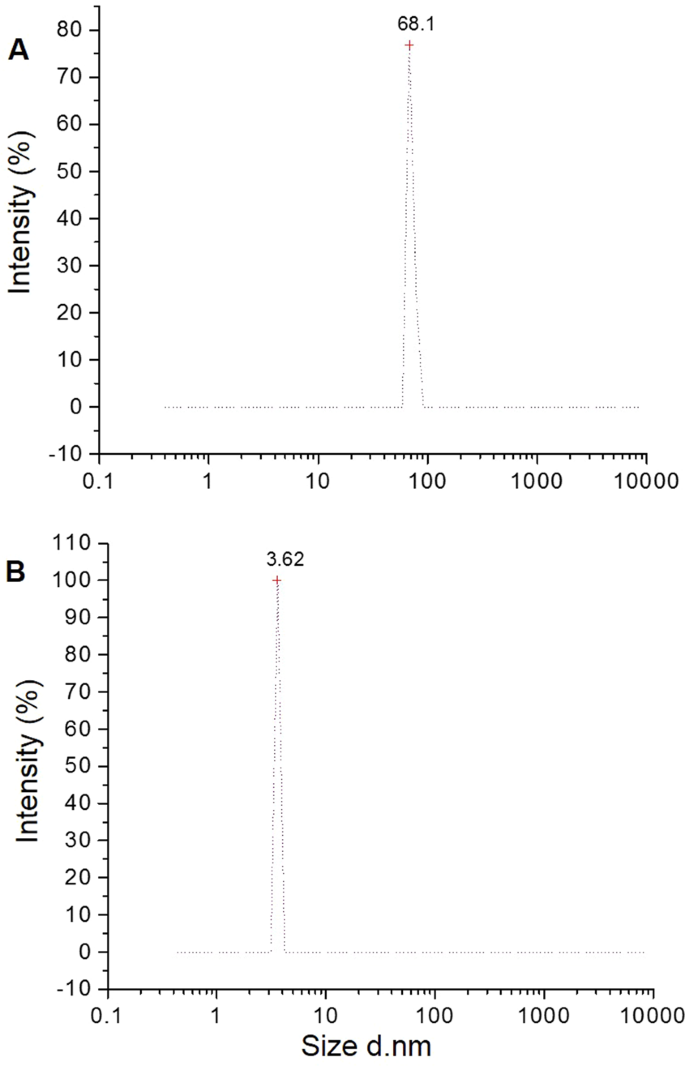
DLS indicating average size of ZnO NPs. ( A ) Cassia fistula mediated ZnO NPs. ( B ) Melia azadarach mediated ZnO NPs.
Figure 4 shows Scanning Electron Microscopy (SEM) images of ZnO NPs synthesized from leaf extracts of C. fistula and M. azadarach . The images were recorded at magnification of 10 µm, 1 µm and 100 nm. Topographical view shows that nanoparticles are more or less spherical in nature, clustered together and surface of the aggregates seems to be rough 30 . SEM images also revealed that NPs derived from both plants are entirely pure and it can be concluded that both the plants have tremendous capability to synthesize ZnO NPs. Shape of NPs plays very crucial role in the effectivity against pathogens. Because spherical NPs tend to be very potent during antibacterial activity owing to their ability to easily penetrate into the cell wall of pathogens 31 , therefore, ZnO NPs syntheized from these two plant species can be of great importance in treating clinical pathogens.
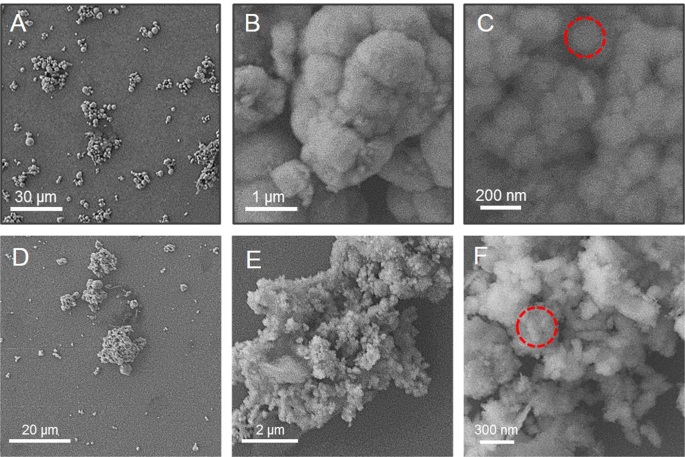
SEM images of ZnO particles showing their morphology at three different resolutions. ( A–C ): Scanning Electron Micrographs of Cassia fistula mediated Zno NPs. ( A ) SEM of Zno NPs captured at 500× magnification. ( B ) SEM of Zno NPs captured at 16,000× magnification. ( C ) SEM of Zno NPs captured at 65,000× magnification. ( D–F ): Scanning Electron Micrographs of Melia azadarach mediated Zno NPs. ( D ) SEM of Zno NPs captured at 800× magnification. ( E ) SEM of Zno NPs captured at 8,000× magnification. ( F ) SEM of Zno NPs captured at 30,000× magnification. Red dotted circles in ( C,F ) indicate the NPs circumference.
Antibacterial activity of ZnO NPs
The bactericidal activities of C. fistula and M. azadarach mediated ZnO NPs were tested against two main clinical pathogens; a (Gram-negative pathogen) E. coli and b (Gram-positive pathogen) S. aureus . Figure 5 illustrates zones of inhibition of E. coli and S. aureus against standard drugs and biosynthesed ZnO NPS at concentrations ranging from 50 µg/mL (10 µL) to 1000 µg/mL (200 µL). The mean values of zone of inhibition (mm) of three replicates are presented in (Table 1 ). Comparison between standard antibiotics and biosynthesed NPs showed strong antibacterial effect of NPs as compared to standard drugs (Table 2 ). In E. coli , zone of inhibition of standard drugs ranged from 15–20 mm while that of ZnO NPs was 16–40 mm. S. aureus was resistant to a variety of standard drugs and zone of inhibition for rest of the standard drugs was ranged from 4–13 mm while that of ZnO NPs was 14–37 mm in range. (Table 3 ) shows zones of inhibition of various standard drugs and standard drug potency according to WHO standards.
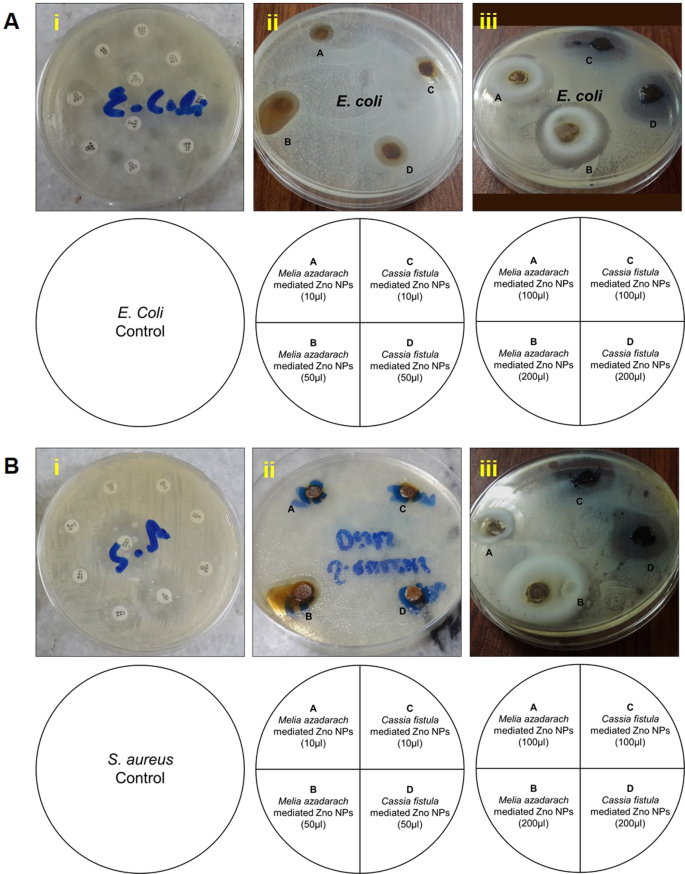
Resistance level of two clinical pathogens against (i) standard drugs, (ii) ZnO NPs 10 µL, 50 µL and (iii) ZnO NPs 100 µL, 200 µL. ( A ) Inhibition zones of ZnO NPs against E. coli growth. ( B ) Inhibition zones of ZnO NPs against S. aureus growth. Lower panel in both part A and B illustrates the labelling of petri plates.
Both the E. coli and S. aureus showed minimum inhibitory concentration (MIC) at 10 µL for the synthesized ZnO NPs. Furthermore, as the concentration of NPs increased so did the zone of inhibition. It is evident from the recordeded images and statistical data that zone of inhibition of C. fistula mediated ZnO NPs was more significant against E. coli ( ∼ 44 mm) as compared to S. aureus (Fig. 5 , Table 2 ). The mild inhibitory effect of C. fistula mediated ZnO NPs on S. aureus when compared to E. coli can be attributed to the differences in membrane strutures of Gram-positive and Gram-negative bacteria. The most disntinctive feature of Gram-positive bacterium is the thickness of cell wall due to the prescence of peptidoglycan layer. It has also been reported that ZnO NPs may damge bacterial cell membrane resulting lysis of intracellular contents and ultimately proved to be lethal for the bacterial cell 32 . Lower efficacy of C. fistula mediated ZnO NPs against S. aureus compared to the Gram-negative species might be due to the resistance of cell wall in Gram-positve species 33 . By contrast, the zone of inhibition of M. azadarach mediated ZnO NPs was compareable against both the pathogens. However, it is important to note that the zone of inhibition of M. azadarch mediated ZnO NPs was significantly greater in comparison to C. fistula mediated ZnO NPs against S. aureus (Fig. 5 , Table 2 ). These results suggest that the use of M. azadarch mediated synthesis of ZnO NPs can be more efficient against Gram-positive pathogens like S. aureus . This might be due to the presence of higher number of phenolic compounds and rare secondary metabolites such as nimbinene, meliacin, quercertin and rutin in M. azadarch .
As a schematic layout of this whole study, a model has been given in Fig. 6 that shows the graphical representation of the synthesis of ZnO NPs using leaf extarcts of C. fistula and M. azadarach as reducing agents and zinc acetate as a precursor salt.
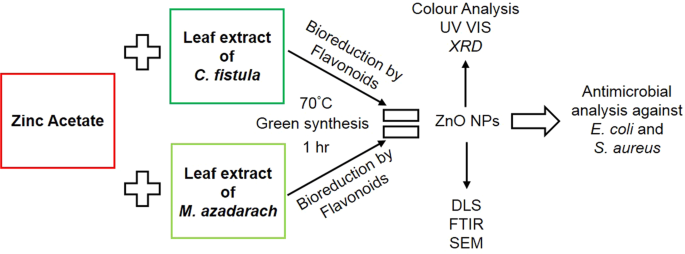
Schematic model of ZnO NP synthesis from the leaf extracts of Cassia fistula and Melia azedarach and their antibacterial activity analysis.
Leaf extracts of C. fistula and M. azadarach showed excellent potential as reducing agents in the formation of NPs. Structural and optical studies conducted using UV, FTIR, XRD, DLS and SEM analysis confirmed the formation of efficient ZnO NPs. Antibacterial analysis revealed that ZnO NPs synthesized from leaf extracts exhibited significant capability of inhibition against the clinical pathogens when compared to traditional drugs. Moreover, some plant extratcs are more effective than that of others in synthesizing NPs and biological activities due to their diverse biochemical compositions. In conclusion, synthesis of NPs using extratcs of medicinal plants can have useful medicinal applciations in treatment of numerous human infectious pathogens. However, further studies will be required to validate the efficacy of these NPs in medical applications and their capacity to overcome the risks associated with conventional drugs.
Synthesis of Nanoparticles
All the glassware were autoclaved before use. To prepare leaf extract, fresh leaves of C. fistula and M. azadarach were thoroughly washed with tap water followed by distilled water (d.H 2 O) to remove any contamination. The leaves were air dried for a week at room temperature ( ∼ 37°C). About 5 g of leaves from each of C. fistula and M. azadarach were ground to fine powder with the help of pestle and mortar. This powder was mixed in 500 mL of d.H 2 O and then heated at 70°C for 30 minutes. The mixture was filtered first by muslin cloth and then using Whatman filter paper No.1. As a result, pale yellow and red colored solutions were obtained as leaf extracts of C. fistula and M. azadarach respectively which were stored at 4 °C.
0.01 M zinc acetate dihydrate (Zn (C 2 H 3 O 2 ) 2 .2H 2 O) solution was prepared in d.H 2 O. For synthesis of ZnO nanoparticles, 95 mL of 0.01 M zinc acetate dihydrate (Zn (C 2 H 3 O 2 ) 2 .2H 2 O) solution was mixed separately with 5 mL plant extract of each of C. fistula and M. azadarach in individual 250 mL flasks. These mixtures were incubated at 70°C for 1 hour with continuous shaking at 150 rpm. This led to the settlement of bio-reduced salt at the bottom of the flask which appeared as white precipitate. The supernatant was decanted and powdery precipitate was transferred to 1.5 mL centrifuge tubes. Both the samples were subjected to washing with d.H 2 O by centrifugation at 3000 rpm for 30 minutes. Washing step was repeated thrice to ensure removal of impurities 22 .
Characterization of NPs
Optical Spectroscopy . To measure the optical parameters, ZnO synthesized nanoparticles were dispersed in d.H 2 O. The absorption spectrum of synthesized NPs was measured using UV–VIS-NIR spectrophotometer (UV-1601, Shimadzu, Japan) in wavelength range between 200–800 nm. The d.H 2 O was used as a reference. Energy gap or band gap was calculated using the following equation
where Eg is the bulk band expressed in eV. Lambda ( 𝜆 ) is peak absorbance wavelength in nm. Therefore, the energy gap for ZnO ranges from 4.27–3.87 eV 34 .
FTIR Analysis . The surface chemistry of NPs was analyzed by FTIR spectroscopy. The functional groups attached to the surface of NPs were detected in the range of 4000–400 nm. The samples were prepared by dispersing the ZnO NPs uniformly in a matrix of dry KBr which was then compressed to form a transparent disc. KBr pellet was used as a standard 35 .
XRD Analysis . X-ray diffractrometer (PAN analytical X-Pert PRO) was used to study the surface morphology, size and crystalline nature of ZnO NPs. The diffraction pattern was obtained using CuKα radiation with wavelength of λ = 1.541 A°. A thin film of the sample was made by putting a small amount of sample on a glass plate for XRD studies. The scanning was done in 2θ value range of 4° to 80° at 0.02 min −1 and 1 second time constant. The instrument was operated at a current of 30 mA and voltage of 40 kV. Scherrer’s equation was used to calculate the average grain size of synthesized NPs which is as under
where D represents the crystallite size, λ stands for the wavelength (1.5406 Å for Cu Kα), β symbolizes the full-width at half-maximum (FWHM) of main intensity peak after subtraction of the equipment broadening and θ is used as a diffraction angle in radians.
DLS Analysis . The particle size distribution of the samples was obtained through Particle Size Analyzer (Zetasizer Ver. 7.11 Malvern). The liquid samples of ZnO NPs was diluted ten times using Milli-Q water, centrifuged and then transferred to cuvette for analysis. The zeta potential of ZnO NPS was determined in water as dispersant.
SEM Imaging . The samples of ZnO NPs were dispersed in methanol (evaporating solvent) at a concentration of 1 mg/20 mL. A single drop of aqueous solution of ZnO NPs was placed on the carbon coated grid to prepare a thin film. Extra solution was removed with the help of blotting paper and the grid was allowed to dry under mercury lamp for around five minutes. The morphological measurements of the ZnO NPs samples were recorded with field emission scanning electron microscope (JEOL, Model: JSM-7600F) in the range of 0.1 nm to 10,000 nm. The data collected from all techniques was analyzed in Origin software version 9.1.
Antimicrobial analysis
To check the bactericidal potential of the NPs, pure cultures of Escherichia coli (EPEC-A (P16), and Staphylococcus aureus [(MRSA belonging to clonal complex 8 (CC8) and sequence type 239 (ST239)] were obtained from the Department of Microbiology, Pakistan Institute of Medical Sciences (PIMS), Islamabad. Disc diffusion method was used to carry out the antibacterial assay of NPs on Muller Hinton Agar (MHA) medium containing petri plates. Contamination test was carried out by incubating the plates over night at room temperature. After confirmation of no contamination, bacterial cultures were streaked on to these MHA plates.
Stock solution of NPs was prepared in d.H 2 O at a concentration of 5 mg/mL. Further, four working dilutions i.e. 50 µg/mL (10 µL), 250 µg/mL (50 µL), 500 µg/mL (100 µL) and 1000 µg/mL (200 µL) were made to find out minimum inhibitory concentration (MIC). The Minimum Inhibitory Concentration (MIC) of the ZnO NPs was determined based on batch cultures containing varying concentrations of ZnO NPs in suspension (10–200 µg/mL). Bacterial concentrations were determined by measuring optical density (OD) at 600 nm.
To examine the bactericidal effect of NPs on clinical strains, approximately 10 8 CFU of each strain was cultured on nutrient agar plates. Following disc diffusion method, the sterile discs were dipped in ZnO nanoparticles solution at varying concentrations from 50 µg/mL to 1000 µg/mL. Discs were placed onto the MHA plates and incubated at 37 °C. Control samples were prepared by placing standard medicine discs onto MHA plates containing bacterial isolates. Standard medicines used for E. coli were Ceftazidime, Imipenem, Cefoperazone, Amoxicillin, and Cefixime, whereas, Erythromycin, Gentamycin, Vancomycin, Chloramphenicol, Lanzolid were used for S. aureus . Mean values of inhibitory zone diameter were recorded in three experimental repeats. The average values of inhibition zones were calculated as Mean ± Standard Deviations. The data was statistically analyzed using Origin software version 9.1 36 .
Kavitha, K. S. et al . Plants as green source towards synthesis of nanoparticles. Int. Res. J. Biol. Sci. 2 , 66–76 (2013).
Google Scholar
Malik, P., Shankar, R., Malik, V., Sharma, N. & Mukherjee, T.K. Green chemistry based benign routes for nanoparticle synthesis. J. Nanopart . https://doi.org/10.1155/2014/302429 (2014).
Article Google Scholar
Kalpana, V. N. & Rajeswari, V. D. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl . https://doi.org/10.1155/2018/3569758 (2018).
Makarov, V. V. et al . “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae 6 , 35–44 (2014).
Article CAS Google Scholar
Arya, V. Living systems: Eco-friendly nanofactories. Dig. J. Nanomater Bios. 5 , 9–21 (2010).
Biswas, B., Rogers, K., Mclaughlin, F., Daniels, D. & Yadav, A. Antimicrobial activities of leaf extracts of Guava ( Psidium guajava L.) on two Gram-negative and Gram-positive bacteria. Int. J. Microbiol . https://doi.org/10.1155/2013/746165 (2013).
Mittal, A. K., Chisti, Y. & Banerjee, U. C. Synthesis of metallic nanoparticles using plant extratcs. Biotechnol. Adv . http://dx.doi.org/10.1016/ (2013).
Rajan, R., Chandran, K., Harper, S. L., Yun, S. I. & Kalaichelvan, P. T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crop Prod. 70 , 356–373 (2015).
Ahmed, S., Ahmad, M., Swami, B. L. & Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 7 , 17–28 (2016).
Fan, Z. Y. & Lu, J. G. Zinc oxide nanostructures: Synthesis and properties. J. Nanosci. Nanotechnol. 5 , 1561–1573 (2005).
Sushma, N. J., Mahitha, B., Mallikarjuna, K. & Deva, P. R. B. Bio-inspired ZnO nanoparticles from Ocimum tenuiflorum and their in vitro antioxidant activity. Appl. Phys. A 122 , 544 (2016).
Article ADS Google Scholar
Ramesh, P., Rajendran, A. & Sundaram, M. Green syntheis of zinc oxide nanoparticles using flower extract Cassia Auriculata . J. Nanosci. Nanotechnol. 2 , 41–45 (2014).
Divya, M. J., Sowmia, C., Joona, K. & Dhanya, K. P. Synthesis of zinc oxide nanoparticle from hibiscus rosa-sinensis leaf extract and investigation of its antimicrobial activity. Res. J. Pharm. Biol. Chem. Sci. 4 , 1137–1142 (2013).
CAS Google Scholar
Awwad, A., Albiss, B. & Ahmad, A. L. Green synthesis, characterization and optical properties of zinc oxide nanosheets using Olea europea leaf extract. Adv. Mater. Lett. 5 , 520–524 (2014).
Gunalan, S., Sivaraj, R. & Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Progress in Natural Science: Materials International 22 , 693–700 (2012).
Santoshkumar, J., Kumar, S. V. & Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technologies 3 , 459–465 (2017).
Al-Dhabi, N. A. & Arasu, M. V. Environmentally friendly green approach for the production of Zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials 8 , 500 (2018).
Shah, R. K., Boruah, F. & Parween, N. Synthesis and characterization of ZnO nanoparticles using leaf extract of Camelia sinensis and evaluation of their antimicrobial efficacy. Int . J. Curr. Microbiol. Appl. Res. 4 , 444–450 (2019).
Veerachari, U. & Bopaiah, A. K. Preliminary phyto-chemical evaluation of the leaf extract of five cassia species. J. Chem . Pharm. Res. 3 , 574–584 (2011).
Bahorun, T., Neergheen, V. S. & Aruoma, O. I. Phytochemical constituents of Cassia fistula . Afri. J. Biotechnol. 4 , 1530–1540 (2005).
Azam, M. M., Mamoon-ur-Rasheed, A. N. M., Towfique, N. M., Sen, M. K. & Nasrin, S. Pharmacological potentials of Melia azedarach L. - A review. Am. J. Bios. 1 , 44–49 (2013).
Rajiv, P., Rajeshwari, S. & Venckatesh, R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochimica Acta Part A: Mol. Biomol. Spectr. 112 , 384–387 (2013).
Article ADS CAS Google Scholar
Saware, K. & Venkataraman, A. Biosynthesis and characterization of stable silver nanoparticles using Ficus religiosa leaf extract: A mechanism perspective. J. Clust. Sci. 25 , 1157–1171 (2014).
Jain, S. & Mehata, M. S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 7 , 15867, https://doi.org/10.1038/s41598-017-15724-8 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar
Zhang, D. H., Xue, Z. Y. & Wang, P. Q. The mechanisms of blue emission from ZnO films deposited on glass substrate by r.f. magnetron sputtering. J. Phys. D Appl. Phys. 35 , 2837 (2002).
Bhatte, K. D., Sawant, D. N., Pinjari, D. V., Pandit, A. B. & Bhanage, B. M. One pot green synthesis of nano sized zinc oxide by sonochemical method. Mater. Lett. 77 , 93–95 (2012).
Das, D., Nath, B. C., Phukon, P. & Dolui, S. K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids. Surf. B Biointerfaces. 101 , 430–433 (2013).
Honary, S., Barabadi, H., Gharaei, E. & Naghibi, F. Green synthesis of copper oxide nanoparticles using Penicillium aurantiogriseum , Penicillium citrinum and Penicillium waksmani . Dig. J. Nanomater Bios. 7 , 999–1005 (2012).
Feng, Q. et al . A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus . J. Biomed. Mater. Res. 15 , 662–668 (2000).
Pan, K. & Zhong, Q. Organic nanoparticles in foods: Fabrication, characterization, and utilization. Annu. Rev. Food Sci. T. 7 , 245–266 (2016).
Wiley, B., Sun, Y., Mayers, B. & Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem. A Eur. J. 11 , 454–463 (2006).
Ruparelia, J. P., Chatterjee, A. K., Duttagupta, S. P. & Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomaterialia 4 , 707–716 (2008).
Kim, J. S. et al . Antimicrobial effects of silver nanoparticles. Nanomedicine: NBM 3 , 95–101 (2007).
Brus, L. Electronic wave functions in semiconductor clusters: experiment and theory. J. Phys. Chem. 90 , 2555–2560 (1986).
Wolkers, W. & Oldenhof, H. In situ FTIR assessment of dried Lactobacillus bulgaricus : KBr disk formation affects physical properties. Spectroscopy 15 , 89–99 (2005).
Herrera, M., Carrion, P., Baca, P., Liebana, J. & Castillo, A. In vitro antibacterial activity of glass-ionomer cements. Microbios 104 , 141–148 (2001).
CAS PubMed Google Scholar
Download references
Acknowledgements
This work was supported by the International Partnership Program of the Chinese Academy of Sciences [grant number 134111KYSB20180021), the National Natural Science Foundation of China [grant numbers 41590871], and the International Science & Technology Cooperation Program of China [grant number 2013DFG22820]. The authors highly acknowldege the techinal support for this research from the Department of Microbiology, Pakistan Institute of Medical Sciences (PIMS), Islamabad and Pakistan Institute of Nuclear Science and Technology (PINSTECH), Islamabad, Pakistan.
Author information
Authors and affiliations.
Department of Environmental Science, International Islamic University Islamabad, Islamabad, Pakistan
Minha Naseer
Department of Plant Breeding and Genetics, University of Agriculture Faisalabad, Faisalabad, Pakistan
Usman Aslam
Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, 11A Datun Road, Chaoyang District, Beijing, 100101, P.R. China
Bushra Khalid
The Abdus Salam International Centre for Theoretical Physics, Trieste, Italy
Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, 100029, China
Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters, Nanjing University of Information Science & Technology, Nanjing, 210044, China
You can also search for this author in PubMed Google Scholar
Contributions
The research was designed and performed by M.N. M.N. also wrote the manuscript. U.A. formatted the pictures in current form and critically reviewed and revised the manuscript. B.K. and B.C. critically reviewed the manuscript.
Corresponding authors
Correspondence to Usman Aslam or Bin Chen .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Naseer, M., Aslam, U., Khalid, B. et al. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci Rep 10 , 9055 (2020). https://doi.org/10.1038/s41598-020-65949-3
Download citation
Received : 23 September 2019
Accepted : 22 January 2020
Published : 03 June 2020
DOI : https://doi.org/10.1038/s41598-020-65949-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Synthesis of metal oxide nanoparticles using punica granatum extract for the removal of cationic and anionic dyes from wastewater.
- Ghulam Mustafa
- Tahir Rasheed
Arabian Journal for Science and Engineering (2024)
Semi-industrial Bio-fabrication of ZnO/MnO2 Nanocomposite Using Endophytic Streptomyces coelicolor: Characterization, Statistical Design, Exponential Pulse Fed-Batch Fermentation, and Its Antimicrobial Application
- Shahira H. EL-Moslamy
- Ahmed H. Rezk
- Hassan Shokry Hassan
Anti-cancer Activity of Biogenic Nat-ZnO Nanoparticles Synthesized Using Nyctanthes arbor-tristis (Nat) Flower Extract
- Siva Chander Chabattula
- Piyush Kumar Gupta
- Rama Shanker Verma
Applied Biochemistry and Biotechnology (2024)
Green synthesis of silver and zinc oxide nanoparticles for novel application to enhance shelf life of fruits
- Maria Zafar
- Tahir Iqbal
Biomass Conversion and Biorefinery (2024)
Solution combustion method for synthesis of ZnO NPs from Syzygium hemisphericum bark extract and a comparative analysis of the same with the crude bark extract for biomedical applications
- C. H. Sushmitha
- G. Krishnakumar
- K. Meghana Navada
Chemical Papers (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Previous Article
- Next Article
A review on biosynthesis zinc oxide nanoparticles by using leaves extract
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Reprints and Permissions
- Cite Icon Cite
- Search Site
Fatini Abdul Karim , Ainun Rahmahwati Ainuddin , Zakiah Kamdi , Siti Aida Ibrahim , Rosniza Hussin; A review on biosynthesis zinc oxide nanoparticles by using leaves extract. AIP Conf. Proc. 19 January 2024; 2925 (1): 020031. https://doi.org/10.1063/5.0182825
Download citation file:
- Ris (Zotero)
- Reference Manager
A nanoparticle is a branch of nanotechnology that deals with nano-scale materials with very small particle sizes ranging from 1 to 100 nm. Metal oxide nanoparticles are more promising than the many other nanoparticles available because they have unique physical, chemical, and biological properties. Zinc oxide is one of the abundantly produced metal oxides after silicon dioxide and titanium dioxide. However, these methods of production are typically costly, labour-intensive, and can harm the environment and living organisms. Therefore, green synthesis (biosynthesis) is a good alternative where plants are used to assist nanoparticles synthesis which has eco-friendly benefits compared to chemical and physical methods. This biosynthesis method uses simple procedures, easily accessible raw materials, and a conducive environment for the synthesis process, where the precursors are safe and reduce the possibilities of harmful by-products being produced. Therefore, this review paper is focused on summaries of the biosynthesis of Zinc oxide nanoparticles from leaf extract such as Mangifera Indica (Mango), Ixora Cocconea (Jungle Geranium) , Corymbia Citridora (Lemon-scented Gum) as a new development of green technology beneficial to the environment and to the plant itself. It also describes the progress made in the understanding of the mechanism routes reported in this review.
Sign in via your Institution
Citing articles via, publish with us - request a quote.

Sign up for alerts
- Online ISSN 1551-7616
- Print ISSN 0094-243X
- For Researchers
- For Librarians
- For Advertisers
- Our Publishing Partners
- Physics Today
- Conference Proceedings
- Special Topics
pubs.aip.org
- Privacy Policy
- Terms of Use
Connect with AIP Publishing
This feature is available to subscribers only.
Sign In or Create an Account
Synthesis and characterization of zinc oxide prepared with ammonium hydroxide and photocatalytic application of organic dye under ultraviolet illumination
- Original Paper
- Published: 13 May 2017
- Volume 148 , pages 1177–1183, ( 2017 )
Cite this article
- Sasimonton Moungsrijun 1 ,
- Supphadate Sujinnapram 1 &
- Sutthipoj Sutthana ORCID: orcid.org/0000-0002-9818-384X 1
579 Accesses
8 Citations
Explore all metrics
ZnO was successfully synthesized using a simple precipitation method with zinc acetate dihydrate as the starting material and ammonium hydroxide solution as the oxidizer. Morphological analysis showed ZnO particle formation with a Zn/O atomic ratio of 0.75–0.92. The synthesized ZnO product was examined using X-ray diffraction measurement (XRD) with the corresponding major peaks implying a hexagonal wurtzite structure. The average crystal size was 18.26–20.49 nm and the specific surface area was 18.17–21.67 m 2 /g. The optical band gap was estimated in the range of 2.95–3.21 eV. For photocatalytic activity, the synthesized ZnO was dispersed in two kinds of organic dyes (eosin-Y and methyl orange) with an initial concentration of 10 mg/cm 3 . The activity was carried out under ultraviolet illumination and the absorbance was measured. The best degradation efficiency was observed with a maximum degradation rate constant of 6.75 × 10 −3 /min and 1.73 × 10 −3 /min for eosin-Y and methyl orange, respectively. The degradation efficiency exhibited a direct correlation to the SSA, with the high SSA provided by the nanostructure size exhibiting better degradation efficiency. Therefore, ZnO nanostructures synthesized from zinc acetate dihydrated using an ammonium hydroxide oxidizer was shown to be a potential material for photocatalytic activity.
Graphical abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Photocatalytic degradation of malachite green dye by modified zno nanomaterial.
S Meena, Dipti VAYA & B K Das
Synthesis and characterization of Zn3V2O8 nanoparticles: mechanism and factors influencing crystal violet photodegradation
Fatin Tagnaouti Moumnani, Khadija Khallouk, … Abdelhak Kherbeche
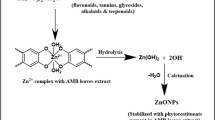
Influence of synthetic parameters on the enhanced photocatalytic properties of ZnO nanoparticles for the degradation of organic dyes: a green approach
Dhananjayan Badma Priya, Dhakshanamurthy Thirumalai & Indira Viswambaran Asharani
Danwittayakul S, Jaisai M, Dutta J (2015) Appl Catal B-Environ 163:1
Article CAS Google Scholar
Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, Bhatnagar A (2016) Chem Eng J 306:1116
Zheng L, Zheng Y, Chen C, Zhan Y, Lin X, Zheng Q, Wei K, Zhu J (2009) Inorg Chem 48:1819
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Appl Catal B-Environ 202:217
Aslam M, Charfi A, Lesage G, Heran M, Kim J (2017) Chem Eng J 307:897
Jo WK, Tayade RJ (2014) Chin J Catal 35:1781
Thepnurat M, Chairuangsri T, Hongsith N, Ruankham P, Choopun S (2015) ACS Appl Mater Interfaces 7:24177
Hong LY, Ke HW, Tsai CE, Lin HN (2016) Mater Lett 185:243
Kim YK, Hwang SH, Kim S, Park H, Lim SK (2017) Sensor Actuat B-Chem 240:1106
Ponhan W, Thepnurat M, Phadungdhitidhada S, Wongratanaphisan D, Choopun S (2016) Surf Coat Tech 306:41
Ruankham P, Choopun S, Wongratanaphisan D, Sagawa T (2016) Integr Ferroelectr 175:113
Chava RK, Kang M (2017) J Alloys Compd 692:67
Qin J, Zhang X, Yang C, Cao M, Ma M, Liu R (2017) Appl Surf Sci 392:196
Li W, Gao S, Li L, Jiao S, Yu Q, Li H, Wang J, Yu Q, Zhang Y, Wang D (2016) Mater Lett 185:161
Wang J, Pei C, Cheng L, Wan W, Zhao Q, Yang H, Liu S (2016) Sensor Actuat B-Chem 223:650
Lang J, Wang J, Zhang Q, Li X, Han Q, Wei M, Sui Y, Wang D, Yang J (2016) Ceram Int 42:14175
Ng SS, Ooi PK, Yaakob S, Abdullah MJ, Hassan HA, Hassan Z (2014) Sains Malays 43:1077
CAS Google Scholar
Jia T, Fu F, Long F, Min Z, Zhao J, Chen J, Li J (2016) Ceram Int 42:13893
Mamaghani AH, Haghighat F, Lee CS (2017) Appl Catal B-Environ 203:247
Khuili M, Fazouan N, El Makarim HA, El Halani G, Atmani EH (2016) J Alloys Compd 688:368
Zhang P, Li X, Wu X, Zhao T, Wen L (2016) J Alloys Compd 673:405
Rajamanickam D, Dhatshanamurthi P, Shanthi M (2015) Spectrochim Acta A 138:489
Nouri A, Fakhri A (2015) Spectrochim Acta A 138:563
Zhang L, Dai CH, Zhang XX, Liu YN, Yan JH (2016) Trans Nonferrous Met Soc China 26:2380
Akhil K, Jayakumar J, Gayathri G, Khan SS (2016) J Photoch Photobio B 160:32
Wang Y, Zhu L, Wang M, Zhang M, Yang Y, Zhu Q, Lei Y (2016) Int J Hydrogen Energy 41:15703
Mageshwari K, Mali SS, Sathyamoorthy R, Patil PS (2013) Powder Technol 249:456
Shen Y, Wang W, Xiao K (2016) J Environ Chem Eng 4:1259
Rezaei M, Salem S (2016) Spectrochim Acta A 167:41
Download references
Acknowledgements
This work was supported by the Kasetsart University Research and Development Institute (KURDI), Kasetsart University, Bangkok, Thailand.
Author information
Authors and affiliations.
Department of Physics, Faculty of Liberal Arts and Science, Kasetsart University Kamphaeng Saen Campus, Nakhon Pathom, 73140, Thailand
Sasimonton Moungsrijun, Supphadate Sujinnapram & Sutthipoj Sutthana
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sutthipoj Sutthana .
Rights and permissions
Reprints and permissions
About this article
Moungsrijun, S., Sujinnapram, S. & Sutthana, S. Synthesis and characterization of zinc oxide prepared with ammonium hydroxide and photocatalytic application of organic dye under ultraviolet illumination. Monatsh Chem 148 , 1177–1183 (2017). https://doi.org/10.1007/s00706-017-1959-z
Download citation
Received : 21 November 2016
Accepted : 19 March 2017
Published : 13 May 2017
Issue Date : July 2017
DOI : https://doi.org/10.1007/s00706-017-1959-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanostructures
- Photocatalytic activity
- Organic dyes
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
1. Introduction. Zinc oxide, with its unique physical and chemical properties, such as high chemical stability, high electrochemical coupling coefficient, broad range of radiation absorption and high photostability, is a multifunctional material [1,2].In materials science, zinc oxide is classified as a semiconductor in group II-VI, whose covalence is on the boundary between ionic and covalent ...
Zinc oxide nanoparticles (ZnO-NPs) are the most commonly used metal oxide nanoparticles because their distinctive optical and chemical properties can be easily modified by altering the morphology and the wide bandgap (3.37 eV) and high excitation binding energy (60 meV) to simulate the ZnO-NPs to be a potent photocatalytic and photo-oxidizing ...
C-O stretching was absorbed in 1033 cm −1 which is further the zinc oxide nanoparticles synthesized from shilajit exhibited a characteristic peak at 3428 cm −1, 2921 cm −1, 1633 cm −1 ...
1. Introduction. Zinc oxide has been used in diverse applications for thousands of years [1] and could reasonably be considered to be a mature engineering material [2] with annual production now approaching one and a half million tons [3].Nevertheless, there has been a steep rise in the number of scientific publications addressing this material in the last decade indicating significant new ...
Zinc oxide can also be utilized to construct a biosensor that specifically can detect Bisphenol A, i.e., BPA in a plastic bottle proved by Kunene et al. [90], in this they employ electrode (carbon screen printed) which are modified with MWCNTs and functionalized by the help of silver doped zinc oxide hybrid and the enzyme named-laccase was ...
Zinc oxide (ZnO) is a distinctive inorganic material exhibiting various properties like piezoelectric, pyroelectric, semiconducting, optoelctronics and catalysis [].ZnO is an n-type semiconductor with band gap energy of 3.37 eV at room temperature and 60 meV excitation binding energy [].ZnO is non-toxic, compatible with skin, and also used as a UV blocker in sunscreen products [].
Zinc oxide (ZnO), magnesium oxide (MgO) and titanium dioxide (TiO 2) are substances recognized as safe when used as food additives or drug deliverers according to the Food and Drug Administration ...
Abbasi, B. A. et al. Bioactivities of Geranium wallichianum leaf extracts conjugated with zinc oxide nanoparticles. Biomolecules 10 , 38-68 (2019). Article Google Scholar
Green approach. Biosynthesis of nanoparticles is an approach of synthesizing nanoparticles using microorganisms and plants having biomedical applications. This approach is an environment-friendly, cost-effective, biocompatible, safe, green approach [22]. Green synthesis includes synthesis through plants, bacteria, fungi, algae etc.
The zinc oxide (ZnO) is an important n-type semiconductor material having direct bandgap of 3.37 eV, large excitons binding energy (60 meV) and high electron mobility [1,2,3,4].These characteristics demonstrates use of ZnO nanostructures in the field of optoelectronics, medications, solar cells, automotive, cosmetics, gas sensors, field emission and photocatalysis [3,4,5,6,7,8,9].
The synthesis of nano- and micro-sized particles, as well as an eco-friendly approach with non-toxic chemicals, is essential for the development of medicine in nanotechnology. This study focuses on the green synthesis of zinc oxide nanoparticles (ZnO NPs) using the aqueous leaf extract of an endemic plant, Jatropha maheshwarii. Microscopic and spectroscopic techniques such as UV-visible ...
ABSTRACT. Green synthesis of nanoparticles by biological systems especially plant extracts has become an emerging field in nanotechnology. In this study, zinc oxide nanoparticles were synthesized using Laurus nobilis L. leaves aqueous extract and two different zinc salts (zinc acetate and zinc nitrate) as precursors. The synthesized nanoparticles were characterized by Ultraviolet-Visible ...
Nanotechnology in modern material science is a research hot spot due to its ability to provide novel applications in the field of dentistry. Zinc Oxide Nanoparticles (ZnO NPs) are metal oxide nanoparticles that open new opportunities for biomedical applications that range from diagnosis to treatment. The domains of these nanoparticles are wide and diverse and include the effects brought about ...
Zinc oxide is an essential ingredient of many enzymes, sun screens, and ointments for pain and itch relief. Its microcrystals are very efficient light absorbers in the UVA and UVB region of spectra due to wide bandgap. ... Article Google Scholar Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol ...
Development of plant based nanoparticles has many advantages over conventional physico-chemical methods and has various applications in medicine and biology. In present study, zinc oxide (ZnO ...
Therefore, this review paper is focused on summaries of the biosynthesis of Zinc oxide nanoparticles from leaf extract such as Mangifera Indica (Mango), Ixora Cocconea (Jungle Geranium), Corymbia Citridora (Lemon-scented Gum) as a new development of green technology beneficial to the environment and to the plant itself. It also describes the ...
Sunscreen safety and efficacy is generally evaluated based upon the properties of the individual chemicals in a formulation. However, the photostability of sunscreens has been shown to be highly dependent on the mixture of chemicals present. To better understand how sunscreen formulation influences stability, and to establish a foundation for probing the influence of zinc oxide additives, we ...
Google Scholar) documents a tight and uniform distribution of ultramicronized ZnO particle diameters, approximately 100-300 nm with a mean of 251 nm. ... (SPF 30, containing 15% zinc oxide) versus the application of popular conventional zinc oxide-based mineral sunscreens (sunscreen C, SPF 36, containing 21.6% zinc oxide, and sunscreen D, ...
Article CAS Google Scholar Zheng L, Zheng Y, Chen C, Zhan Y, Lin X, Zheng Q, Wei K, Zhu J (2009) Inorg Chem 48:1819 ... S. & Sutthana, S. Synthesis and characterization of zinc oxide prepared with ammonium hydroxide and photocatalytic application of organic dye under ultraviolet illumination. Monatsh Chem 148, 1177-1183 (2017). https: ...