- Search Menu
- Advance Articles
- Thematic Issues
- Clinical Practice Guidelines
- Supplements
- Endocrine Reviews
- Endocrinology
- Journal of the Endocrine Society
- The Journal of Clinical Endocrinology & Metabolism
- JCEM Case Reports
- Molecular Endocrinology
- Endocrine Society Journals
- Author Guidelines
- Submission Site
- Open Access
- Why Publish with the Endocrine Society
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About The Journal of Clinical Endocrinology & Metabolism
- Editorial Board
- Author Resources
- Reviewer Resources
- Rights & Permissions
- Other Society Publications
- Member Access
- Journals Career Network
- Terms and Conditions
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Anorexia nervosa, eating disorders not otherwise specified (ednos), acknowledgments, abbreviations:.
- < Previous
Endocrine Manifestations of Eating Disorders
- Article contents
- Figures & tables
- Supplementary Data
Michelle P. Warren, Endocrine Manifestations of Eating Disorders, The Journal of Clinical Endocrinology & Metabolism , Volume 96, Issue 2, 1 February 2011, Pages 333–343, https://doi.org/10.1210/jc.2009-2304
- Permissions Icon Permissions
The endocrinopathies associated with eating disorders involve multiple systems and mechanisms designed to preserve energy and protect essential organs. Those systems that are most affected are in need of significant energy, such as the reproductive and skeletal systems. The changes in neuropeptides and in the hypothalamic axis that mediate these changes also receive input from neuroendocrine signals sensitive to satiety and food intake and in turn may be poised to provide significant energy conservation. These adaptive changes are described, including the thyroid, GH, and cortisol axes, as well as the gastrointestinal tract.
Articles were found via PubMed search for both original articles and reviews summarizing current understanding of the endocrine changes of eating disorders based on peer review publications on the topic between 1974 and 2009.
The signals that control weight and food intake are complex and probably involve multiple pathways that appear to have as a central control the hypothalamus, in particular the medial central area. The hypothalamic dysfunction of eating disorders provides a reversible experiment of nature that gives insight into understanding the role of various neuropeptides signaling nutritional status, feeding behavior, skeletal repair, and reproductive function.
The endocrinopathies seen with eating disorders have fascinated scientists for decades. They present strong evidence for diffuse hypothalamic dysfunction and provide a reversible experiment of nature that gives insight into understanding the role of various neuropeptides signaling nutritional status. In turn, these central signals of nutritional status may be important mediators in the maintenance of normal reproduction and skeletal integrity.
Eating disorders include the syndrome of anorexia nervosa, which is characterized by a classic triad of amenorrhea, weight loss, and psychiatric disturbance ( 1 – 3 ). Bulimia nervosa is often related to previous anorectic behavior and is characterized by gorging followed by periods of severe food restriction. Unusual methods are employed to lose weight, including vomiting and abuse of diuretics or laxatives. Some eating disorders are classified as an eating disorder not otherwise specified or “EDNOS” and may be associated with excessive exercise ( 4 ). All of these conditions are more common in women when thinness is advantageous, such as professional dance and certain competitive athletics including gymnastics and long-distance running ( 5 ).
Hormonal changes
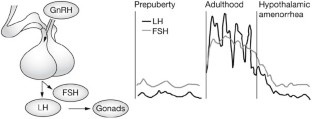
The most extensively studied eating disorder is anorexia nervosa. Typically, hypothalamic amenorrhea is accompanied by low levels of gonadotropins and a profound estrogen deficiency. Amenorrhea may be absent, however, and there is presently considerable debate as to the appropriateness of the criteria for anorexia nervosa due to the presence of multiple endocrine and metabolic abnormalities in some patients without amenorrhea ( 6 ). Some of the lowest levels of LH seen in secondary amenorrhea have been observed in this syndrome ( 7 ), although the LH level may be higher if there are other endocrinopathies present such as an underlying polycystic ovarian syndrome (PCOS), an association that has recently been reported ( 8 ). Twenty-four-hour studies of gonadotropin secretion, which reflect GnRH secretion, reveal persistent low levels throughout the day and night with the absence of the normal pulsatile peaks of LH every 60–90 min ( 9 ). With recovery (weight gain), sleep-associated episodic secretion of LH occurs similar to the peripubertal child, and with full recovery the normal pulsatile activity occurs without sleep-associated spikes ( 9 ). However, as the GnRH pulse generator recovers, a variety of LH patterns may occur ( 9 ).
Additional hormone changes of the eating disorders are summarized in Table 1 . Low leptin levels have been reported ( 10 , 11 ). Leptin is a small anorexigenic polypeptide made by many tissues including fat cells and is thought to be a signal to the brain of nutritional status. It also changes rapidly with food restriction. Its administration in nutritionally restricted animals will cause the return of LH pulsatility, and administration of recombinant leptin has led to return of LH pulsatility and ovulation in some patients with hypothalamic amenorrhea ( 12 ). Leptin also has a diurnal pattern of secretion, with rising levels during the day and falling at night. Fasting will delay this rise ( 13 , 14 ). Low basal and pulsatile secretion of leptin has been reported in anorexia nervosa and hypothalamic amenorrhea, and weight gain appears to result in a surge of secretion leading to higher than normal levels ( 15 , 16 ). However, there is a large variability in levels with overlap with normal.
Changes in hormonal mediators in anorexia nervosa, bulimia, and EDNOS
↑, Increase; ↓, decrease; ?, undetermined.
Other neuropeptides
Ghrelin, in contrast to leptin, is an orexigenic peptide secreted from the oxyntic cells of the stomach that causes decreased gonadotropin pulsatility in animals and humans ( 17 ). It has been found to be elevated in women with disordered eating and amenorrhea ( 18 ) and may be a better discriminator of immediate energy availability than leptin ( 19 ), which was found to be normal in anorexia whereas ghrelin was elevated.
Peptide YY (PYY), like leptin, is an anorexigenic peptide that is secreted in response to caloric intake and is derived from the gut. It is thought to act at the level of the hypothalamus by binding to the Y2 receptor, causing presynaptic inhibition of neuropeptide Y neurons with resultant stimulation of proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus. PYY is low in obesity, and its administration in the rodent decreases food intake ( 20 ). Patients with anorexia nervosa have been found to have elevated levels. This may predispose patients with this syndrome to reduce food intake ( 15 ).
Thus, these neuropeptides secreted by the fat cells, the stomach, and gut, respectively, are most likely interacting signals reflecting nutritional homeostasis as reflected in fat composition and the sum of energy available and in turn appropriateness for reproduction. The neuropeptides are also most likely involved in the regulation of signals reaching the medial central hypothalamus from the arcuate nucleus, the center responsible for the important episodic stimulation of GnRH. This interaction intimately ties metabolic status to reproduction. Both leptin and ghrelin interact with POMC and agouti-related peptide/neuropeptide Y, key peptides in the arcuate nucleus, and appear to modulate food intake. Both POMC and agouti-related peptide send projections into other areas of the hypothalamus, including the lateral hypothalamus and the periventricular nucleus. Thus, there are multiple integrated signals that may be affected with nutritional restriction and may affect other systems such as the skeleton and the reproductive system ( 21 , 22 ) ( Fig. 1 ).
![anorexia nervosa hormonal changes Adipose tissue- and gut-derived hormones and reproduction. [Reproduced with permission from Budak et al.: Fertil Steril 85:1563–1581, 2006 (21).] CART, Cocaine and amphetamine-regulated transcript; NPY, neuropeptide Y; AgRP, Agouti-related protein.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/96/2/10.1210_jc.2009-2304/3/m_zeg0021178640001.jpeg?Expires=1714564216&Signature=k2NxxQG5pZ09aBE8jd-N4HguIhPMbIHNOtKB0INxvBHWPSQGHLiXyDoPNFPHusCoQq~cDn-X3wnWMyexTLDqrM1qv6orZVEqMaTmeLO3yG3Li-UL-xtehb2dDK3-yoF6o1XiJ2Lx2iXahQoXRklKFz3cDlEWsLXJseM7yzoYrW-fftJIbPhFFqKVRliSQliKYRfFGK1576~qA6dPPrWNEJlWQvGBGYdkh3fXcDXVbIf4OFQrFew45t7A4pdFCr2VovutxDhTqfCGu~apKasymLX6tWuVKQ07Es5toPCKh2npnKHFYKI48j4aRHHu0XBfNUNtSH6H~cSYNd5AMl8aIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Adipose tissue- and gut-derived hormones and reproduction. [Reproduced with permission from Budak et al. : Fertil Steril 85:1563–1581, 2006 ( 21 ).] CART, Cocaine and amphetamine-regulated transcript; NPY, neuropeptide Y; AgRP, Agouti-related protein.
The adrenal axis
The suppressed gonadotropin secretion in anorexia is accompanied by low T 3 , low IGF-I, and high cortisol levels, the latter finding differentiating anorexia nervosa from pituitary insufficiency. Diurnal variation of cortisol is preserved, but at a higher set point as shown in 24-h studies of cortisol secretion. Elevated cortisol is found in multiple sites including the serum, urine, and saliva ( 23 ), and corticotropin-releasing factor (CRH) is elevated in cerebral spinal fluid ( 24 ), suggesting a centrally driven mechanism causing the elevated cortisol. However, the ACTH response is inappropriately low and suggests feedback at the pituitary level, whereas failure of dexamethasone to fully suppress cortisol suggests impairment in feedback or an altered set point. Increased secretory bursts and half-life of cortisol have been found and appear to account for the elevated levels ( 23 ). At the present time, the balance of evidence suggests that an altered set point occurs with a diurnal variation maintained at a higher level ( 25 ). Recent studies suggesting an absence of diurnal variation have infrequent sampling of cortisol ( 26 , 27 ) and thus may not be accurate. Chronic elevation of cortisol would be expected to be associated with Cushingoid-like features, but this is not the case. However, the weight gain associated with recovery in anorexia is marked by an accumulation of fat in a central (truncal) pattern denoting accumulation of visceral fat and documented by magnetic resonance imaging and dual-energy x-ray absorptiometry ( 28 , 29 ). In one study, cortisol predicted increases in body fat ( 30 ), and cortisol levels may be instrumental in this pattern of fat distribution. Insulin levels are usually normal, but sensitivity may be increased ( 31 , 32 ).
The GH axis
Increased GH levels accompanied by decreased IGF-I suggest an acquired resistance to GH that reverses with refeeding ( 33 – 35 ) as well as a reduction in the bioactivity of IGF-I ( 36 ). In the starved state, GH shows increased basal secretory rates as well as increased secretory pulses. This appears to be an adaptation to starvation because IGF-I secretion is blocked by the liver and negative feedback is attenuated ( 33 , 34 ). GH levels are also negatively correlated with markers of nutritional status including body mass index (BMI), fat mass, and leptin ( 37 ).
The thyroid axis
Also typical in anorexia are changes seen with the euthyroid sick syndrome ( 37 ). T 3 levels are low, whereas rT 3 is elevated. In some patients, T 4 is also decreased ( 37 , 38 ). TSH levels are normal or occasionally slightly reduced, suggesting a hypothalamic origin of the suppressed thyroid function. With weight gain, T 3 levels rise and rT 3 levels fall, and one study showed a correlation with rising metabolic rate suggesting that T 3 is involved in controlling metabolic rate ( 37 , 39 ). Interestingly, anorexia presents with many clinical features of hypothyroidism (bradycardia, hypothermia, delayed ankle reflexes), features that conserve energy. Treatment with thyroid hormone is inappropriate and leads to undesirable weight loss and loss of muscle mass ( 37 ). It should be noted that in starving patients, a lowered metabolic rate, an increase in cortisol (which stimulates gluconeogenesis and decreases peripheral glucose utilization), and a decrease in gonadotropins (with a loss of fertility) are all appropriate adaptations.
The gonadal axis
The primary changes in this axis are described under hormonal changes. Secretion of androgens including in particular testosterone is deficient in this syndrome, suggesting that gonadal sources are compromised ( 40 ). Although smaller longitudinal studies suggest compromise with improvement in recovery ( 41 ), adrenal precursors appear to be normal in large cross-sectional studies ( 40 , 42 ). Low estradiol levels are also seen in anorexia due to a lack of ovarian stimulation. However, estrogen metabolism is also altered. Estradiol, which normally undergoes 16α-hydroxylation, is channeled to 2-hydroxylation and the formation of a catechol estrogen (2-hydroxyestrone) in the undernourished state ( 43 ). This compound has no intrinsic biological activity and acts as an antiestrogen. Thus, the very low estrogen levels seen in anorexia are compounded by an endogenously produced antiestrogen. The lack of adipose tissue may also contribute to the hypoestrogenic state by limiting the extraovarian sources of estrogen because fat converts androstenedione to estrone and testosterone to estradiol.
Adipose tissue
Most studies have also noted increased levels of adiponectin in anorexia nervosa ( 44 ), which appears to be inversely related to BMI. The increase in this adipokine may be related to the increased insulin sensitivity seen in anorexia. Although some studies report normal or increased insulin sensitivity and some report normal insulin sensitivity, they may be explained by different methodologies. For example, when homeostasis model of assessment for insulin resistance is examined under basal conditions, increased insulin sensitivity ( 31 , 45 ) is demonstrated, whereas use of the euglycemic hyperinsulinemic clamp technique shows decreased ( 46 ) or normal insulin sensitivity ( 32 , 47 ). SHBG is high, perhaps related to the insulin sensitivity, and appears to be a marker of nutritional recovery ( 48 , 49 ). Another adipokine affecting fuel homeostasis and insulin action is resistin, which is decreased in some studies on anorexia ( 32 ) although its role in fuel homeostasis is unclear.
Osteoporosis is a leading and severe complication of anorexia nervosa. Most of the endocrinopathies described above likely contribute to the bone loss including low T 3 , estradiol, testosterone, IGF-I, high cortisol, and various neuropeptides. In addition, it has recently been recognized that bone is a highly metabolically active tissue requiring energy ( 50 ), and the process of forming new bone is appropriately suppressed in situations where nutritional status is compromised. Mechanisms that link bone formation to the adipose cells have recently been recognized and open new vistas to the understanding and treatment of bone loss. In addition, the skeleton itself interacts with body weight, has feedback from energy expenditure, and is involved in glucose homeostasis. In summary, the etiology of the low bone mass in anorexia nervosa is multifactorial ( 51 ), reflecting endocrine dysfunction in several areas ( 52 ) ( Fig. 2 ).
![anorexia nervosa hormonal changes Hormonal adaptation in anorexia nervosa. [Reproduced with permission from Jayasinghe et al.: BJOG 115:304–315, 2008 (52).] FLI, Free leptin index; sOB-r soluble leptin receptor; IGFBP1&2, IGF-binding protein 1 and 2.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/96/2/10.1210_jc.2009-2304/3/m_zeg0021178640002.jpeg?Expires=1714564216&Signature=I8NHuiH0AM6v4OiBjxmwMKKPDus~Rg3eINWbk9oPct38b6RnPKSxTzyZYskwcb-4Ef1bErZx0QM3BikFBUl6WtfA99vKBDKG7nHfE8I7P69cgQnzYVPRTZb77v2~s7OWWbV4W7nTjLHS-GSZE6~nNWHCqRaOY9iYgeAOsCyCzv68o6GMlebcNpC47yvqT~55gyuFVIZqTfMKcNyEEUWcPnxthh8QO1LouGl0XcV3FPM4zlfcJtHhW8isE2GV1-1MdcMQqD~cBpb1s9KHIRywioNyhhf0k9xe1TMAIEaq3FxI~Y8R28cKQlawTAN1pwSPC8fGmrezja0qIC0SksweiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Hormonal adaptation in anorexia nervosa. [Reproduced with permission from Jayasinghe et al. : BJOG 115:304–315, 2008 ( 52 ).] FLI, Free leptin index; sOB-r soluble leptin receptor; IGFBP1&2, IGF-binding protein 1 and 2.
Osteopenia, or low bone mass, and osteoporosis are among the most severe and common complications of anorexia nervosa ( 53 – 55 ) and are associated with fragility fractures, even in the young population where this condition generally surfaces ( 56 ). The cumulative risk of fracture is 57% for a follow-up of 40 yr after diagnosis ( 57 ), and microarchitecture is abnormal ( 58 ). Bone mass may not fully return to normal levels even with weight gain, calcium supplementation, and hormonal therapy. Young patients most likely never reach their optimal peak bone mass, which puts them at risk for severe osteoporosis later in life ( 59 ). Severe complications such as collapse of the femoral head and hip fracture and other fractures have been reported at a young age ( 60 – 65 ).
Considerable confusion in the pathogenesis of bone loss in anorexia nervosa has led to the widespread belief that estrogen replacement will prevent bone loss. Multiple randomized studies have shown, however, that neither estrogen replacement nor oral contraceptive therapy is effective and, in fact, bone loss and fractures may continue in treated women ( 66 , 67 ).
These observations would suggest that estrogen deficiency alone cannot explain the skeletal findings in anorexia nervosa. In the pure hypoestrogenic model, both formation and resorption of bone increase. In contrast, anorexia has been associated with an uncoupling of markers of bone turnover and with a suppression of bone formation that reverses with refeeding. This appears to be the dominant mechanism ( 68 ). Bone resorption on the other hand is increased and does not normalize until menses return, associated with endogenous estrogen secretion ( 8 ) ( Fig. 3 ). However, it is possible that the restoration of menses is associated with improvement in the secretion of other important neuropeptides that may positively affect bone mass.
![anorexia nervosa hormonal changes Mean (±sd) changes in osteocalcin and urine N- telopeptide (NTX) concentrations from admission for treatment of anorexia nervosa until recovery of 90% of ideal body weight (IBW) for subjects who regained menses and subjects who remained amenorrheic. Bold lines represent ±2 sd values from the mean osteocalcin (a marker of formation) value of 6.20 ± 1.90 ng/ml and mean NTX (a marker of resorption) value of 37.00 ± 6.00 nmol/mmol creatinine (Cr) for reference control subjects. (reference controls; L. Audi, personal communication, 23 November 2004). [Adapted from J. Dominguez et al.: Am J Clin Nutr 86:92–99, 2007 (8).]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/96/2/10.1210_jc.2009-2304/3/m_zeg0021178640003.jpeg?Expires=1714564216&Signature=ZfBvsF32Leo562SryTMZeYB7zHJiDfT3Jx7swqCjfp~vpXR4CX4RT~Tw0ot0hIN-gqHn4KZn6BZosNy3C9B3WfJKohbKnFurdZNSXtpuNDxY5vlmsJf9VYYaHgc0WFU2J7RlermK7AgmnB7sWaDhPqnoMhBIvHAFbYSKe51RkNHNtqEAemxlSl~sq9uBhrudaufdpW2TLDpVHZvxCP-F5zB~HCkr0QbiK-yTUkAZl2oIIYxTKVXX-IiqGWTM--aqdzhvAGaG~YHjJdK6pKR5eiXByuHPjAj5GNOi25RwjPJjvcqH~zKHYw-jOp6blZOWmVL27qpWVRxaHfa5yUo~pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Mean (± sd ) changes in osteocalcin and urine N - telopeptide (NTX) concentrations from admission for treatment of anorexia nervosa until recovery of 90% of ideal body weight (IBW) for subjects who regained menses and subjects who remained amenorrheic. Bold lines represent ±2 sd values from the mean osteocalcin (a marker of formation) value of 6.20 ± 1.90 ng/ml and mean NTX (a marker of resorption) value of 37.00 ± 6.00 nmol/mmol creatinine (Cr) for reference control subjects. (reference controls; L. Audi, personal communication, 23 November 2004). [Adapted from J. Dominguez et al. : Am J Clin Nutr 86:92–99, 2007 ( 8 ).]
Vitamin D levels in anorexia are generally normal, as is the vitamin D binding protein ( 69 ), although the serum binding capacity was diminished in a small study ( 70 ). PTH levels are also generally normal, suggesting that the impressive bone loss seen in anorexia is not due to deficiencies in vitamin D and a secondary hyperparathyroidism ( 71 , 72 ).
Androgens, which also have an anabolic effect on bone, are also known to be depressed in anorexia nervosa. However, a 1-yr randomized study of oral dehydroepiandrosterone did not demonstrate significant effect of treatment ( 73 ). A 3-wk study with testosterone administration also did not have consistent effects on bone, but mood was improved ( 74 ).
Cortisol excess may also affect bone loss by inhibiting bone formation. Markers of bone formation are inversely correlated with cortisol levels as well as spine bone mineral density (BMD) ( 23 ).
Recently, it has been suggested that a bone-adipose axis exists and that various adipokines such as leptin, adiponectin, and resistin may be involved in bone physiology ( 51 , 75 ). In addition, the skeleton itself may have effects on body weight control and glucose homeostasis and is a regulator of energy balance ( 50 , 75 ). Leptin, which is found in adipose tissue, is low in anorexia ( 17 , 37 ) and has been found to have contradictory effects on bone ( 37 , 50 , 76 – 78 ). However, in a study of patients with hypothalamic amenorrhea given recombinant leptin, an increase in osteocalcin (a marker of bone formation) was seen ( 12 ). Adiponectin (which is elevated in anorexia) is thought to indirectly affect induction of osteoclast formation via receptor activator for nuclear factor κB ligand stimulation and inhibition of osteoprotegerin production in osteoblasts, although human studies are very preliminary ( 32 ). Resistin, which is low in anorexia ( 32 ), is thought to stimulate osteoclasts and osteoblastic proliferation, although its effect in humans is unclear.
The role of leptin in regulation of bone mass is unclear but probably involves peripheral secretion signaling hypothalamic neurons and ultimately a hypothalamic relay involving the sympathetic nervous system, which suggests that feedback systems exist that are sensitive to energy demands ( 50 , 51 , 76 ). In this system, leptin stimulates the β2-adrenegic receptor in bone, decreasing osteoblast proliferation and bone formation ( 76 ). Another possible role of leptin is mediated through activation of cocaine amphetamine-regulated transcript neurons expressed in the hypothalamus, which appear to inhibit bone loss ( 79 , 80 ). Thus, several systems may be involved in the cross-regulation of bone and energy, and because the data are highly preliminary, the exact dysfunction in anorexia nervosa is not understood. In addition to the bone-adipose tissue connection is a gut-skeleton connection with serotonin as an important mediator ( 81 ). The fact that energy requirements involving neuropeptides are key regulators in both the skeleton and the gut suggests that these systems may be interconnected.
Gastrointestinal peptides
The gut appears to be an important source of neuropeptide signals. The cells that secrete these signals include the L cells in the small intestine and colon that secrete peptides such as glucagon-like peptide 1 and PYY, which has been associated with low bone density in anorexia ( 20 , 44 , 82 ). Ghrelin, which is elevated in anorexia, is secreted from the stomach, which at present has no known effect on the skeleton ( 50 ). Noteworthy is that these peptides travel to the brain to control metabolism and food intake, suggesting that the brain uses these peripheral signals to measure nutritional status, energy intake, and energy needs. Receptors for these peptides are present in the hypothalamus and the hindbrain, and the arcuate nucleus appears to behave as a control center. The signals for normal GnRH pulsatility also emanate from the arcuate nucleus, integrating nutrition and reproduction.
In terms of treatment, the most significant predictor of recovery as measured by return of menses is the BMI ( 22 ). For underweight subjects, nutritional counseling is paramount with psychological or psychiatric therapy if eating disorders are present. Increases in BMI are accompanied by increases in bone density ( 12 , 58 ). Thus, reestablishing a normal weight is the first aim in recovery, although normalization of BMD may not occur. With refeeding, parameters of bone turnover return rapidly to normal. The effects of weight gain are robust and to date no pharmacological intervention has approached the improvement seen with refeeding ( 83 ). In a controlled hospital environment, increases of 3 to 4% in BMD have been seen in as little as 3 months ( 8 ). Numerous pharmacological interventions have been tried. Some have shown a small effect. In some cases, intervention has been questioned because of the possible suppression of bone formation. Interventions have included dehydroepiandrosterone ( 73 ) and testosterone replacement ( 74 ), oral contraceptives ( 84 ), bisphosphonates ( 85 , 86 ), and IGF-I ( 87 ).
The bisphosphonates are relatively contraindicated because of their long half-life in the bone but may be used with caution in compassionate situations when low bone mass is associated with fragility fractures and refeeding is not feasible. These compounds are labeled category C because of toxic effects in pregnant rats ( 88 , 89 ). Young women may eventually recover their reproductive potential and become pregnant and may put a developing fetus at risk if these compounds leach from bone.
The GH resistance noted in anorexia may have direct or indirect effects (via IGF-I) on bone. In a randomized study of women with anorexia nervosa, the administration of recombinant IGF-I had a small bone-sparing effect, and the within-group change was positive only in combination with an oral contraceptive ( 87 ). Nutritional restriction, particularly protein restriction, may be minimizing the powerful anabolic effects of IGF-I on bone ( 90 ). Animals deprived of protein show a rapid fall in IGF-I, which is accompanied by a decrease in osteocalcin. This effect is not reversed by the exogenous administration of IGF-I/IGF binding protein 3, indicating resistance in the face of protein deficiency ( 91 ). In fact, with presently available pharmacological interventions, the literature suggests that fractures and bone loss may continue in the face of weight loss or poor nutrition ( 56 , 62 , 67 , 84 , 92 , 93 ).
Bulimia, or bulimia nervosa, is an eating disorder marked by recurrent episodes of binge eating that may be followed by restrictive eating and inappropriate behavior to prevent weight gain (self-induced vomiting, laxatives, diuretics, and/or excessive exercise). This syndrome is also marked by a lack of impulse control, impulsive behavior, alcohol or drug use, promiscuity, and stealing or shoplifting. Anorectic behavior often precedes bulimia and may determine the presence of endocrine findings. The prevalence of bulimia is 1–1.5% of the population. Bulimia is more common in men than anorexia, but like anorexia the majority of patients are women with an incidence of 2% in community-based samples and 4–13% in college-aged groups ( 3 ). Patients tend to be older than those with anorexia nervosa, usually between 17 and 25 yr of age.
Patients with bulimia nervosa are sometimes classified as having the purging or nonpurging type, with the latter being less severe and the former having more psychiatric comorbidity, more metabolic disturbances, and lower weight. Depression is also common. Often the bulimic behavior evolves from the completely restricting anorexia nervosa. Weight fluctuates but is generally not at low levels, but metabolic issues cause serious health problems. Bulimics have a wide variety of medical problems including tooth decay, parotid enlargement, carpopedal spasm, stomach rupture, metabolic alkalosis, hypercarotenemia, hypokalemia, and pancreatitis.
Bulimia has not been studied as extensively as anorexia and because the weight may remain within a reasonable range, this type of problem is often difficult to diagnose. Multiple neuroendocrine abnormalities exist but are less pronounced than in anorexia. Bulimic patients may present with menstrual irregularity, but the incidence is highly variable ( 94 – 97 ), ranging from 37 to 64%, with amenorrhea occurring in 5–40% ( 94 , 95 , 98 ). This variability may be due to the fact that patients have adequate estrogen secretion and present with an anovulatory syndrome and irregular bleeding. With a preceding history of anorexia, however, the incidence of amenorrhea is much higher (77%) ( 98 ). The menstrual disorder and amenorrhea may also develop when weight is within a reasonable range. Because bulimic behavior is often secretive, patients will often not admit to this behavior, even when questioned directly ( 94 ). Eventually the protein calorie malnutrition and the metabolic problems may make the diagnosis obvious. However, laboratory values may remain normal, and these should not be relied upon to make a diagnosis. Risk factors for the development of menstrual dysfunction include the lowest reported BMI, a history of anorexia, or weight loss. More subtle associations include low calorie and dietary fat intake, frequent vomiting or binge eating, increased exercise, and low T 4 levels ( 95 , 97 – 99 ). The menstrual irregularity may persist with fluctuations in body weight, depression, and smoking suggesting a metabolic stress on the hypothalamic-pituitary-ovarian axis ( 96 ). Low LH and FSH levels along with low estradiol levels have been found in normal-weight women with bulimia nervosa as well as reduced LH patterns over a 24-h period ( 100 ).
Another interesting observation is the association of an abnormal polycystic ovarian morphology and bulimia, which is seen with high frequency (76–100%) ( 97 ). Criteria for polycystic ovarian morphology in these studies have used the Adams criteria (more than 10 follicles measuring 2–6 cm) ( 101 ), which is more liberal than the Rotterdam criteria (12 follicles measuring 2–9 mm and an ovarian volume of 10 ml) ( 102 ). Patients with PCOS may also have a high frequency of bulimia (6%) ( 103 – 106 ), although standard criteria for PCOS were not used and further work is needed. In a 9-yr follow-up of eight patients with bulimia, polycystic ovary morphology was seen at baseline in seven of nine patients. Five patients who were still bulimic continued to show polycystic ovary morphology by the Adams criteria, whereas three who had resolution of their bulimia had normal ovarian morphology ( 107 ). This is the first report of resolution of this morphology. It also suggests that binge eating may lead to abnormal insulin secretion and ovarian morphology ( 105 ). Hyperinsulinemia has been described in controlled experiments in normal women submitted to binge eating ( 108 ). PCOS may also lead in turn to bulimia by increasing insulin levels ( 97 ).
Other axes besides the hypothalamic-pituitary-ovarian have been found to be affected. The hypothalamic-pituitary-thyroidal axis is also abnormal. Bulimia nervosa has been associated with a decreased resting metabolic rate, which has been attributed to lower T 3 levels. There is a delayed peak and blunted response of thyrotropin-releasing hormone (TRH) to TSH. However, the data are conflicting, and others have noted normal thyroid indices and responses to TRH ( 109 ). One carefully done study examined bulimics at baseline and after 3 wk of abstinence from bulimic behavior. T 3 levels were normal at baseline but were significantly lower than matched controls. After abstinence, there was a decline in T 3 and T 4 levels and an increase in TSH. This suggests that the metabolic rate may be higher during bingeing episodes due to the higher thyroid indices and the indices fall when bingeing is absent, perhaps due to the low caloric intake of bulimics when not bingeing. Other studies have shown a lower resting metabolic rate and T 3 levels after abstinence. Interestingly, low T 4 levels have been associated with poor outcome in bulimics ( 110 ).
The hypothalamic-pituitary-adrenal (HPA) axis also shows dysregulation, and because satiety appears to be abnormal, the neurotransmitters involved in food intake and satiety have been the focus of research. In particular, the limbic-HPA axis appears to play an important role in hunger and satiety signaling. Both normal and increased circadian secretion of cortisol has been reported in bulimia nervosa. A recent study of eight bulimics with 3 wk of abstinence from purging or overeating showed decreased HPA activity with lower cortisol levels between 0600 and 1400 h and hyperreactivity to CRH ( 111 ). The heterogeneous results published in the literature may relate in part to whether the subjects are studied during an active bingeing interval or after a significant time of abstinence from this behavior. Morphological changes have also been reported, including increased visceral fat and adrenal gland volume ( 112 ).
Bulimia is associated with decreased leptin ( 113 ). In normal subjects, binge eating drastically alters the diurnal secretion of leptin, leading to a progressive and lower than normal fall in leptin during the day and a delay in the nocturnal rise in leptin after the evening meal. Associated with this change in leptin dynamics is an exaggerated insulin secretion and an increase in fasting glucose ( 108 ). As would be expected when leptin levels are decreased, ghrelin is increased in bulimia nervosa, with responses to ghrelin injection being fairly normal ( 114 ). Unlike anorexia nervosa where ghrelin injection causes a decrease in GH, bulimia is marked by a normal increase in GH, prolactin, ACTH, cortisol, and glucose, with a decrease in insulin and increases in appetite and food intake.
Bone density is also altered in bulimia, but this appears to be determined primarily by the presence of previous anorexia ( 115 ).
EDNOS have not been extensively studied but may appear more frequently in females with menstrual dysfunction than realized ( 116 – 118 ). This group includes those patients whose weight has not fallen to less than 85% of ideal body weight, women with a short interval of amenorrhea (less than 3 months), patients who purge regularly without bingeing or vice versa, and some patients who present the female athletic triad (disordered eating, amenorrhea, and osteoporosis). In particular, athletic women without eating disorders are often noted to report abnormal eating behavior that cannot be categorized as anorexia or bulimia but may have characteristics of both. Patients with all of the characteristics of anorexia nervosa and normal menses may also fall into this category. Disciplines where it is advantageous to be thin present particular risk, and some endocrine changes seen in anorexia are reported. A hypoestrogenic hypothalamic amenorrhea may develop with normal to low gonadotropins. Twenty-four-hour secretion of gonadotropins is altered with decreased pulses of LH and FSH. The underlying etiology is a chronic negative caloric balance. Findings specific to women with the female athletic triad include low T 3 levels and an increase in reverse T 3 . IGF-I levels may be low. Cortisol levels are higher than matched controls, and leptin is low whereas ghrelin is high, although there is much overlap ( 17 , 19 , 116 , 119 , 120 ). PYY elevation has also been associated with amenorrhea ( 121 ). Bone density may also be affected and show lower than normal levels. Fractures, particularly stress fractures, are a significant problem ( 122 ). Some reversal of the bone loss occurs with return of menses, but similar to anorexia nervosa, the use of hormone replacement does not guarantee a bone-sparing effect and may mask an important indicator of recovery. Although the use of oral contraceptives or hormones is commonly prescribed, the emerging evidence indicates that fractures may continue, and the problem is not due to hypoestrogenism but is mainly a nutritional issue ( 8 , 93 , 123 – 126 ). In fact, normal women put on an experimental protocol of exercise and food restriction were found to have rapid effects on indices of bone formation (within 5 d) and, if the restriction is severe enough, on bone resorption ( 127 ). These experiments show that the skeleton is exquisitely sensitive to nutritional restriction.
Eating disorders present with extensive hormonal dysfunctions that appear to give an unusual insight on the body's metabolic adjustments to food restriction and negative energy balance. In the case of bulimia, they also suggest that prolonged abnormal eating patterns may induce metabolic alterations possibly associated with chronically induced hyperinsulinemia. These syndromes are unfortunate, but unique metabolic aberrations associated with eating disorders may provide keys to understanding the neuroendocrine control of weight loss, reproduction, and nutritionally induced osteoporosis. At present, the most effective treatment to prevent bone loss and fractures is nutritional rehabilitation and normalization of aberrant eating patterns.
Disclosure Summary: M.P.W. received honorarium, consultancy fee, educational grant, and research support from Wyeth, and Wyeth activities include Advisory Board, speaker, and research; received consultancy fee from Pfizer; received speaker honorarium from Merck and Amgen; received consultancy fee from Depomed; received consultancy fee and consulted for Barr Laboratories; received consultancy fee and consulted for Bradley Pharmaceuticals; received speaker honorarium and clinical trial support from Novartis; received speaker honorarium from Novo Nordisk; received clinical trial support from Solvay Pharmaceuticals; received speaker honorarium from Upsher Smith; received speaker honorarium, consultancy fee, and consulted for Warner Chilcott; received consultancy fee and consulted for QuatRx; received consultancy fee and consulted for Council on Menopause Management; received royalty from Wolters Kluwer; received clinical trial support from Ferring Pharmaceuticals; received consultancy fee from and is member of Advisory Board for Yoplait; received consultancy fee and gave expert review for Medical Malpractice Insurance Pool.
Bone mineral density
body mass index
corticotropin-releasing hormone
eating disorder(s) not otherwise specified
hypothalamic-pituitary-adrenal
polycystic ovarian syndrome
proopiomelanocortin
thyrotropin-releasing hormone.
Warren MP 1985 Anorexia nervosa and related eating disorders. Clin Obstet Gynecol 28 : 588 – 597
Google Scholar
Klein DA , Walsh BT 2003 Eating disorders. Int Rev Psychiatry 15 : 205 – 216
Warren MP 1993 Anorexia and bulimia . In: , Sciarra JJ ed. Gynecology and obstetrics . Philadelphia : Lippincott ; 1 – 15
Google Preview
Psychiatric AA 2000 Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR) . 4th ed. Washington, DC : American Psychiatric Association
Warren MP 1999 Health issues for women athletes: exercise-induced amenorrhea. J Clin Endocrinol Metab 84 : 1892 – 1896
Attia E , Roberto CA 2009 Should amenorrhea be a diagnostic criterion for anorexia nervosa? Int J Eat Disord 42 : 581 – 589
Golden NH , Jacobson MS , Schebendach J , Solanto MV , Hertz SM , Shenker IR 1997 Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 151 : 16 – 21
Dominguez J , Goodman L , Sen Gupta S , Mayer L , Etu SF , Walsh BT , Wang J , Pierson R , Warren MP 2007 Treatment of anorexia nervosa is associated with increases in bone mineral density, and recovery is a biphasic process involving both nutrition and return of menses. Am J Clin Nutr 86 : 92 – 99
Boyar RM , Katz J , Finkelstein JW , Kapen S , Weiner H , Weitzman ED , Hellman L 1974 Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med 291 : 861 – 865
Köpp W , Blum WF , von Prittwitz S , Ziegler A , Lübbert H , Emons G , Herzog W , Herpertz S , Deter HC , Remschmidt H , Hebebrand J 1997 Low leptin levels predict amenorrhea in underweight and eating disordered females. Mol Psychiatry 2 : 335 – 340
Hebebrand J , Muller TD , Holtkamp K , Herpertz-Dahlmann B 2007 The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 12 : 23 – 35
Welt CK , Chan JL , Bullen J , Murphy R , Smith P , DePaoli AM , Karalis A , Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351 : 987 – 997
Elimam A , Marcus C 2002 Meal timing, fasting and glucocorticoids interplay in serum leptin concentrations and diurnal profile. Eur J Endocrinol 147 : 181 – 188
Schoeller DA , Cella LK , Sinha MK , Caro JF 1997 Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest 100 : 1882 – 1887
Hebebrand J , Blum WF , Barth N , Coners H , Englaro P , Juul A , Ziegler A , Warnke A , Rascher W , Remschmidt H 1997 Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Mol Psychiatry 2 : 330 – 334
Misra M , Miller KK , Kuo K , Griffin K , Stewart V , Hunter E , Herzog DB , Klibanski A 2005 Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289 : E373 – E381
Christo K , Cord J , Mendes N , Miller KK , Goldstein MA , Klibanski A , Misra M 2008 Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol (Oxf) 69 : 628 – 633
Misra M , Miller KK , Kuo K , Griffin K , Stewart V , Hunter E , Herzog DB , Klibanski A 2005 Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289 : E347 – E356
Schneider LF , Warren MP 2006 Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil Steril 86 : 1744 – 1749
Misra M , Miller KK , Tsai P , Gallagher K , Lin A , Lee N , Herzog DB , Klibanski A 2006 Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 91 : 1027 – 1033
Budak E , Fernández Sánchez M , Bellver J , Cerveró A , Simón C , Pellicer A 2006 Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3–36 with the reproductive system. Fertil Steril 85 : 1563 – 1581
Badman MK , Flier JS 2007 The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 132 : 2103 – 2115
Misra M , Miller KK , Almazan C , Ramaswamy K , Lapcharoensap W , Worley M , Neubauer G , Herzog DB , Klibanski A 2004 Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 89 : 4972 – 4980
Hotta M , Shibasaki T , Masuda A , Imaki T , Demura H , Ling N , Shizume K 1986 The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab 62 : 319 – 324
Boyar RM , Hellman LD , Roffwarg H , Katz J , Zumoff B , O'Connor J , Bradlow HL , Fukushima DK 1977 Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med 296 : 190 – 193
dos Santos E , dos Santos JE , Ribeiro RP , Rosa E , Silva AC , Moreira AC , Silva de Sá MF 2007 Absence of circadian salivary cortisol rhythm in women with anorexia nervosa. J Pediatr Adolesc Gynecol 20 : 13 – 18
Putignano P , Dubini A , Toja P , Invitti C , Bonfanti S , Redaelli G , Zappulli D , Cavagnini F 2001 Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 145 : 165 – 171
Mayer L , Walsh BT , Pierson RN , Heymsfield SB , Gallagher D , Wang J , Parides MK , Leibel RL , Warren MP , Killory E , Glasofer D 2005 Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr 81 : 1286 – 1291
Misra M , Soyka LA , Miller KK , Grinspoon S , Levitsky LL , Klibanski A 2003 Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. Am J Clin Nutr 77 : 1361 – 1367
Misra M , Prabhakaran R , Miller KK , Tsai P , Lin A , Lee N , Herzog DB , Klibanski A 2006 Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res 59 : 598 – 603
Karczewska-Kupczewska M , Straczkowski M , Adamska A , Nikołajuk A , Otziomek E , Górska M , Kowalska I 2010 Insulin sensitivity, metabolic flexibility, and serum adiponectin concentration in women with anorexia nervosa. Metabolism 59 : 473 – 477
Dostálová I , Smitka K , Papezová H , Kvasnicková H , Nedvídková J 2007 Increased insulin sensitivity in patients with anorexia nervosa: the role of adipocytokines. Physiol Res 56 : 587 – 594
Misra M , Miller KK , Bjornson J , Hackman A , Aggarwal A , Chung J , Ott M , Herzog DB , Johnson ML , Klibanski A 2003 Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 88 : 5615 – 5623
Støving RK , Veldhuis JD , Flyvbjerg A , Vinten J , Hangaard J , Koldkjaer OG , Kristiansen J , Hagen C 1999 Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab 84 : 2056 – 2063
Golden NH , Kreitzer P , Jacobson MS , Chasalow FI , Schebendach J , Freedman SM , Shenker IR 1994 Disturbances in growth hormone secretion and action in adolescents with anorexia nervosa. J Pediatr 125 : 655 – 660
Støving RK , Chen JW , Glintborg D , Brixen K , Flyvbjerg A , Hørder K , Frystyk J 2007 Bioactive insulin-like growth factor (IGF) I and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab 92 : 2323 – 2329
Lawson EA , Klibanski A 2008 Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab 4 : 407 – 414
Croxson MS , Ibbertson HK 1977 Low serum triiodothyronine (T3) and hypothyroidism in anorexia nervosa. J Clin Endocrinol Metab 44 : 167 – 174
Leslie RD , Isaacs AJ , Gomez J , Raggatt PR , Bayliss R 1978 Hypothalamo-pituitary-thyroid function in anorexia nervosa: influence of weight gain. Br Med J 2 : 526 – 528
Miller KK , Lawson EA , Mathur V , Wexler TL , Meenaghan E , Misra M , Herzog DB , Klibanski A 2007 Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 92 : 1334 – 1339
Zumoff B , Walsh BT , Katz JL , Levin J , Rosenfeld RS , Kream J , Weiner H 1983 Subnormal plasma dehydroisoandrosterone to cortisol ratio in anorexia nervosa: a second hormonal parameter of ontogenic regression. J Clin Endocrinol Metab 56 : 668 – 672
Estour B , Germain N , Diconne E , Frere D , Cottet-Emard JM , Carrot G , Lang F , Galusca B 2010 Hormonal profile heterogeneity and short-term physical risk in restrictive anorexia nervosa. J Clin Endocrinol Metab 95 : 2203 – 2210
Warren MP 1983 Effects of undernutrition on reproductive function in the human. Endocr Rev 4 : 363 – 377
Rosen CJ , Klibanski A 2009 Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med 122 : 409 – 414
Fukushima M , Nakai Y , Taniguchi A , Imura H , Nagata I , Tokuyama K 1993 Insulin sensitivity, insulin secretion, and glucose effectiveness in anorexia nervosa: a minimal model analysis. Metabolism 42 : 1164 – 1168
Pannacciulli N , Vettor R , Milan G , Granzotto M , Catucci A , Federspil G , De Giacomo P , Giorgino R , De Pergola G 2003 Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab 88 : 1748 – 1752
Castillo M , Scheen A , Lefebvre PJ , Luyckx AS 1985 Insulin-stimulated glucose disposal is not increased in anorexia nervosa. J Clin Endocrinol Metab 60 : 311 – 314
Estour B , Pugeat M , Lang F , Dechaud H , Pellet J , Rousset H 1986 Sex hormone binding globulin in women with anorexia nervosa. Clin Endocrinol (Oxf) 24 : 571 – 576
Barbe P , Bennet A , Stebenet M , Perret B , Louvet JP 1993 Sex-hormone-binding globulin and protein-energy malnutrition indexes as indicators of nutritional status in women with anorexia nervosa. Am J Clin Nutr 57 : 319 – 322
Confavreux CB , Levine RL , Karsenty G 2009 A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 310 : 21 – 29
Gómez-Ambrosi J , Rodríguez A , Catalán V , Frühbeck G 2008 The bone-adipose axis in obesity and weight loss. Obes Surg 18 : 1134 – 1143
Jayasinghe Y , Grover SR , Zacharin M 2008 Current concepts in bone and reproductive health in adolescents with anorexia nervosa. BJOG 115 : 304 – 315
Miller KK , Grinspoon SK , Ciampa J , Hier J , Herzog D , Klibanski A 2005 Medical findings in outpatients with anorexia nervosa. Arch Intern Med 165 : 561 – 566
Miller KK , Lee EE , Lawson EA , Misra M , Minihan J , Grinspoon SK , Gleysteen S , Mickley D , Herzog D , Klibanski A 2006 Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab 91 : 2931 – 2937
Grinspoon S , Miller K , Coyle C , Krempin J , Armstrong C , Pitts S , Herzog D , Klibanski A 1999 Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab 84 : 2049 – 2055
Cohen A , Fleischer J , Freeby MJ , McMahon DJ , Irani D , Shane E 2009 Clinical characteristics and medication use among premenopausal women with osteoporosis and low BMD: the experience of an osteoporosis referral center. J Womens Health (Larchmt) 18 : 79 – 84
Lucas AR , Melton LJ , Crowson CS , O'Fallon WM 1999 Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc 74 : 972 – 977
Lawson EA , Miller KK , Bredella MA , Phan C , Misra M , Meenaghan E , Rosenblum L , Donoho D , Gupta R , Klibanski A 2010 Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone 46 : 458 – 463
Soyka LA , Misra M , Frenchman A , Miller KK , Grinspoon S , Schoenfeld DA , Klibanski A 2002 Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 87 : 4177 – 4185
Raymond CA 1987 Long-term sequelae pondered in anorexia nervosa. JAMA 257 : 3324 – 3325
Vestergaard P , Emborg C , Støving RK , Hagen C , Mosekilde L , Brixen K 2002 Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders—a nationwide register study. Int J Eat Disord 32 : 301 – 308
Warren MP , Shane E , Lee MJ , Lindsay R , Dempster DW , Warren LF , Hamilton WG 1990 Femoral head collapse associated with anorexia nervosa in a 20-year-old ballet dancer. Clin Orthop Relat Res 251 : 171 – 176
Rigotti NA , Neer RM , Skates SJ , Herzog DB , Nussbaum SR 1991 The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 265 : 1133 – 1138
Kaplan FS , Pertschuk M , Fallon M , Haddad J 1986 Osteoporosis and hip fracture in a young woman with anorexia nervosa. Clin Orthop Relat Res 212 : 250 – 254
Hay PJ , Hall A , Delahunt JW , Harper G , Mitchell AW , Salmond C 1989 Investigation of osteopaenia in anorexia nervosa. Aust N Z J Psychiatry 23 : 261 – 268
Levine RL 2002 Endocrine aspects of eating disorders in adolescents. Adolesc Med 13 : 129 – 143 , vii
Sim LA , McGovern L , Elamin MB , Swiglo BA , Erwin PJ , Montori VM 2010 Effect on bone health of estrogen preparations in premenopausal women with anorexia nervosa: a systematic review and meta-analyses. Int J Eat Disord 43 : 218 – 225
Hotta M , Fukuda I , Sato K , Hizuka N , Shibasaki T , Takano K 2000 The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab 85 : 200 – 206
Haagensen AL , Feldman HA , Ringelheim J , Gordon CM 2008 Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int 19 : 289 – 294
Olmos JM , Riancho JA , Amado JA , Freijanes J , Menéndez-Arango J , González-Macías J 1991 Vitamin D metabolism and serum binding proteins in anorexia nervosa. Bone 12 : 43 – 46
Rigotti NA , Nussbaum SR , Herzog DB , Neer RM 1984 Osteoporosis in women with anorexia nervosa. N Engl J Med 311 : 1601 – 1606
Carmichael KA , Carmichael DH 1995 Bone metabolism and osteopenia in eating disorders. Medicine (Baltimore) 74 : 254 – 267
Gordon CM , Grace E , Emans SJ , Feldman HA , Goodman E , Becker KA , Rosen CJ , Gundberg CM , LeBoff MS 2002 Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab 87 : 4935 – 4941
Miller KK , Grieco KA , Klibanski A 2005 Testosterone administration in women with anorexia nervosa. J Clin Endocrinol Metab 90 : 1428 – 1433
Karsenty G 2006 Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 4 : 341 – 348
Wolf G 2008 Energy regulation by the skeleton. Nutr Rev 66 : 229 – 233
Lee NK , Karsenty G 2008 Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 19 : 161 – 166
Cirmanová V , Bayer M , Stárka L , Zajícková K 2008 The effect of leptin on bone: an evolving concept of action. Physiol Res 57 ( Suppl 1 ): S143 – S151
Elefteriou F , Ahn JD , Takeda S , Starbuck M , Yang X , Liu X , Kondo H , Richards WG , Bannon TW , Noda M , Clement K , Vaisse C , Karsenty G 2005 Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434 : 514 – 520
Singh MK , Elefteriou F , Karsenty G 2008 Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. Endocrinology 149 : 3933 – 3941
Oury F , Karsenty G 2009 [Intestinal serotonin and regulation of bone mass]. Med Sci (Paris) 25 : 445 – 446
Utz AL , Lawson EA , Misra M , Mickley D , Gleysteen S , Herzog DB , Klibanski A , Miller KK 2008 Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone 43 : 135 – 139
Hotta M , Shibasaki T , Sato K , Demura H 1998 The importance of body weight history in the occurrence and recovery of osteoporosis in patients with anorexia nervosa: evaluation by dual x-ray absorptiometry and bone metabolic markers. Eur J Endocrinol 139 : 276 – 283
Liu SL , Lebrun CM 2006 Effect of oral contraceptives and hormone replacement therapy on bone mineral density in premenopausal and perimenopausal women: a systematic review. Br J Sports Med 40 : 11 – 24
Miller KK , Grieco KA , Mulder J , Grinspoon S , Mickley D , Yehezkel R , Herzog DB , Klibanski A 2004 Effects of risedronate on bone density in anorexia nervosa. J Clin Endocrinol Metab 89 : 3903 – 3906
Golden NH , Iglesias EA , Jacobson MS , Carey D , Meyer W , Schebendach J , Hertz S , Shenker IR 2005 Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 90 : 3179 – 3185
Grinspoon S , Thomas L , Miller K , Herzog D , Klibanski A 2002 Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 87 : 2883 – 2891
Patlas N , Golomb G , Yaffe P , Pinto T , Breuer E , Ornoy A 1999 Transplacental effects of bisphosphonates on fetal skeletal ossification and mineralization in rats. Teratology 60 : 68 – 73
Minsker DH , Manson JM , Peter CP 1993 Effects of the bisphosphonate, alendronate, on parturition in the rat. Toxicol Appl Pharmacol 121 : 217 – 223
Bonjour JP 2005 Dietary protein: an essential nutrient for bone health. J Am Coll Nutr 24 : 526S – 536S
Bourrin S , Ammann P , Bonjour JP , Rizzoli R 2000 Dietary protein restriction lowers plasma insulin-like growth factor I (IGF-I), impairs cortical bone formation, and induces osteoblastic resistance to IGF-I in adult female rats. Endocrinology 141 : 3149 – 3155
Golden NH , Lanzkowsky L , Schebendach J , Palestro CJ , Jacobson MS , Shenker IR 2002 The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 15 : 135 – 143
Golden NH 2007 Eating disorders in adolescence: what is the role of hormone replacement therapy? Curr Opin Obstet Gynecol 19 : 434 – 439
Pirke KM , Fichter MM , Chlond C , Schweiger U , Laessle RG , Schwingenschloegel M , Hoehl C 1987 Disturbances of the menstrual cycle in bulimia nervosa. Clin Endocrinol (Oxf) 27 : 245 – 251
Cantopher T , Evans C , Lacey JH , Pearce JM 1988 Menstrual and ovulatory disturbance in bulimia. BMJ 297 : 836 – 837
Gendall KA , Bulik CM , Joyce PR , McIntosh VV , Carter FA 2000 Menstrual cycle irregularity in bulimia nervosa. Associated factors and changes with treatment. J Psychosom Res 49 : 409 – 415
Vyver E , Steinegger C , Katzman DK 2008 Eating disorders and menstrual dysfunction in adolescents. Ann NY Acad Sci 1135 : 253 – 264
Poyastro Pinheiro A , Thornton LM , Plotonicov KH , Tozzi F , Klump KL , Berrettini WH , Brandt H , Crawford S , Crow S , Fichter MM , Goldman D , Halmi KA , Johnson C , Kaplan AS , Keel P , LaVia M , Mitchell J , Rotondo A , Strober M , Treasure J , Woodside DB , Von Holle A , Hamer R , Kaye WH , Bulik CM 2007 Patterns of menstrual disturbance in eating disorders. Int J Eat Disord 40 : 424 – 434
Schweiger U , Pirke KM , Laessle RG , Fichter MM 1992 Gonadotropin secretion in bulimia nervosa. J Clin Endocrinol Metab 74 : 1122 – 1127
Pirke KM , Dogs M , Fichter MM , Tuschl RJ 1988 Gonadotrophins, oestradiol and progesterone during the menstrual cycle in bulimia nervosa. Clin Endocrinol (Oxf) 29 : 265 – 270
Adams J , Franks S , Polson DW , Mason HD , Abdulwahid N , Tucker M , Morris DV , Price J , Jacobs HS 1985 Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 2 : 1375 – 1379
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome. Fertil Steril 81 : 19 – 25
McCluskey S , Evans C , Lacey JH , Pearce JM , Jacobs H 1991 Polycystic ovary syndrome and bulimia. Fertil Steril 55 : 287 – 291
McCluskey SE , Lacey JH , Pearce JM 1992 Binge-eating and polycystic ovaries. Lancet 340 : 723
Raphael FJ , Rodin DA , Peattie A , Bano G , Kent A , Nussey SS , Lacey JH 1995 Ovarian morphology and insulin sensitivity in women with bulimia nervosa. Clin Endocrinol (Oxf) 43 : 451 – 455
Jahanfar S , Eden JA , Nguyent TV 1995 Bulimia nervosa and polycystic ovary syndrome. Gynecol Endocrinol 9 : 113 – 117
Morgan JF , McCluskey SE , Brunton JN , Hubert Lacey J 2002 Polycystic ovarian morphology and bulimia nervosa: a 9-year follow-up study. Fertil Steril 77 : 928 – 931
Taylor AE , Hubbard J , Anderson EJ 1999 Impact of binge eating on metabolic and leptin dynamics in normal young women. J Clin Endocrinol Metab 84 : 428 – 434
Spalter AR , Gwirtsman HE , Demitrack MA , Gold PW 1993 Thyroid function in bulimia nervosa. Biol Psychiatry 33 : 408 – 414
Gendall KA , Joyce PR , Carter FA , McIntosh VV , Bulik CM 2003 Thyroid indices and treatment outcome in bulimia nervosa. Acta Psychiatr Scand 108 : 190 – 195
Birketvedt GS , Drivenes E , Agledahl I , Sundsfjord J , Olstad R , Florholmen JR 2006 Bulimia nervosa—a primary defect in the hypothalamic-pituitary-adrenal axis? Appetite 46 : 164 – 167
Ludescher B , Leitlein G , Schaefer JE , Vanhoeffen S , Baar S , Machann J , Claussen CD , Schick F , Eschweiler GW 2009 Changes of body composition in bulimia nervosa: increased visceral fat and adrenal gland size. Psychosom Med 71 : 93 – 97
Monteleone P , Di Lieto A , Tortorella A , Longobardi N , Maj M 2000 Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiatry Res 94 : 121 – 129
Fassino S , Daga GA , Mondelli V , Pierò A , Broglio F , Picu A , Giordano R , Baldi M , Arvat E , Ghigo E , Gianotti L 2005 Hormonal and metabolic responses to acute ghrelin administration in patients with bulimia nervosa. Psychoneuroendocrinology 30 : 534 – 540
Naessén S , Carlström K , Glant R , Jacobsson H , Hirschberg AL 2006 Bone mineral density in bulimic women—influence of endocrine factors and previous anorexia. Eur J Endocrinol 155 : 245 – 251
Warren MP , Voussoughian F , Geer EB , Hyle EP , Adberg CL , Ramos RH 1999 Functional hypothalamic amenorrhea: hypoleptinemia and disordered eating. J Clin Endocrinol Metab 84 : 873 – 877
De Souza MJ , Hontscharuk R , Olmsted M , Kerr G , Williams NI 2007 Drive for thinness score is a proxy indicator of energy deficiency in exercising women. Appetite 48 : 359 – 367
De Souza MJ , Lee DK , VanHeest JL , Scheid JL , West SL , Williams NI 2007 Severity of energy-related menstrual disturbances increases in proportion to indices of energy conservation in exercising women. Fertil Steril 88 : 971 – 975
Schneider LF , Monaco SE , Warren MP 2008 Elevated ghrelin level in women of normal weight with amenorrhea is related to disordered eating. Fertil Steril 90 : 121 – 128
De Souza MJ , Leidy HJ , O'Donnell E , Lasley B , Williams NI 2004 Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab 89 : 3536 – 3542
Scheid JL , Williams NI , West SL , VanHeest JL , De Souza MJ 2009 Elevated PYY is associated with energy deficiency and indices of subclinical disordered eating in exercising women with hypothalamic amenorrhea. Appetite 52 : 184 – 192
Constantini NW , Warren MP 1994 Special problems of the female athlete. Baillieres Clin Rheumatol 8 : 199 – 219
Zanker CL , Cooke CB , Truscott JG , Oldroyd B , Jacobs HS 2004 Annual changes of bone density over 12 years in an amenorrheic athlete. Med Sci Sports Exerc 36 : 137 – 142
Cobb KL , Bachrach LK , Sowers M , Nieves J , Greendale GA , Kent KK , Brown BW , Pettit K , Harper DM , Kelsey JL 2007 The effect of oral contraceptives on bone mass and stress fractures in female runners. Med Sci Sports Exerc 39 : 1464 – 1473
Vescovi JD , Jamal SA , De Souza MJ 2008 Strategies to reverse bone loss in women with functional hypothalamic amenorrhea: a systematic review of the literature. Osteoporos Int 19 : 465 – 478
Fredericson M , Kent K 2005 Normalization of bone density in a previously amenorrheic runner with osteoporosis. Med Sci Sports Exerc 37 : 1481 – 1486
Ihle R , Loucks AB 2004 Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res 19 : 1231 – 1240
Email alerts
More on this topic, related articles in pubmed, citing articles via.
- About The Journal of Clinical Endocrinology & Metabolism
- About the Endocrine Society
- Recommend to Your Librarian
- Advertising and Corporate Services
Affiliations
- Online ISSN 1945-7197
- Print ISSN 0021-972X
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 10 June 2008
Endocrine abnormalities in anorexia nervosa
- Elizabeth A Lawson 1 &
- Anne Klibanski 1
Nature Clinical Practice Endocrinology & Metabolism volume 4 , pages 407–414 ( 2008 ) Cite this article
1068 Accesses
79 Citations
Metrics details
Anorexia nervosa (AN) is a psychiatric disease associated with notable medical complications and increased mortality. Endocrine abnormalities, including hypogonadotropic hypogonadism, hypercortisolemia, growth hormone resistance and sick euthyroid syndrome, mediate the clinical manifestations of this disease. Alterations in anorexigenic and orexigenic appetite-regulating pathways have also been described. Decreases in fat mass result in adipokine abnormalities. Although most of the endocrine changes that occur in AN represent physiologic adaptation to starvation, some persist after recovery and might contribute to susceptibility to AN recurrence. In this Review, we summarize key endocrine alterations in AN, with a particular focus on the profound bone loss that can occur in this disease. Although AN is increasingly prevalent among boys and men, the disorder predominantly affects girls and women who are, therefore, the focus of this Review.
Anorexia nervosa is associated with characteristic endocrine abnormalities
Most of the changes are physiologic adaptations to starvation
Some endocrine abnormalities do not normalize with recovery, and may confer a predisposition to disease development and relapse
Endocrine pathways mediate the medical complications of anorexia nervosa, including severe osteopenia
Weight gain and menstrual recovery are the only known effective, available therapies for bone loss in anorexia nervosa
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
Purchase on Springer Link
Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Functional hypogonadism among patients with obesity, diabetes, and metabolic syndrome
Marne Louters, Michelle Pearlman, … Amy Pearlman
Skeletal disorders associated with the growth hormone–insulin-like growth factor 1 axis
Gherardo Mazziotti, Andrea G. Lania & Ernesto Canalis

Long-term cardiovascular consequences of adolescent anorexia nervosa
Gabriella A. C. Springall, Michelle Caughey, … Michael M. H. Cheung
Hoek HW and van Hoeken D (2003) Review of the prevalence and incidence of eating disorders. Int J Eat Disord 34 : 383–396
Article Google Scholar
Keel PK et al . (2003) Predictors of mortality in eating disorders. Arch Gen Psychiatry 60 : 179–183
American Psychiatric Association (2000) DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders , edn 4. Washington, DC: American Psychiatric Press
Crisp A and Stonehill E (1971) Relation between aspects of nutritional disturbance and menstrual activity in primary anorexia nervosa. BMJ 3 : 149–151
Article CAS Google Scholar
Gendall KA et al . (2006) The psychobiology and diagnostic significance of amenorrhea in patients with anorexia nervosa. Fertil Steril 85 : 1531–1535
Jacoangeli F et al . (2006) Amenorrhea after weight recovery in anorexia nervosa: role of body composition and endocrine abnormalities. Eat Weight Disord 11 : e20–e26
Misra M et al . (2006) Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res 59 : 598–603
Miller KK et al . (2004) Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab 89 : 4434–4438
Swenne I (2004) Weight requirements for return of menstruations in teenage girls with eating disorders, weight loss and secondary amenorrhea. Acta Paediatr 93 : 1449–1455
Miller KK et al . (2007) Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab 92 : 1334–1339
Gordon CM et al . (2002) Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr 141 : 64–70
Miller KK et al . (2007) Androgen deficiency: association with increased anxiety and depression symptom severity in anorexia nervosa. J Clin Psychiatry 68 : 959–965
Miller KK et al . (2005) Testosterone administration in women with anorexia nervosa. J Clin Endocrinol Metab 90 : 1428–1433
Misra M et al . (2004) Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics 114 : 1574–1583
Grinspoon S et al . (1999) Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab 84 : 2049–2055
CAS PubMed Google Scholar
Nussbaum M et al . (1985) Short stature in anorexia nervosa patients. J Adolesc Health Care 6 : 453–455
Misra M et al . (2004) Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 89 : 4972–4980
Putignano P et al . (2001) Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 145 : 165–171
dos Santos E et al . (2007) Absence of circadian salivary cortisol rhythm in women with anorexia nervosa. J Pediatr Adolesc Gynecol 2007 : 13–18
Gold PW et al . (1986) Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med 314 : 1335–1342
Hotta M et al . (1986) The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab 62 : 319–324
Laessle RG et al . (1992) Cortisol levels and vigilance in eating disorder patients. Psychoneuroendocrinology 17 : 475–484
Giordano G et al . (2001) Volume measurement with magnetic resonance imaging of hippocampus-amygdala formation in patients with anorexia nervosa. J Endocrinol Invest 24 : 510–514
McLoughlin DM et al . (2000) Metabolic abnormalities associated with skeletal myopathy in severe anorexia nervosa. Nutrition 16 : 192–196
Grinspoon S et al . (2000) Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med 133 : 790–794
Grinspoon S et al . (2001) Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr 73 : 865–869
Mayer L et al . (2005) Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr 81 : 1286–1291
Stoving RK et al . (1999) Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab 84 : 2056–2063
Misra M et al . (2003) Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab 88 : 5615–5623
Counts DR et al . (1992) The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab 75 : 762–767
Støving RK et al . (2007) Bioactive insulin-like growth factor (IGF) I and IGF-binding protein-1 in anorexia nervosa. J Clin Endocrinol Metab 92 : 2323–2329
Golden NH et al . (1994) Disturbances in growth hormone secretion and action in adolescents with anorexia nervosa. J Pediatr 125 : 655–660
Gianotti L et al . (2000) Effects of recombinant human insulin-like growth factor I administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH secretion in anorexia nervosa. J Clin Endocrinol Metab 85 : 2805–2809
Gianotti L et al . (1999) Effect of somatostatin infusion on the somatotrope responsiveness to growth hormone-releasing hormone in patients with anorexia nervosa. Biol Psychiatry 45 : 334–339
Grinspoon S et al . (2002) Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab 87 : 2883–2891
Croxson MS and Ibbertson HK (1977) Low serum triiodothyronine (T3) and hypothyroidism in anorexia nervosa. J Clin Endocrinol Metab 44 : 167–174
Leslie RD et al . (1978) Hypothalamo-pituitary-thyroid function in anorexia nervosa: influence of weight gain. BMJ 2 : 526–528
Kiyohara K et al . (1989) Decreased thyroidal triiodothyronine secretion in patients with anorexia nervosa: influence of weight recovery. Am J Clin Nutr 50 : 767–772
Stoving RK et al . (2001) Evidence of diffuse atrophy of the thyroid gland in patients with anorexia nervosa. Int J Eat Disord 29 : 230–235
Onur S et al . (2005) L-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa during weight gain. Eur J Endocrinol 152 : 179–184
Bannai C et al . (1988) Assessment of the relationship between serum thyroid hormone levels and peripheral metabolism in patients with anorexia nervosa. Endocrinol Jpn 35 : 455–462
Inui A (2001) Eating behavior in anorexia nervosa—an excess of both orexigenic and anorexigenic signaling. Mol Psychiatry 6 : 620–624
Grinspoon S et al . (1996) Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab 81 : 3861–3863
Misra M et al . (2005) Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289 : E373–381
Hebebrand J et al . (1997) Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short- term weight restoration. Mol Psychiatry 2 : 330–334
Gendall KA et al . (1999) Leptin, neuropeptide Y, and peptide YY in long-term recovered eating disorder patients. Biol Psychiatry 46 : 292–299
Kopp W et al . (1997) Low leptin levels predict amenorrhea in underweight and eating disordered females. Mol Psychiatry 2 : 335–340
Cirmanová V et al . The effect of leptin on bone—an evolving concept of action. Physiol Res , in press
Misra M et al . (2006) Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 91 : 1027–1033
Pfluger PT et al . (2007) Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab 92 : 583–588
Nakahara T et al . (2007) Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry 10.1016/j.biopsych.2007.08.005
Otto B et al . (2001) Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 145 : 669–673
Misra M et al . (2005) Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289 : E347–E356
Vestergaard P et al . (2002) Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders—A nationwide register study. Int J Eat Disord 32 : 301–308
Biller BMK et al . (1989) Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 68 : 548–554
Miller KK et al . (2006) Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab 91 : 2931–2937
Klibanski A et al . (1995) The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 80 : 898–904
Golden NH et al . (2002) The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 15 : 135–143
Munoz M et al . (2002) The effects of estrogen administration on bone mineral density in adolescents with anorexia nervosa. Eur J Endocrinol 146 : 45–50
Robinson E et al . (2000) Use of hormone replacement therapy to reduce the risk of osteopenia in adolescent girls with anorexia nervosa. J Adolesc Health 26 : 343–348
Lawson EA et al . (2007) Hormonal and nutritional effects on cardiovascular risk markers in young women. J Clin Endocrinol Metab 92 : 3089–3094
Gordon CM et al . (2002) Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab 87 : 4935–4941
Bachrach LK et al . (1991) Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 72 : 602–606
Dominguez J et al . (2007) Treatment of anorexia nervosa is associated with increases in bone mineral density, and recovery is a biphasic process involving both nutrition and return of menses. Am J Clin Nutr 86 : 92–99
Download references
Acknowledgements
This work was supported by grants from the NIH.
Author information
Authors and affiliations.
Department of Medicine, EA Lawson is a Clinical and Research Fellow, and A Klibanski is a Professor of Medicine in the Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.,
Elizabeth A Lawson & Anne Klibanski
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Anne Klibanski .
Ethics declarations
Competing interests.
A Klibanski acts as a consultant for Tercica.
EA Lawson declared no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lawson, E., Klibanski, A. Endocrine abnormalities in anorexia nervosa. Nat Rev Endocrinol 4 , 407–414 (2008). https://doi.org/10.1038/ncpendmet0872
Download citation
Received : 01 February 2008
Accepted : 30 April 2008
Published : 10 June 2008
Issue Date : July 2008
DOI : https://doi.org/10.1038/ncpendmet0872
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Nutritional rickets presenting with developmental regression: a rare presentation of rickets.
- Chariklia Pieridou
BMC Pediatrics (2023)
Endokrine Veränderungen bei Essstörungen
- Sabine Elisabeth Segerer
Journal für Gynäkologische Endokrinologie/Schweiz (2023)
Directly measured free 25-hydroxy vitamin D levels show no evidence of vitamin D deficiency in young Swedish women with anorexia nervosa
- Martin Carlsson
- Lars Brudin
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2018)
A role for intestinal TLR4-driven inflammatory response during activity-based anorexia
- Liliana Belmonte
- Najate Achamrah
- Moïse Coëffier
Scientific Reports (2016)
Anorexia nervosa
- Janet Treasure
- Stephan Zipfel
- Elisabet Wentz
Nature Reviews Disease Primers (2015)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
The Biology of Anorexia Nervosa
A New Narrative Overview
- Living reference work entry
- First Online: 20 September 2022
- Cite this living reference work entry

- Kamil Skowron 3 ,
- Magdalena Kurnik-Łucka 3 &
- Krzysztof Gil 3
75 Accesses
Anorexia nervosa, one of the most deadly mental disorders, is a pathophysiologically complex mosaic of numerous extensively investigated pathways, and the current state of knowledge cannot provide us with one definite answer regarding its etiopathogenesis. Although classified as a psychiatric disorder, traditionally viewed as a consequence of the psychological features, the model explaining the onset and development of anorexia is multifactorial with growing interest in its metabolic origin. The clinical presentation varies in severity, but ultimately every organ in the diseased body is affected. There are many biological alterations that simply act in accordance with severe malnutrition and weight loss; nevertheless, there is an evident dysfunction in the course of adaptive pathways that induce or at least potentiate those changes. Based on human and animal studies, the most relevant streams in AN pathophysiology seem to point toward a relatively adequate peripheral response that fails to properly stimulate feeding-related neurohormonal brain circuits. This metabolic origin of AN is supported by molecular identifications of specific genetic polymorphisms. Endocrine adaptations involve among others hyperghrelinemia, hypoleptinemia, hypogonadotropic hypogonadism, and CRH hypersecretion. Those hormonal shifts interact not only with appetite-regulating brain regions but also affect energy expenditure, physical activity, behavior, cognition, as well as rewarding/motivational drive. This narrative review aims to present emerging biological concepts underlying anorexia nervosa.
Anorexia nervosa
- Malnutrition
- Activity-based anorexia
- Neurobiology
- Hypoleptinemia
- Hypothalamus
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Abbreviations
Agouti-related peptide
Arcuate nucleus
Body mass index
Cocaine- and amphetamine-regulated transcript
Corticotropin-releasing hormone
Gonadotropin-releasing hormone
Hypothalamic-pituitary-gonadal
Kisspeptin receptor
Leptin gene
Lateral hypothalamic area
Magnetic resonance imaging
Neuropeptide Y
Proopiomelanocortin
Soluble leptin receptor
Triiodothyronine
Abdel-Hamid TK (2002) Modeling the dynamics of human energy regulation and its implications for obesity treatment. Syst Dyn Rev 18:431–471
Article Google Scholar
Adolphs R (2002) Neural systems for recognizing emotion. Curr Opin Neurobiol 12(2):169–177
Article CAS PubMed Google Scholar
Al-Lahham SH, Roelofsen H, Priebe M et al (2010) Regulation of adipokine production in human adipose tissue by propionic acid. Eur J Clin Investig 40(5):401–407
Article CAS Google Scholar
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn. American Psychiatric Publishing, Washington, DC.
Book Google Scholar
Appelhans BM (2010) Circulating leptin moderates the effect of stress on snack intake independent of body mass. Eat Behav 11(3):152–155
Article PubMed PubMed Central Google Scholar
Auger N, Potter BJ, Ukah UV et al (2021) Anorexia nervosa and the long-term risk of mortality in women. World Psychiatry 20(3):448–449
Ballauff A, Ziegler A, Emons G et al (1999) Serum leptin and gonadotropin levels in patients with anorexia nervosa during weight gain. Mol Psychiatry 4(1):71–75
Barbano MF, Wang HL, Morales et al (2016) Feeding and reward are differentially induced by activating GABAergic lateral hypothalamic projections to VTA. J Neurosci 36(10):2975–2985
Google Scholar
Brownson RC, Boehmer TK, Luke DA (2005) Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health 26:421–443
Article PubMed Google Scholar
Bulik C, Yilmaz Z, HArdaway A (2015) Genetics and epigenetics of eating disorders. Adv Genomics Genet 5:131
Burghardt PR, Love TM, Stohler CS et al (2012) Leptin regulates dopamine responses to sustained stress in humans. J Neurosci 32(44):15369–15376
Article CAS PubMed PubMed Central Google Scholar
Candler T, Kühnen P, Prentice AM, Silver M (2019) Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front Neuroendocrinol 54:100773
Caro JF, Kolaczynski JW, Nyce et al (1996) Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348:159–161
Castellano JM, Navarro VM, Fernández-Fernández R et al (2005) Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146(9):3917–3925
Chesney E, Goodwin GM, Fazel S (2014) Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 13:153–160
Clarke G, Stilling RM, Kennedy PJ et al (2014) Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol 28:1221–1238
Article PubMed PubMed Central CAS Google Scholar
Claudino AM, Silva de Lima M, Hay PP et al (2006) Antidepressants for anorexia nervosa. Cochrane Database Syst Rev 1:CD004365
Considine RV, Sinha MK, Heiman ML et al (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
de Araujo IE, Ferreira JG, Tellez LA et al (2012) The gut-brain dopamine axis: a regulatory system for caloric intake. Physiol Behav 106(3):394–399
de Roux N, Genin E, Carel JC et al (2003) Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci 100(19):10972–10976
Dempfle A, Herpertz-Dahlmann B, Timmesfeld N et al (2013) Predictors of the resumption of menses in adolescent anorexia nervosa. BMC Psychiatry 13:308
Duncan L, Yilmaz Z, Gaspar H et al (2017) Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry 173:850–858
Eckel LA (2011) The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104(4):517–524
Edakubo S, Fushimi K (2020) Mortality and risk assessment for anorexia nervosa in acute-care hospitals: a nationwide administrative database analysis. BMC Psychiatry 20(1):19
Fetissov SO, Hökfelt T (2019) On the origin of eating disorders: altered signalling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Curr Opin Pharmacol 48:82–91
Fetissov SO, Harro J, Jaanisk M et al (2005) Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci U S A 102:14865–14870
Fetissov SO, Sinno MH, Coëffier M et al (2008) Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition 24:348–359
Flament MF, Bissada H, Spettigue W (2012) Evidence-based pharmacotherapy of eating disorders. Int J Neuropsychopharmacol 15(2):189–207
Florent V, Baroncini M, Jissendi-Tchofo P et al (2020) Hypothalamic structural and functional imbalances in anorexia nervosa. Neuroendocrinology 110(6):552–562
Frolich J, Palm CVB, Stoving RK (2016) To the limit of extreme malnutrition. Nutrition 32:146–148
Galusca B, Prévost G, Germain N et al (2015) Neuropeptide Y and α-MSH circadian levels in two populations with low body weight: anorexia nervosa and constitutional thinness. PLoS One 10(3):e0122040
Gioldasi S, Karvela A, Rojas-Gil AP et al (2019) Metabolic association between leptin and the corticotropin releasing hormone. Endocr Metab Immune Disord Drug Targets 19(4):458–466
Hebebrand J, Blum WF, Barth N et al (1997) Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Mol Psychiatry 2(4):330–334
Hebebrand J, Muller T, Holtkamp K et al (2007) The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 12:23–35
Hebebrand J, Milos G, Wabitsch M et al (2019) Clinical trials required to assess potential benefits and side effects of treatment of patients with anorexia nervosa with recombinant human leptin. Front Psychol 10:769
Hofmann T, Elbelt U, Haas V (2017) Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite 108:141–150
Howard D, Negraes P, Voineskos AN et al (2020) Molecular neuroanatomy of anorexia nervosa. Sci Rep 10(1):11411
Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC (2019) Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients 11(11):2704
Article CAS PubMed Central Google Scholar
Jacoangeli F, Masala S, Staar Mezzasalma F et al (2006) Amenorrhea after weight recover in anorexia nervosa: role of body composition and endocrine abnormalities. Eat Weight Disord 11:e20–e26
Jiang T, Soussignan R, Carrier E, Royet JP (2019) Dysfunction of the mesolimbic circuit to food odors in women with anorexia and bulimia nervosa: a fMRI study. Front Hum Neurosci 13:117
Kaye W (2008) Neurobiology of anorexia and bulimia nervosa purdue ingestive behavior research center symposium influences on eating and body weight over the lifespan: children and adolescents. Physiol Behav 94(1):121–135
Keski-Rahkonen A, Raevuori A, Hoek HW (2018) Epidemiology of eating disorders: an update. In: Annual review of eating disorders, 1st edn. CRC Press, London, pp 66–76
Kinney JM (1995) Influence of altered body weight on energy expenditure. Nutr Rev 53:265–268
Kurnik-Łucka M, Skowron K, Gil K (2020) In search for perfection: an activity-based rodent model of anorexia. In: Animal models of eating disorders, Neuromethods, vol 161. Humana, New York, pp 363–377
Chapter Google Scholar
Mantzoros C, Flier JS, Lesem MD et al (1997) Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab 82(6):1845–1851
CAS PubMed Google Scholar
McArdle WD, Katch FI, Katch VL (1996) Exercise physiology: energy, nutrition, and human performance, 4th edn. Williams & Wilkins, Baltimore, p 442
McIlwraith EK, Loganathan N, Belsham DD (2018) Phoenixin expression is regulated by the fatty acids palmitate, docosahexaenoic acid and oleate, and the endocrine disrupting chemical bisphenol A in immortalized hypothalamic neurons. Front Neurosci 12:838
Mehler PS, Brown C (2015) Anorexia nervosa – medical complications. J Eat Disord 3:11
Miller KK, Wexler TL, Zha AM et al (2007) Androgen deficiency: association with increased anxiety and depression symptom severity in anorexia nervosa. J Clin Psychiatry 68:959–965
Misra M, Katzman DK, Cord J et al (2008) Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab 93:3029–3036
Misra M, Golden NH, Katzman DK (2016) State of the art systematic review of bone disease in anorexia nervosa. Int J Eat Disord 49:276–292
Miyatake N, Murakami H, Kawakami R et al (2014) Circulating leptin levels are associated with physical activity or physical fitness in Japanese. Environ Health Prev Med 19(5):362–366
Montague CT, Prins JB, Sanders L et al (1997) Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 46(3):342–347
Morton G, Cummings D, Baskin D et al (2006) Central nervous system control of food intake and body weight. Nature 443(7109):289–295
Muoio DM, Lynis Dohm G (2002) Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab 16(4):653–666
Myers MG, Cowley MA, Munzberg H (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556
Olivares JL, Vázquez M, Fleta J et al (2005) Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Pediatr 164(6):383–386
Park HK, Ahima RS (2015) Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64(1):24–34
Paz-Filho G, Wong ML, Licinio J (2010) The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract 64(13):1808–1812
Peters T, Antel J, Naaresh R et al (2021) Suggestive evidence for causal effect of leptin levels on risk for anorexia nervosa: results of a Mendelian randomization study. Front Genet 12:733606
Rajala MW, Patterson CM, Opp JS et al (2014) Leptin acts independently of food intake to modulate gut microbial composition in male mice. Endocrinology 155(3):748–757
Rome ES, Ammerman S (2015) Medical complications of eating disorders: an update. J Adolesc Health 33(6):418–426
Ruscica M, Macchi C, Gandini S et al (2016) Free and bound plasma leptin in anorexia nervosa patients during a refeeding program. Endocrine 51:380–383
Sachs KV, Harnke B, Mehler PS et al (2016) Cardiovascular complications of anorexia nervosa: a systematic review. Int J Eat Disord 49(3):238–248
Sato Y, Fukudo S (2015) Gastrointestinal symptoms and disorders in patients with eating disorders. Clin J Gastroenterol 8(5):255–263
Schmalbach I, Herhaus B, Pässler S et al (2020) Cortisol reactivity in patients with anorexia nervosa after stress induction. Transl Psychiatry 10:275
Schorr M, Miller KK (2017) The endocrine manifestations of anorexia nervosa: mechanisms and management. Nat Rev Endocrinol 13(3):174–186
Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D (1996) Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593
Simon JJ, Stopyra MA, Mönning E et al (2020) Neuroimaging of hypothalamic mechanisms related to glucose metabolism in anorexia nervosa and obesity. J Clin Investig 130(8):4094–4103
CAS PubMed PubMed Central Google Scholar
Skowron K, Aleksandrovych V, Kurnik-Łucka M et al (2018) Folia Med Cracov 58(3):115–125
PubMed Google Scholar
Skowron K, Jasiński K, Kurnik-Łucka M et al (2020a) Hypothalamic and brain stem neurochemical profile in anorectic rats after peripheral administration of kisspeptin-10 using 1 H-NMR spectroscopy in vivo. NMR Biomed 33(7):e4306
Skowron K, Kurnik-Łucka M, Dadański E et al (2020b) Backstage of eating disorder-about the biological mechanisms behind the symptoms of anorexia nervosa. Nutrients 12(9):2604
Skowron K, Kurnik-Łucka M, Jurczyk M et al (2021) Is the activity-based anorexia model a reliable method of presenting peripheral clinical features of anorexia nervosa? Nutrients 13(8):2876
Steinhausen HC (2002) The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 159:1284–1293
Støving RK (2019) Mechanisms in endocrinology: anorexia nervosa and endocrinology: a clinical update. Eur J Endocrinol 180(1):R9–R27
Støving RK, Vinten J, Handberg A et al (1998) Diurnal variation of the serum leptin concentration in patients with anorexia nervosa. Clin Endocrinol 48:761–768
Stroe-Kunold E, Buckert M, Friederich H-C et al (2016) Time course of leptin in patients with anorexia nervosa during inpatient treatment: longitudinal relationships to BMI and psychological factors. PLoS One 11:e0166843231
Teegala SB, Sheng Z, Dalal MS (2020) Lateral hypothalamic orexin glucose-inhibited neurons may regulate reward-based feeding by modulating glutamate transmission in the ventral tegmental area. Brain Res 1731:145808
Terrill SJ, Hyde KM, Kay KE et al (2016) Ventral tegmental area orexin 1 receptors promote palatable food intake and oppose postingestive negative feedback. Am J Physiol Regul Integr Comp Physiol 311(3):R592–R599
Watson HJ, Yilmaz Z, Thornton LM et al (2019) Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 51:1207–1214
Westmoreland P, Krantz MJ, Mehler PS (2016) Medical complications of anorexia nervosa and bulimia. Am J Med 129:30–37
Zaibi MS, Stocker CJ, O’Dowd J et al (2010) Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584(11):2381–2386
Download references
Author information
Authors and affiliations.
Department of Pathophysiology, Collegium Medicum Jagiellonian University, Krakow, Poland
Kamil Skowron, Magdalena Kurnik-Łucka & Krzysztof Gil
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Krzysztof Gil .
Editor information
Editors and affiliations.
Department of Biomedical Sciences, University of Westminster, London, UK
Vinood Patel
Dept Nutrition & Dietetics, King's College London, London, UK
Victor Preedy
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry.
Skowron, K., Kurnik-Łucka, M., Gil, K. (2022). The Biology of Anorexia Nervosa. In: Patel, V., Preedy, V. (eds) Eating Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-67929-3_28-1
Download citation
DOI : https://doi.org/10.1007/978-3-030-67929-3_28-1
Received : 16 February 2022
Accepted : 17 March 2022
Published : 20 September 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-67929-3
Online ISBN : 978-3-030-67929-3
eBook Packages : Springer Reference Medicine Reference Module Medicine
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Suggestions
Hormones: what they do and what happens in anorexia nervosa.

Written by Mariya Bershad and Laurel Mayer, MD.
Day in and day out, signals from our brain tell us when to eat, doze off, stress out and even feel butterflies. Many of these signals can be attributed to hormones—chemical messengers that travel through the bloodstream and communicate with the rest of the body by interacting with specific receptors.
Hormones influence the way our bodies function, as well as our thoughts, feelings, and actions—however, they are themselves responsive to our behavior and environment. What results is this complex system in which our brain, body, and behavior are constantly interacting.
It is, therefore, no surprise that scientists are interested in studying the role of hormones in a disorder like anorexia nervosa, which itself is characterized by an interplay of physiology, psychology, and behavior. For example, how does a hormonally-mediated signal like hunger work in a person with anorexia nervosa? But first, here’s a review of Hormones 101.
Hormones Involved in Energy, Reproduction, and Growth
Released from: the adrenal gland.
What does it do? Cortisol—which typically acts as part of the response to stress— aids in the metabolism of fat, protein, and carbohydrates. Specifically, it stimulates the formation of glucose (i.e. blood sugar, or readily available energy). Cortisol levels cycle throughout the day, and have been found to have big impacts on memory, sleep, mood, and acid secretion in the stomach and kidneys.
Released from: the lining of the stomach.
What does it do? Ghrelin stimulates appetite, food intake, use of carbohydrates and the release of growth hormone; it also activates pathways that reinforce rewards (e.g. food and some drugs), and inhibits fat utilization and physical activity. Production of ghrelin increases before a meal and then slows down afterward.
Released from: pancreatic beta cells.
What does it do? The net result of insulin release should be a decrease in blood sugar (i.e. glucose) levels when they are elevated, such as when carbohydrates are consumed. When insulin binds to receptors on fat and muscle, it causes glucose transporters to fuse with the cell membranes, allowing for the entry of glucose into these tissues. It also causes glucose to be stored as glycogen in the liver and inhibits the breakdown of fat for energy.
Released from: fat cells (i.e. adipocytes).
What does it do? Leptin, like ghrelin, acts on feeding centers in a brain region called the hypothalamus—but in an inhibitory way, tending to lead to decreases in appetite and food intake. It is also involved in energy expenditure and modulating control of the hormones related to reproduction (discussed below).
While leptin is sometimes referred to as a “satiety hormone,” its primary function seems to be to tell the body whether fat stores are sufficient for growth and reproduction. If fat stores are inadequate (e.g. below a “threshold” that is developmentally and genetically set), we see effects like increased hunger and food consumption, low energy output, and infertility; however, when levels are at or above this threshold, leptin’s actions may be less influential. Therefore, it may actually be more useful in giving cues related to hunger, rather than satiety.
Peptide YY3-36 (PYY)
Released from: cells in the small intestine and colon.
What does it do? In the presence of food, PYY also acts on hypothalamic feeding centers to inhibit hunger and food intake.
Thyroid Hormones (T 3 and T 4 )
Released from: the thyroid gland.
What do they do? T 3 —the metabolically active thyroid hormone—increases heart rate, ventilation (breathing) rate, and basal metabolic rate. It also influences sympathetic activity (i.e. our fight-or-flight response).
Reproductive Hormones
Both male and female sex hormones play a huge role in growth, development and sexual differentiation. Here is how.
Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH)
Released from: the pituitary gland in the brain.
What do they do? In females, LH and FSH levels rise and fall and regulate the menstrual cycle. In addition, LH stimulates the production of testosterone (below) in males.
Released from: the ovaries of females and other neuroendocrine tissues.
What does it do? Estradiol—the primary female sex hormone—stimulates the development of secondary sex characteristics of females and the female reproductive system (e.g. reproductive-organ development, bone-shape changes).
Testosterone
Released from: the testicles of males (primarily), the ovaries of females and other endocrine (i.e. hormone-producing) tissues.
What does it do? Testosterone—the primary male sex hormone—stimulates muscle-mass growth, increases bone density, bone maturation and linear growth. It also promotes the development of secondary sex characteristics of males (e.g. sex-organ development, facial-hair growth, deepening of the voice).
What happens in anorexia nervosa?
Anorexia nervosa significantly impacts both the body and the mind . The low weight state leads to a variety of medical consequences, including bone loss (osteopenia) and the loss of one’s menstrual period (amenorrhea). Many of these symptoms can be explained by the way hormones respond to the signals that we send our body when we restrict food intake for an extended period.
- Amenorrhea: Anorexia nervosa is associated with profound decreases in reproductive hormones (e.g. LH, FSH, and estrogen). The mechanism may be related to leptin levels, which are lower than expected based on height and weight, and seem to send the signal that energy availability is low, and our bodies aren’t ready for reproduction.
- Osteoporosis: Poor bone health is possibly one of the most compelling consequences as people who develop anorexia nervosa at a young age and may never reach peak bone mass. Severe complications, like bone fractures, can occur even as late as 40 years after diagnosis. The combination of low leptin and sex-hormone levels, high cortisol levels and poor nutrition can explain this feature.
- Thyroid levels: It is not uncommon for T3 levels to be low in people with anorexia nervosa. This manifests as bradycardia (slow heart rate) and hypothermia (low body temperature)—features that serve to preserve energy.
- Behavioral/psychological symptoms (e.g., anxiety, hyperactivity, impulsivity): Hormones that activate our fight-or-flight response (i.e. cortisol) are typically elevated in anorexia nervosa. This could have something to do with behavioral features of the illness that promote quick decisions and a high state of alert.
Perhaps the most fundamental question about hormones and anorexia nervosa of interest to researchers is about the impact of physiology in the development of the disorder. Is there a hormonal perturbation that promotes a low-weight state or predisposes one to the illness? Studies have looked at hormones like PYY that may predispose patients to reduce food intake, but in general, there is no evidence to conclude that this is the case. However, the hormonal irregularities experienced by individuals with anorexia nervosa are what one would expect in those who are starved. The question then becomes—what causes individuals with anorexia nervosa to overcome such a strong biological drive to regain lost weight? Whether it has to do with delaying reward , habit formation or differences in taste preference or something else we haven’t thought of yet, is a big focus of research today and is crucial to fully understand the illness.
© The Feed, 2013-present. Unauthorized use and/or duplication of this material without express and written permission from this blog’s authors is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to the article’s author and The Feed with appropriate and specific direction to the original content.
Share this:.
- Anorexia Nervosa
Mariya Bershad, BA
I have been on Growth Hormone, and without it I struggled to eat enough through loss of appetite, and found myself exercising more so I could feel hungrier. But if I exercised too much with low HGH, I found myself too tired to be hungry. I found myself regularly monitoring my food intake, but that was cos my appetite was so weak, cos I was deprived of replacement hormone, and my weight dropped. When my hormones were replaced, the problem went away.
[…] distress that accompanies eating disorders can take a toll on the mind and body. It is not uncommon for people to experience moments of feeling helpless, confused, and overwhelmed […]
[…] Though eating disorders are, by definition, disorders of the mind, they can be incredibly hard on the body. The physiological toll varies by individual and condition but in general includes heart health, reproductive functioning, and changes to hormones. […]
[…] are dangerous and can lead to injuries and other problems associated with being underweight such as menstrual dysfunction, low bone density, and cardiovascular problems – all problems which threaten to disrupt all […]
Leave a Reply Cancel reply
You might be interested in.

What to Know about Eating Disorder Risk in the US Military

What Brain Stimulation is Teaching Us about Anorexia Nervosa

Psychedelic-Assisted Therapy for Eating Disorders

Learning from People with Lived Experience

Habit Strength and Eating Behavior

Welcome to NYSPI: Our History and Mission

NY Times Retro Report Video: Myths & Misperceptions about Eating Disorders


Can Smartphone Technology Help in the Treatment of Binge Eating?
Discover more from the feed.
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
- Patient Care & Health Information
- Diseases & Conditions
- Anorexia nervosa
Anorexia (an-o-REK-see-uh) nervosa — often simply called anorexia — is an eating disorder characterized by an abnormally low body weight, an intense fear of gaining weight and a distorted perception of weight. People with anorexia place a high value on controlling their weight and shape, using extreme efforts that tend to significantly interfere with their lives.
To prevent weight gain or to continue losing weight, people with anorexia usually severely restrict the amount of food they eat. They may control calorie intake by vomiting after eating or by misusing laxatives, diet aids, diuretics or enemas. They may also try to lose weight by exercising excessively. No matter how much weight is lost, the person continues to fear weight gain.
Anorexia isn't really about food. It's an extremely unhealthy and sometimes life-threatening way to try to cope with emotional problems. When you have anorexia, you often equate thinness with self-worth.
Anorexia, like other eating disorders, can take over your life and can be very difficult to overcome. But with treatment, you can gain a better sense of who you are, return to healthier eating habits and reverse some of anorexia's serious complications.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
The physical signs and symptoms of anorexia nervosa are related to starvation. Anorexia also includes emotional and behavioral issues involving an unrealistic perception of body weight and an extremely strong fear of gaining weight or becoming fat.
It may be difficult to notice signs and symptoms because what is considered a low body weight is different for each person, and some individuals may not appear extremely thin. Also, people with anorexia often disguise their thinness, eating habits or physical problems.
Physical symptoms
Physical signs and symptoms of anorexia may include:
- Extreme weight loss or not making expected developmental weight gains
- Thin appearance
- Abnormal blood counts
- Dizziness or fainting
- Bluish discoloration of the fingers
- Hair that thins, breaks or falls out
- Soft, downy hair covering the body
- Absence of menstruation
- Constipation and abdominal pain
- Dry or yellowish skin
- Intolerance of cold
- Irregular heart rhythms
- Low blood pressure
- Dehydration
- Swelling of arms or legs
- Eroded teeth and calluses on the knuckles from induced vomiting
Some people who have anorexia binge and purge, similar to individuals who have bulimia. But people with anorexia generally struggle with an abnormally low body weight, while individuals with bulimia typically are normal to above normal weight.
Emotional and behavioral symptoms
Behavioral symptoms of anorexia may include attempts to lose weight by:
- Severely restricting food intake through dieting or fasting
- Exercising excessively
- Bingeing and self-induced vomiting to get rid of food, which may include the use of laxatives, enemas, diet aids or herbal products
Emotional and behavioral signs and symptoms may include:
- Preoccupation with food, which sometimes includes cooking elaborate meals for others but not eating them
- Frequently skipping meals or refusing to eat
- Denial of hunger or making excuses for not eating
- Eating only a few certain "safe" foods, usually those low in fat and calories
- Adopting rigid meal or eating rituals, such as spitting food out after chewing
- Not wanting to eat in public
- Lying about how much food has been eaten
- Fear of gaining weight that may include repeated weighing or measuring the body
- Frequent checking in the mirror for perceived flaws
- Complaining about being fat or having parts of the body that are fat
- Covering up in layers of clothing
- Flat mood (lack of emotion)
- Social withdrawal
- Irritability
- Reduced interest in sex
When to see a doctor
Unfortunately, many people with anorexia don't want treatment, at least initially. Their desire to remain thin overrides concerns about their health. If you have a loved one you're worried about, urge her or him to talk to a doctor.
If you're experiencing any of the problems listed above, or if you think you may have an eating disorder, get help. If you're hiding your anorexia from loved ones, try to find a person you trust to talk to about what's going on.
The exact cause of anorexia is unknown. As with many diseases, it's probably a combination of biological, psychological and environmental factors.
- Biological. Although it's not yet clear which genes are involved, there may be genetic changes that make some people at higher risk of developing anorexia. Some people may have a genetic tendency toward perfectionism, sensitivity and perseverance — all traits associated with anorexia.
- Psychological. Some people with anorexia may have obsessive-compulsive personality traits that make it easier to stick to strict diets and forgo food despite being hungry. They may have an extreme drive for perfectionism, which causes them to think they're never thin enough. And they may have high levels of anxiety and engage in restrictive eating to reduce it.
- Environmental. Modern Western culture emphasizes thinness. Success and worth are often equated with being thin. Peer pressure may help fuel the desire to be thin, particularly among young girls.
Risk factors
Anorexia is more common in girls and women. However, boys and men have increasingly developed eating disorders, possibly related to growing social pressures.
Anorexia is also more common among teenagers. Still, people of any age can develop this eating disorder, though it's rare in those over 40. Teens may be more at risk because of all the changes their bodies go through during puberty. They may also face increased peer pressure and be more sensitive to criticism or even casual comments about weight or body shape.
Certain factors increase the risk of anorexia, including:
- Genetics. Changes in specific genes may put certain people at higher risk of anorexia. Those with a first-degree relative — a parent, sibling or child — who had the disorder have a much higher risk of anorexia.
- Dieting and starvation. Dieting is a risk factor for developing an eating disorder. There is strong evidence that many of the symptoms of anorexia are actually symptoms of starvation. Starvation affects the brain and influences mood changes, rigidity in thinking, anxiety and reduction in appetite. Starvation and weight loss may change the way the brain works in vulnerable individuals, which may perpetuate restrictive eating behaviors and make it difficult to return to normal eating habits.
- Transitions. Whether it's a new school, home or job; a relationship breakup; or the death or illness of a loved one, change can bring emotional stress and increase the risk of anorexia.
Complications
Anorexia can have numerous complications. At its most severe, it can be fatal. Death may occur suddenly — even when someone is not severely underweight. This may result from abnormal heart rhythms (arrhythmias) or an imbalance of electrolytes — minerals such as sodium, potassium and calcium that maintain the balance of fluids in your body.
Other complications of anorexia include:
- Heart problems, such as mitral valve prolapse, abnormal heart rhythms or heart failure
- Bone loss (osteoporosis), increasing the risk of fractures
- Loss of muscle
- In females, absence of a period
- In males, decreased testosterone
- Gastrointestinal problems, such as constipation, bloating or nausea
- Electrolyte abnormalities, such as low blood potassium, sodium and chloride
- Kidney problems
If a person with anorexia becomes severely malnourished, every organ in the body can be damaged, including the brain, heart and kidneys. This damage may not be fully reversible, even when the anorexia is under control.
In addition to the host of physical complications, people with anorexia also commonly have other mental health disorders as well. They may include:
- Depression, anxiety and other mood disorders
- Personality disorders
- Obsessive-compulsive disorders
- Alcohol and substance misuse
- Self-injury, suicidal thoughts or suicide attempts
There's no guaranteed way to prevent anorexia nervosa. Primary care physicians (pediatricians, family physicians and internists) may be in a good position to identify early indicators of anorexia and prevent the development of full-blown illness. For instance, they can ask questions about eating habits and satisfaction with appearance during routine medical appointments.
If you notice that a family member or friend has low self-esteem, severe dieting habits and dissatisfaction with appearance, consider talking to him or her about these issues. Although you may not be able to prevent an eating disorder from developing, you can talk about healthier behavior or treatment options.
- Sim LA (expert opinion). Mayo Clinic, Rochester, Minn. Jan. 31, 2018.
- Anorexia nervosa. In: Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th ed. Arlington, Va.: American Psychiatric Association; 2013. http://dsm.psychiatryonline.org. Accessed Nov. 13, 2017.
- Hales RE, et al. Anorexia nervosa. In: The American Psychiatric Publishing Textbook of Psychiatry. 6th ed. Washington, D.C.: American Psychiatric Publishing; 2014. http://psychiatryonline.org. Accessed Nov. 13, 2017.
- Klein D, et al. Anorexia nervosa in adults: Clinical features, course of illness, assessment, and diagnosis. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Mehler P. Anorexia nervosa in adults and adolescents: Medical complications and their management. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Mehler P. Anorexia nervosa in adults: Evaluation for medical complications and criteria for hospitalization to manage these complications. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Pike K. Anorexia nervosa in adults: Cognitive behavioral therapy (CBT). https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Walsh BT. Anorexia nervosa in adults: Pharmacotherapy. https://www.uptodate.com/contents/search. Accessed Nov. 13, 2017.
- Anorexia nervosa. Merck Manual Professional Version. http://www.merckmanuals.com/professional/psychiatric-disorders/eating-disorders/anorexia-nervosa. Accessed Nov. 13, 2017.
- Harrington BC, et al. Initial evaluation, diagnosis, and treatment of anorexia nervosa and bulimia nervosa. American Family Physician. 2015;91:46.
- Brockmeyer T, et al. Advances in the treatment of anorexia nervosa: A review of established and emerging interventions. Psychological Medicine. In press. Accessed Nov. 13, 2017.
- Davis H, et al. Pharmacotherapy of eating disorders. Current Opinion in Psychiatry. 2017;30:452.
- Herpertz-Dahlmann B. Treatment of eating disorders in child and adolescent psychiatry. Current Opinion in Psychiatry. 2017;30:438.
- Fogarty S, et al. The role of complementary and alternative medicine in the treatment of eating disorders: A systematic review. Eating Behaviors. 2016;21:179.
- Eating disorders. National Alliance on Mental Illness. https://www.nami.org/Learn-More/Mental-Health-Conditions/Eating-Disorders/Overview. Accessed Nov. 13, 2017.
- Lebow J, et al. Is there clinical consensus in defining weight restoration for adolescents with anorexia nervosa? Eating Disorders. In press. Accessed Dec. 4, 2017.
- Lebow J, et al. The effect of atypical antipsychotic medications in individuals with anorexia nervosa: A systematic review and meta-analysis. International Journal of Eating Disorders. 2013;46:332.
- Five things to know about safety of dietary supplements for children and teens. National Center for Complementary and Integrative Health. https://nccih.nih.gov/health/tips/child-supplements. Accessed Feb. 9, 2018.
Associated Procedures
- Acupuncture
- Bone density test
- Complete blood count (CBC)
- Electrocardiogram (ECG or EKG)
- Liver function tests
- Psychotherapy
Mayo Clinic in Rochester, Minnesota, has been recognized as one of the top Psychiatry hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
A review of endocrine changes in anorexia nervosa
Affiliation.
- 1 Department of Endocrinology and Centre for Eating Disorders, Odense University Hospital, Odense C, Denmark.
- PMID: 10221746
- DOI: 10.1016/s0022-3956(98)00049-1
Anorexia nervosa is a syndrome of unknown etiology. It is associated with multiple endocrine abnormalities. Hypothalamic monoamines (especially serotonin), neuropeptides (especially neuropeptide Y and cholecystokinin) and leptin are involved in the regulation of human appetite, and in several ways they are changed in anorexia nervosa. However, it remains to be clarified whether the altered appetite regulation is secondary or etiologic. Increased secretion of corticotropin-releasing hormone and proopiomelanocortin seems to be secondary to starvation, however, there is evidence that it may maintain and intensify anorexia, excessive physical activity and amenorrhea. Hypothalamic amenorrhea, which is a diagnostic criterion in anorexia nervosa, is not solely related to the low body weight and exercise. Growth hormone resistance with low production of insulin-like growth factor I and high growth hormone secretion reflect the nutritional deprivation. The nutritional therapy of patients with anorexia nervosa might be improved by administering an anabolic agent such as growth hormone or insulin-like growth factor I. So far none of the endocrine abnormalities have proved to be primary, however, there is increasing evidence that some of these might participate in a vicious circle.
Publication types
- Research Support, Non-U.S. Gov't
- Anorexia Nervosa / metabolism*
- Hypothalamo-Hypophyseal System / metabolism
- Hypothalamus / metabolism*
- Pituitary Diseases / diagnosis
- Pituitary Diseases / metabolism*
- Pituitary Gland, Anterior / metabolism
- Pituitary Gland, Posterior / metabolism
- Pituitary-Adrenal System / metabolism
- Open access
- Published: 29 September 2023
Mechanisms and predictors of menses resumption once normal weight is reached in anorexia nervosa
- Bogdan Galusca 1 , 2 , 3 ,
- Aurélia Gay 2 , 3 , 4 ,
- Gwenaëlle Belleton 1 , 3 ,
- Martin Eisinger 1 , 3 ,
- Catherine Massoubre 2 , 3 , 4 ,
- François Lang 2 , 3 , 4 ,
- Dominique Grouselle 5 ,
- Bruno Estour 1 , 2 , 3 &
- Natacha Germain 2 , 3 , 4
Journal of Eating Disorders volume 11 , Article number: 172 ( 2023 ) Cite this article
2099 Accesses
Metrics details
In cases of Anorexia Nervosa (AN), achieving weight gain recovery beyond the lower limits set by the World Health Organization and normalizing classical nutritional markers appears to be essential for most patients. However, this is not always adequate to restore menstrual cycles. This discrepancy can cause concern for both patients and healthcare providers, and can impact the medical management of these individuals. Thus, the purpose of this study was to assess the ability of anthropometric and hormonal factors to predict the resumption of menstrual cycles in individuals with anorexia nervosa upon reaching a normal body weight.
Patients with AN who had achieved a normal Body Mass Index but had not yet resumed their menstrual cycles (referred to as ANRec) were evaluated on two occasions: first at visit 1 and then again 6 months later, provided their body weight remained stable over this period (visit 2). Among the 46 ANRec patients who reached visit 2, they were categorized into two groups: 20 with persistent amenorrhea (PA-ANRec) and 26 who had regained their menstrual cycles (RM-ANRec). Anthropometric measurements, several hormone levels, Luteinizing Hormone (LH) pulsatility over a 4-h period, and LH response to gonadotropin-releasing hormone injection (LH/GnRH) were then compared between the two groups at visit 1.
Patients in the RM-ANRec group exhibited higher levels of follicular stimulating hormone, estradiol, inhibin B, LH/GnRH, and lower levels of ghrelin compared to those in the PA-ANRec group. Analysis of Receiver Operating Characteristic curves indicated that having ≥ 2 LH pulses over a 4-h period, LH/GnRH levels ≥ 33 IU/l, and inhibin B levels > 63 pg/ml predicted the resumption of menstrual cycles with a high degree of specificity (87%, 100%, and 100%, respectively) and sensitivity (82%, 80%, and 79%, respectively).
Conclusions
These three hormonal tests, of which two are straightforward to perform, demonstrated a high predictive accuracy for the resumption of menstrual cycles. They could offer valuable support for the management of individuals with AN upon achieving normalized weight. Negative results from these tests could assist clinicians and patients in maintaining their efforts to attain individualized metabolic targets.
Trial registration
IORG0004981.
Plain English summary
Once a minimally normal weight has been reached during eating disorder recovery for female patients with anorexia nervosa (AN), the persistence of amenorrhea can be a cause for concern both patient and practitioner. In our study, we have discovered that positive results in biological blood tests, which can be conveniently conducted in an ambulatory setting, offer valuable predictive insights. Specifically, parameters such as LH pulse numbers exceeding 2, LH response to GnRH injection surpassing 33 UI/L, or Inhibin B levels in the blood exceeding 63 pg/mL, can accurately predict the resumption of menstrual cycles in the upcoming months, provided that the patient does not experience weight loss or engage in intense exercise. Conversely, negative results from these tests at this critical juncture in the recovery process can serve as valuable tools to encourage and motivate both the healthcare provider and the patient. By maintaining their efforts and continuing to increase their weight, patients can work towards a more comprehensive restoration of their menstrual cycles.
Anorexia Nervosa (AN) is an eating disorder characterized by self-starvation leading to weight loss, undernutrition, and subsequent adaptive typical hormonal changes [ 1 , 2 ]. Some of these changes are responsible for a functional gonadal axis blockade [ 3 ]. Specifically, the combination of weight loss, blunted leptin [ 4 ], high cortisol [ 5 ] and high ghrelin plasma levels [ 6 , 7 ] results in a functional hypothalamic amenorrhea through the inhibition of GnRH pulses [ 8 , 9 ]. Consequently, amenorrhea was systematically associated with weight loss in the previous DSM IV definition of AN [ 10 , 11 ]. Nevertheless, one major modification included in the revised DSM 5 definition was the removal of amenorrhea [ 12 ], potentially impacting the differential diagnosis of thinness [ 13 ].
However, resumption of menstrual cycles during weight gain remains highly relevant for nutritional recovery [ 10 ]. While achieving weight gain beyond the lower limits of the World Health Organization's body mass index (BMI) normal range (18.5 kg/m 2 ) and normalizing disrupted nutritional markers is generally considered essential, it does not always guarantee the return of menstruation in patients with AN [ 3 ]. It has been observed that menses typically resume within six months after reaching 90% of standard body weight for high and age [ 14 ]. Meanwhile the delay of menses resumption after weight recovery appears to vary from 6 months [ 14 ] to several years [ 15 ]. However specific individual set-point of weight and body composition enabling menses recovery can be experienced in clinical practice. Additionally intense physical activity may contribute to amenorrhea persistence despite BMI normalization [ 16 ]. Consequently, patients with AN and healthcare professionals alike require well-defined weight gain and physical activity targets, as menses recovery appears to be influenced by factors beyond BMI normalization alone.
Several parameters, including initial and current Follicular Stimulating Hormone (FSH), inhibin B, and anti mullerian hormone (AMH) plasma level [ 17 , 18 ], baseline cortisol plasma level [ 19 ], body weight before the onset of anorexia episode, duration of the illness, and the rate of weight gain [ 20 , 21 ], entered multivariate models to predict menses recovery in the referred studies. However, these studies have not examined the absolute values of these parameters as thresholds or benchmarks to predict menses restoration and propose them as reliable indicators that could impact clinical decision-making.
Considering the collective insights derived from these observations, the focus of this study was to investigate the potential impact of both anthropometric and hormonal factors in the persistence of amenorrhea among individuals with anorexia nervosa who have achieved normalized weight. Specifically, the study aimed to explore whether these factors play a role in the mechanisms underlying the continued absence of menstruation and to assess their potential to predict the likelihood of subsequent resumption of menstrual cycles.
This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments (as revised in 1983). The study received approval from the local Research and Ethics Committee of Saint-Etienne, France (IORG0004981). All subjects provided written informed consent.
Patients and study design
This was a prospective observational study. The study design and flow chart is presented Fig. 1 .
Study design and flow chart of the study
Female patients with restrictive-type anorexia nervosa (AN) included in the study analysis met the criteria of the DSM IV and 5 [ 11 ] at visit 0. The minimal period between puberty onset and AN onset was two years; hence, no patient with primary amenorrhea was included. None of the patients used oral contraceptives throughout the study period. None of these subjects had other documented chronic or congenital diseases, and none were taking any medication at visits 0, 1 and 2 or between visits 1 and 2. Patients with AN with a documented history of constitutional thinness were not included. Patients were followed in an Eating Disorder Reference Center, under the care of both an endocrinologist/nutritionist and a psychiatrist. From visit 0 to visit 1, the primary somatic goal was to assist patients in gaining weight. From visit 1 to visit 2, primary somatic goal was weight maintenance, with no addition of physical activity.
Initially, 52 female patients with AN were recruited from the Eating Disorder Reference Center of Saint-Etienne when they reached a BMI superior to 18.5 kg/m 2 during weight gain but still experiencing amenorrhea (ANRec patients) (visit 1). They were then evaluated 6 months later to determine whether amenorrhea persisted despite body weight stabilization (visit 2). Six ANRec patients were excluded due to reported intense physical activity or weight loss during this period, indicating relapse. Consequently, 46 ANRec patients were ultimately included in the study and categorized into two groups at the end of this 6-month follow-up period: 20 patients with persistent amenorrhea despite weight gain preservation, termed Persistent amenorrhea ANRec (PA-ANRec); and 26 patients who recovered their menses during the 6-month follow-up period, termed Recovered Menses ANRec (RM-ANRec). A procedure timeline for data collection is presented in Table 1 . Historical maximal BMI (before the disease occurred) was also gathered by self-report.
Anthropometry and body composition
Body weight was measured with a digital scale (ProDoc, Detecto, PD200M) to the nearest 0.1 kg, and body height was recorded with a standard wall-mounted stadiometer to the nearest 0.1 cm. Fat mass (FM) measurements were assessed by Dual-energy X-ray Absorptiometry (LUNAR, DPX-L).
Hormonal assessment
Venous blood samples were collected on dry glass tubes containing EDTA, centrifuged, and plasma was aliquoted and kept frozen at − 80 °C before the assay. After an overnight fast, venous blood samples were obtained at 08:00 h for measurement of serum Insulin Like Growth Factor type 1 (IGF-1), Prolactin, TSH, free T3, normetanephrin, metanephrin, Estradiol, FSH, LH, total testosterone, sex hormone binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS) and inhibin B. Samples were collected every four hours over a 24-h period (08 h–12 h–16 h–20 h–24 h–04 h) to assess Growth hormone (GH), ACTH, cortisol, and leptin, both acylated and total ghrelin. The assessment techniques of these parameters were previously described [ 22 , 23 , 24 , 25 ].
FSH and LH responses were also assessed 30 min after intravenous Gonadotropin Releasing Hormone (GnRH) administration (Relefact® LH-RH 100 µg/1 ml, Sanofi Aventis).
LH pulsatile activity was evaluated by blood sampling every ten minutes over four hours in the morning, a day-time known to have the highest frequency of LH pulses.
Statistical analysis
Hormonal values were presented as mean ± SEM. For GH, cortisol, leptin and ghrelin, the mean value of six circadian assessments was determined for each subject. The time series of LH concentrations over the four hours (LH pulsatile activity test) was analyzed for pulse frequency, pulse amplitude and four hours mean concentration using the validated, objective pulse detection algorithm Autodecon [ 26 ]. An LH pulse was defined (and counted) as a significant rise of LH from basal levels every 60 to 90 min, significant enough to be detected within deconvolution analysis (Autodecon).
The two groups, PA-ANRec and RM-ANRec, according to the study design, were compared for all the parameters described in Table 2 (retrospective comparison for visit 0 and 1), BMI at visit 2, and BMI prior to the onset of the disease. For each time point of the study, comparisons between groups were performed using a non-parametric Mann Whitney unpaired t-test. A two-factor repeated measures ANOVA, followed by post hoc test if relevant, was used to compare total and acylated ghrelin during 6-point cycle over 24-h evaluation. A non-parametric Wilcoxon signed rank paired test was used to evaluate changes in all parameters between the time points of the study (visit 1 vs. visit 0).
ROC curve analysis was performed to assess sensitivity, specificity, and accuracy (Area under the curve–AUC) of different hormonal markers at visit 1 to predict menses resumption during the 6-month follow-up period.
Statistical analyses were performed with StatView 4.5 software (Abacus Concepts, Inc., Palo Alto, CA).
Patients’ overall group characteristics
The mean age of total group at visit 0 was 20.0 ± 0.86 years. The mean maximal BMI prior to disease was 19.9 ± 0.3 kg/m 2 . It decreased at Visit 0 (first admission) to a mean value of 15.1 ± 0.33 kg/m 2 . At visit 1 (weight normalization) mean BMI increased to 18.9 ± 0.15 kg/m 2 . The average time elapsed between visit 0 and visit 1 was 1.6 ± 0.23 years. By the end of the study (visit 2, 6-month follow-up) the mean BMI was 19.2 ± 0.38 kg/m 2 .
Anthropometric and hormonal inter-group comparison at visit 0 (undernutrition state)
All anthropometric and hormonal parameters are presented in Table 2 .
At visit 0, no major differences were noticed between groups while patients were undernourished. Gonadal axis evaluation showed no difference between the groups at visit 0.
BMI was similar between groups at visit 0 (Table 2 ) but also before the AN diagnosis (19.7 ± 0.4 kg/m 2 in PA-ANRec vs. 20.3 ± 0.5 kg/m 2 in RM-ANRec, p = 0.6754). The decrease of BMI at this visit (visit 0 BMI–BMI prior to disease) was not different between the groups (− 5.2 ± 0.51 vs. − 4.5 ± 0.54 kgM 2 , p = 0.36).
Anthropometric and hormonal comparison at visit 1 (weight normalisation) and between visit 0 and 1
At visit 1, no differences were noticed between groups for BMI. Although increased when compared to visit 0, BMI at visit 1 was lower than BMI prior to disease. This delta BMI (BMI visit 1–BMI prior to disease) was similar between PA-ANRec and RM-ANRec (− 1.15 ± 0.46 vs. − 0.93 ± 0.37 kg/m 2 , p = 0.71).
Lower cortisol and higher leptin, free T3 and IGF1 were found in both groups when compared to visit 0. At visit 1, RM-ANRec patients also presented with higher plasma levels of FSH, LH and inhibin B and higher LH response to GnRH injection compared to PA-ANRec ( p = 0.0004) (Table 2 ).
LH pulse analysis performed only at this visit showed significantly lower peak frequency (0.8 ± 0.2 vs. 2.0 ± 0.2 peaks/24 h; p = 0.005), area under the curve of LH (639 ± 145 vs. 1067 ± 139; p = 0.04), and mean LH (2.4 ± 0.5 vs. 4.1 ± 0.5 U/l; p = 0.04) and significantly higher inter-pulse interval (686 ± 94 vs. 313 ± 73; p = 0.003) in PA-ANRec patients compared to RM-ANRec patients. Examples of individual LH pulse deconvolution in a PA-ANRec patient and a RM-ANRec patient are presented in Additional file 1 : Fig. S1.
Both total and acylated ghrelin plasma levels evaluated at visit 1 (BMI normalization time-point) were significantly higher in PA-ANRec patients compared to RM-ANRec patients (respectively p = 0.003 and 0.002) (Additional file 2 : Fig. S2).
The time spent between Visit 0 and Visit 1 trended to be higher in PA-ANRec than in RM-ANRec (2.10 ± 0.41 vs. 1.35 ± 0.22 yrs, p = 0.06). The mean time elapsed between visit 1 and menses resumption in RM-ANRec group was 3.71 ± 0.51 months.
Anthropometric comparison at visit 2
Similar BMIs were also found in PA-ANRec and in RM-ANRec by the end of the study (19.0 ± 0.12 vs. 13.3 ± 0.23, p = 0.75). No significant differences were found when compared to visit 1 in both groups.
ROC analysis to predict menses recovery ( Fig. 2 )
Receiver Operator Curve (ROC) analysis for A LH response to GnRH, B Presence of LH pulses and C inhibin B plasma level; Optimal values of sensitivity (Se), specificity (Sp) and accuracy (AUC) corresponding to the red-circle point on the ROC curve are presented
ROC analysis indicated that LH response to GnRH (≥ 33 UI/l) (Fig. 2 A), the number of LH pulses ≥ 2 (Fig. 2 B), but also Inhibin B > 63 pg/ml (Fig. 2 C) showed equivalent high values of sensitivity (79 to 82%) and specificity (87 to 100%) with AUC around 0.90 to predict menses recovery. Estradiol levels, one-point levels of LH, and FSH predicted menses resumption with lower accuracy.
The persistence of amenorrhea following the attainment of a minimally normal weight during the recovery phase poses a significant challenge for both patients with anorexia nervosa and healthcare professionals. The mechanisms contributing to this blockade or dysfunction of the gonadotropic axis appear to be multifaceted, involving various hormonal factors.
Despite normalization of BMI in all patients of this study, our study clearly demonstrates the existence of two distinct populations of patients with regards to gonadal axis functioning which corresponds to their clinical evolution. Indeed, patients with anorexia nervosa who recovered their weight and further on their menses, displayed higher plasma levels of FSH, LH and inhibin B. Moreover, they presented with higher LH response to GnRH injection. Furthermore, LH pulse analysis showed higher peak frequency, higher area under the curve of LH, higher mean LH, and higher inter-pulse interval, indicating a more “awakened” gonadal axis than in those who did not recover their menses during the follow-up period. Remarkably, these differences occur despite the disappearance of undernutrition signs in both groups. Indeed, nutritional parameters such as free T3 and IGF1 reached similar normalized levels in both studied anorexia nervosa groups. Taken together, these findings suggest that other hormonal parameters involved in gonadal axis functioning could explain the differences observed in both biological and clinical evolution between the two studied groups.
Hormones such as leptin, cortisol and prolactin do not seem to contribute the persistence of amenorrhea after weight normalization. Notably, all patients at visit 1 displayed normalized values for leptin, cortisol, and prolactin. While low leptin levels and functional hypercortisolism are known to underlie hypothalamic amenorrhea in undernourished patients with AN, and the administration of recombinant leptin has been shown to restore menses in women with hypothalamic amenorrhea and leptin deficiency [ 27 , 28 ] the normalization of these hormones post-weight recovery implies that they might not be directly associated with the ongoing amenorrhea in this context. It is plausible that other hormones or neuropeptides could be involved.
In our study, circadian levels of acylated and total ghrelin were significantly higher in weight normalized patients with anorexia nervosa with persistent amenorrhea than in those with subsequent menses resumption. An increased level of ghrelin in restrictive-type anorexia nervosa with secondary amenorrhea have been widely established [ 29 ]. These higher levels could account for a persistent negative metabolic balance explained either by undeclared excess of physical activity or by insufficient food intake. This aligns with previous research suggesting that increased ghrelin levels could act as a metabolic signal hindering the return to a cyclic state in women with eating disorders who exhibit normal weight and body fat but still experience amenorrhea [ 7 ]. Moreover, recent evidence highlights ghrelin inhibitory effects on GnRH pulse activity and LH secretion in both animal and human models [ 6 , 30 , 31 ].
Brain imaging data we previously published showed an increased opioid activity in hypothalamic and pituitary areas in undernourished patients with anorexia nervosa when compared to healthy volunteers. In the same study, patients with AN with recovered gonadal activity after weight gain presented with normal opioid activity in these areas, significantly lower than in undernourished patients [ 32 ]. Endogenous opioids and their antagonists modulate GnRH pulse and LH secretion [ 33 ]. In adult men, treatment with morphine resulted in a decrease of LH pulse frequency while administration of naloxone by itself increased the LH pulse frequency [ 33 ]. Interestingly, naltrexone is able to induce ovulation in amenorrheic women [ 34 , 35 , 36 ]. However in undernourished patients with anorexia nervosa mean levels of LH and LH pulse activity were not significantly changed by naltrexone [ 37 ]. We could account here that LH pulse activity restoration in fully recovered patients with anorexia nervosa may be partly due to a decrease in opioid tone. Further interventional studies could address the question of opioid agonist or ghrelin analogue utility in patients with anorexia nervosa with normalized body weight but still amenorrheic.
Collectively, these findings suggest that specific hormonal and neurobiological abnormalities may persist despite BMI normalization or the normalization of classical nutritional markers. These factors could potentially impede complete metabolic recovery and explain the delay in gonadotropic axis recovery.
Interestingly, we previously demonstrated in this very specific population that pulsatile GnRH therapy was a safe and efficient treatment in cases where patients desired to become pregnant, but still did not allowed menses recovery after pregnancy [ 38 ]. This suggests the chronic profile of upper mentioned abnormalities and the importance of their impact on gonadal axis. Undeclared features like intense physical activity or psychological stress may be responsible for this chronic profile of incomplete somatic recovery from AN.
We also evaluated in the current study the potential of certain hormones at the stage when patients with anorexia nervosa achieved a normal BMI but remained amenorrheic to predict future menses resumption. Notably, the differences in gonadal axis between the two groups were evident in our study while all patients were still amenorrheic. This distinguishes our study from many prior ones that found differences in gonadal axis and other hormonal parameters between recovered AN subjects with persistent amenorrhea and those who had already resumed menses [ 17 , 19 , 20 , 21 ]. These previous findings were expected as they compared two clinically distinct conditions. In contrast, our study reveals these differences before clinical changes’ manifest, indicating their potential as predictive markers. In clinical practice, such hormonal evaluations might inform and educate patients about their gonadal status while supporting clinician recommendations to pursue further weight restoration.
For the first time, our study demonstrates that specific parameters of gonadotropic function are reliable tools for predicting menses recovery in weight-normalized but still amenorrheic patients with anorexia nervosa. The presence of at least two LH pulses within a 4-h interval predicts menses resumption accurately within the next 6 months. As LH pulsatile activity is fundamental for gonadotropic function, this test serves as a reference point. Typically, research studies assess LH pulsatile activity over a 24-h span [ 39 , 40 , 41 ]. Our study focused solely on detecting this activity within a shorter 4-h period, as our objective was to identify its presence. During puberty onset, the LH pulse initiates during the night and gradually becomes cyclic throughout the day; however, in adults, LH pulse screening is typically conducted in the morning [ 42 ] as performed it in our study.
Comparable accuracy was observed for the 30-min LH response to GnRH test (≥ 33 UI/l) and for inhibin B plasma levels (≥ 63 pg/ml). While LH pulse evaluation proved to be reliable in predicting menses recovery, its complexity limits its practicality in routine clinics. In contrast, inhibin B is simpler to sample and assay, and the GnRH stimulation test is commonly performed in outpatient settings, indicating their potential as practical routine tools.
It is crucial to bear in mind that the assessment of these predictive markers would lose its relevance if a patient recovering from anorexia nervosa, who had previously regained weight, were to start losing it again due to disruptions in their energy balance or engaging in intense physical exercise. Simultaneously, our study revealed a trend toward a longer time lapse between visit 0 and visit 1 in the PA-ANRec group. One might question the relevance of this time frame, considering that weight gain is seldom linear. However, it is plausible that patients with AN, experiencing a more prolonged period of amenorrhea and weight gain, may require extra time for menstrual resumption following weight normalization. In such instances, it could prove advantageous to reevaluate the gonadal axis every six months.
Our findings underscore the importance of tailored care during the recovery process for individuals with anorexia nervosa. However, while utilizing individualized BMI growth curves as a benchmark may be the most effective approach [ 14 ], it is often impractical. Individual growth BMI curves may not be readily available, especially for post-adolescent young adults. In some cases, these growth curves may be obscured or biased by prior weight fluctuations occurring before the onset of AN. Additionally, this equilibrium set point can change or be influenced by factors such as physical activity. The aim of this work was not to impose a BMI of 18.5 kg/m 2 at all costs as a weight gain objective but to propose it, once it has been reached (without menses recovery), as a milestone to trigger an assessment of the gonadotropic axis in all these situations. We selected this value of 18.5 kg/m 2 as it still represents the lower normal limit for BMI recommended by the World Health Organization (WHO), a value familiar to most patients.
In conclusion, we propose that the presence of at least two LH pulses over 4 h, an LH response to GnRH ≥ 33 UI/l, or inhibin B plasma levels ≥ 63 pg/ml can accurately predict the resumption of menses in weight-normalized but persistently amenorrheic patients with anorexia nervosa, provided there is no weight loss or intense exercise. These evaluations could assist clinicians in patient counseling. Based on this evaluation, clinicians can inform patients if their individual body weight or metabolic set-point has been achieved or not, thereby guiding them towards continued care: extending weight gain to achieve individual body weight equilibrium and subsequent menses resumption, or maintaining weight while awaiting menses to resume.
Availability of data and materials
Data will be made available upon researchers request for meta-analysis.
Abbreviations
Anti mullerian hormone
Anorexia nervosa
Recovered anorexia nervosa patient
Ostéocalcine
Body mass index
Dehydroepiandrosterone sulfate
Diagnostic and statistical manual
Follicle stimulating hormone
Growth hormone
Gonadotrophin releasing hormone
Insulin like growth factor type 1
Luteinizing hormone
Recovered anorexia nervosa patient with persistent amenorrhea
Recovered anorexia nervosa patient with recovered menses
Receiver operating characteristic
Serum cross laps
Sex hormone binding globulin
World Health Organization
Revised diagnostic subgroupings for anorexia nervosa. Nutr Rev. 1994;52(6):213–5
Estour B, Germain N, Diconne E, Frere D, Cottet-Emard JM, Carrot G, et al. Hormonal profile heterogeneity and short-term physical risk in restrictive anorexia nervosa. J Clin Endocrinol Metab. 2010;95(5):2203–10.
Article PubMed Google Scholar
Viricel J, Bossu C, Galusca B, Kadem M, Germain N, Nicolau A, et al. Retrospective study of anorexia nervosa: reduced mortality and stable recovery rates. Presse Med. 2005;34(20 Pt 1):1505–10.
Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, et al. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr. 2007;85(4):967–71.
Estour B, Pugeat M, Lang F, Lejeune H, Broutin F, Pellet J, et al. Rapid escape of cortisol from suppression in response to i.v. dexamethasone in anorexia nervosa. Clin Endocrinol. 1990;33(1):45–52.
Article Google Scholar
Kluge M, Schüssler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92(8):3202.
Schneider LF, Warren MP. Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil Steril. 2006;86(6):1744–9.
Ferin M. Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84(6):1768–74.
Allouche J, Bennet A, Barbe P, Plantavid M, Caron P, Louvet JP. LH pulsatility and in vitro bioactivity in women with anorexia nervosa-related hypothalamic amenorrhea. Acta Endocrinol. 1991;125(6):614–20.
Google Scholar
Association AP. Diagnostic and statistical manual of mental disorders (DSM-5 ® ): American Psychiatric Pub; 2013.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association; 1994. p. 943.
Attia E, Becker AE, Bryant-Waugh R, Hoek HW, Kreipe RE, Marcus MD, et al. Feeding and eating disorders in DSM-5. Am J Psychiatry. 2013;170(11):1237–9.
Estour B, Marouani N, Sigaud T, Lang F, Fakra E, Ling Y, et al. Differentiating constitutional thinness from anorexia nervosa in DSM 5 era. Psychoneuroendocrinology. 2017;84:94–100.
Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med. 1997;151(1):16–21.
Herpertz-Dahlmann BM, Wewetzer C, Schulz E, Remschmidt H. Course and outcome in adolescent anorexia nervosa. Int J Eat Disord. 1996;19(4):335–45.
Mountjoy M, Sundgot-Borgen JK, Burke LM, Ackerman KE, Blauwet C, Constantini N, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 2018;52(11):687–97.
van Elburg AA, Eijkemans MJ, Kas MJ, Themmen AP, de Jong FH, van Engeland H, et al. Predictors of recovery of ovarian function during weight gain in anorexia nervosa. Fertil Steril. 2007;87(4):902–8.
Popovic V, Djurovic M, Cetkovic A, Vojvodic D, Pekic S, Spremovic S, et al. Inhibin B: a potential marker of gonadal activity in patients with anorexia nervosa during weight recovery. J Clin Endocrinol Metab. 2004;89(4):1838–43.
Arimura C, Nozaki T, Takakura S, Kawai K, Takii M, Sudo N, et al. Predictors of menstrual resumption by patients with anorexia nervosa. Eat Weight Disord. 2010;15(4):e226–33.
PubMed Google Scholar
Dei M, Seravalli V, Bruni V, Balzi D, Pasqua A. Predictors of recovery of ovarian function after weight gain in subjects with amenorrhea related to restrictive eating disorders. Gynecol Endocrinol. 2008;24(8):459–64.
Jacoangeli F, Masala S, Staar Mezzasalma F, Fiori R, Martinetti A, Ficoneri C, et al. Amenorrhea after weight recover in anorexia nervosa: role of body composition and endocrine abnormalities. Eat Weight Disord. 2006;11(1):e20–6.
Galusca B, Bossu C, Germain N, Kadem M, Frere D, Lafage-Proust MH, et al. Age-related differences in hormonal and nutritional impact on lean anorexia nervosa bone turnover uncoupling. Osteoporos Int. 2006;17(6):888–96.
Galusca B, Germain N, Estour B. Bone abnormalities in constitutional thinness. Br J Nutr. 2009;102(11):1698–9.
Galusca B, Leca V, Germain N, Frere D, Khalfallah Y, Lang F, et al. Normal inhibin B levels suggest partial preservation of gonadal function in adult male patients with anorexia nervosa. J Sex Med. 2012;9(5):1442–7.
Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, et al. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J Clin Endocrinol Metab. 2010;95(6):3057–62.
Urban RJ, Johnson ML, Veldhuis JD. In vivo biological validation and biophysical modeling of the sensitivity and positive accuracy of endocrine peak detection. I. The LH pulse signal. Endocrinology. 1989;124(5):2541.
Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–97.
Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A. 2011;108(16):6585–90.
Article PubMed PubMed Central Google Scholar
Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88(1):109.
Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 2004;89(11):5718–23.
Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab. 2008;93(9):3633.
Galusca B, Traverse B, Costes N, Massoubre C, Le Bars D, Estour B, et al. Decreased cerebral opioid receptors availability related to hormonal and psychometric profile in restrictive-type anorexia nervosa. Psychoneuroendocrinology. 2020;118:104711.
Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31(1):98–132.
Wildt L, Leyendecker G. Induction of ovulation by the chronic administration of naltrexone in hypothalamic amenorrhea. J Clin Endocrinol Metab. 1987;64(6):1334–5.
Genazzani AD, Petraglia F, Gastaldi M, Volpogni C, Gamba O, Genazzani AR. Naltrexone treatment restores menstrual cycles in patients with weight loss-related amenorrhea. Fertil Steril. 1995;64(5):951–6.
Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod. 2008;23(11):2564–9.
Armeanu MC, Berkhout GM, Schoemaker J. Pulsatile luteinizing hormone secretion in hypothalamic amenorrhea, anorexia nervosa, and polycystic ovarian disease during naltrexone treatment. Fertil Steril. 1992;57(4):762–70.
Germain N, Fauconnier A, Klein J-P, Wargny A, Khalfallah Y, Papastathi-Boureau C, et al. Pulsatile gonadotropin-releasing hormone therapy in persistent amenorrheic weight-recovered anorexia nervosa patients. Fertil Steril. 2017;107(2):502–9.
Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287(12):582–6.
Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci U S A. 1998;95(5):2541–6.
Veldhuis JD. Neuroendocrine mechanisms mediating awakening of the human gonadotropic axis in puberty. Pediatr Nephrol. 1996;10(3):304–17.
Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27(2):101–40.
Download references
Acknowledgements
The authors would like to thank Michael L Johnson and Paula P Veldhuis (Departments of Pharmacology and Medicine, University of Virginia Health System, Charlottesville, VA) for helping using Autodecon software and interpreting LH pulse.
Not applicable. No funding was available for this work.
Author information
Authors and affiliations.
Division of Endocrinology, Endocrinology Department, University Hospital of Saint-Etienne, 42055, Saint-Étienne Cedex 2, France
Bogdan Galusca, Gwenaëlle Belleton, Martin Eisinger & Bruno Estour
EA 7423, Eating Disorders, Addictions and Extreme Body Weight Research Group, Saint-Étienne, France
Bogdan Galusca, Aurélia Gay, Catherine Massoubre, François Lang, Bruno Estour & Natacha Germain
Eating Disorder Reference Center of Saint-Etienne, University Hospital of Saint-Etienne, Saint-Étienne, France
Bogdan Galusca, Aurélia Gay, Gwenaëlle Belleton, Martin Eisinger, Catherine Massoubre, François Lang, Bruno Estour & Natacha Germain
Division of Psychiatry, University Hospital of Saint-Etienne, Saint-Étienne, France
Aurélia Gay, Catherine Massoubre, François Lang & Natacha Germain
UMR 894 INSERM Psychiatry and Neurosciences Center, Paris Descartes University, Paris, France
Dominique Grouselle
You can also search for this author in PubMed Google Scholar
Contributions
BG designed the study, collected the data, analyzed the data and wrote the manuscript. AG collected the data, analyzed the data and edited the manuscript. GB collected the data, analyzed the data and edited the manuscript. ME collected the data, analyzed the data and edited the manuscript. CM collected the data, analyzed the data and edited the manuscript. FL collected the data, analyzed the data and edited the manuscript. DG collected the data, analyzed the data and edited the manuscript. BE designed the study, collected the data, analyzed the data and edited the manuscript. NG designed the study, collected the data, analyzed the data, wrote the manuscript and edited the manuscript and was a major contributor in the writing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Bogdan Galusca .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments (as revised in 1983). Study was approved by the local research and Ethics Committee of Saint-Etienne, France (IORG0004981). All subjects gave a written informed consent.
Consent for publication
The manuscript does not contain any individual data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: fig. s1..
Example of deconvolution of LH pulse for A a patient from Persistent Amenorrhea Recovered Anorexia Nervosa (PA-ANRec) group and B a patient from Recovered Menses Recovered Anorexia Nervosa (RM-ANRec) group at visit 1. The upper graphs present the absolute LH values throughout the LH pulsatility 4-hour test while the lower graphs are issued from the deconvolution analysis. Patient ( A ) present with no pulses while pulses were detected in patient ( B ).
Additional file 2: Fig. S2.
Six-point circadian plasma levels of A Acylated and B Total ghrelin in both groups of the study: Recovered Menses AN after weight recovery (RM-ANRec) (open circles) versus persistent amenorrhea after weight recovery (PA-ANRec) (black triangles).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Galusca, B., Gay, A., Belleton, G. et al. Mechanisms and predictors of menses resumption once normal weight is reached in anorexia nervosa. J Eat Disord 11 , 172 (2023). https://doi.org/10.1186/s40337-023-00893-x
Download citation
Received : 02 December 2022
Accepted : 14 September 2023
Published : 29 September 2023
DOI : https://doi.org/10.1186/s40337-023-00893-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Recovered anorexia nervosa
- Menses resumption
- Body weight set-point
- Predictive markers
Journal of Eating Disorders
ISSN: 2050-2974
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Endocrinol Metab

Endocrine Dysregulation in Anorexia Nervosa Update
Anorexia nervosa is a primary psychiatric disorder with serious endocrine consequences, including dysregulation of the gonadal, adrenal, and GH axes, and severe bone loss. This Update reviews recent advances in the understanding of the endocrine dysregulation observed in this state of chronic starvation, as well as the mechanisms underlying the disease itself.
Evidence Acquisition:
Findings of this update are based on a PubMed search and the author's knowledge of this field.
Evidence Synthesis:
Recent studies have provided insights into the mechanisms underlying endocrine dysregulation in states of chronic starvation as well as the etiology of anorexia nervosa itself. This includes a more complex understanding of the pathophysiologic bases of hypogonadism, hypercortisolemia, GH resistance, appetite regulation, and bone loss. Nevertheless, the etiology of the disease remains largely unknown, and effective therapies for the endocrine complications and for the disease itself are lacking.
Conclusions:
Despite significant progress in the field, further research is needed to elucidate the mechanisms underlying the development of anorexia nervosa and its endocrine complications. Such investigations promise to yield important advances in the therapeutic approach to this disease as well as to the understanding of the regulation of endocrine function, skeletal biology, and appetite regulation.
Anorexia nervosa is a common psychiatric disorder in women and adolescent girls and is characterized by body image distortion and chronic severe undernutrition. Although psychiatric in origin, serious endocrine complications, including bone loss, are a prevalent feature of this disease. This Update reviews published research into the pathophysiology and treatment of endocrine complications of this disorder as well as recent insights into the etiopathology of the eating disorder itself. Although significant progress has been made in the understanding of the disorder and the endocrine physiology of chronic starvation, the fundamental basis of this disease remain unknown. Further research is needed to elucidate the etiology of the disorder and to identify effective treatments for anorexia nervosa and its complications.
Definition and Epidemiology
Sir William Withey Gull, one of Queen Victoria's personal physicians, coined the term “anorexia nervosa” in 1868, providing a name for a syndrome of wasting for which no organic etiology, such as tuberculosis, could be identified ( 1 ). The disorder has since been recognized as psychiatric in origin and is currently diagnosed using standard psychiatric criteria as delineated in the Diagnostic and Statistical Manual IV. These include failure to maintain weight above 85% of ideal, a distorted body image or denial of the seriousness of one's low body weight, fear of gaining weight, and amenorrhea ( 2 ). However, the amenorrhea criterion is under debate and may be excluded from the upcoming psychiatric diagnostic criteria revision (Diagnostic and Statistical Manual V) based on data that demonstrate few psychiatric differences between women with anorexia nervosa who do and do not have amenorrhea ( 3 , 4 ). In addition, it has been recognized that individual susceptibility of the gonadal axis to undernutrition is highly variable, resulting in a subset of extremely low-weight women with all of the psychiatric features of anorexia nervosa who maintain regular menses ( 5 ). There are two subtypes of anorexia nervosa: restricting and binge/purge. The latter is characterized by frequent episodes of purging and is distinguished from bulimia nervosa in that establishment of a diagnosis of anorexia nervosa, bulimic subtype requires low weight. In addition, a significant percentage of patients with a restricting subtype develop purging and/or binge-eating symptoms, with more than 50% developing bulimic behaviors over the course of the disease ( 6 ).
Anorexia nervosa affects 0.3 to 3.% of women ( 7 – 9 ) and is the third most prevalent chronic disease afflicting adolescent girls ( 10 ) in Western societies. It is primarily a disease of young women, but men and older women are affected at lower rates ( 8 , 10 ). The risk of death is relatively high for a young population, with an overall standardized mortality ratio of 11 to 12 ( 11 , 12 ), a substantial portion of which is attributable to a suicide rate 56 times that expected for age and sex ( 12 ). Alcoholism is an independent risk factor for mortality ( 12 ), and other factors associated with an increased risk of death include older age, longer duration since eating disorder onset, history of suicide attempt, diuretic use, patient desire for low body mass index (BMI), and severity of disordered eating symptomology ( 11 ). Although approximately 50% of patients with anorexia nervosa recover fully, 30% sustain only partial recovery, and 20–30% suffer from chronic disease ( 13 ). Therefore, complications of the disease may be chronic and may exert long-lasting and serious health effects. In addition, some sequelae, such as low bone mass, may persist even with weight recovery.
Endocrine Complications of Eating Disorders
Anorexia nervosa is complicated by hypothalamic-pituitary dysregulation, including hypothalamic amenorrhea, hypothalamic-pituitary-adrenal axis dysregulation resulting in hypercortisolemia, and GH resistance ( Table 1 ), all of which contribute to the high prevalence of severe bone loss in adults and adolescents with this disorder. Electrolyte disorders are also common, with hypokalemia in 20% (a consequence of purging), and hyponatremia in 20%, as is a mild transaminitis (12%), anemia (39%), and leukocytopenia (34%) ( 14 ).
Endocrine dysregulation in anorexia nervosa
↑, Increased in women with anorexia nervosa; ↓, decreased.
Gonadal axis dysregulation
Dysregulation of GnRH pulsatility in anorexia nervosa was first characterized in detail by Boyar et al. in 1974 ( 15 ). Boyar's group demonstrated a range of GnRH patterns in amenorrheic young women with anorexia nervosa, which included apulsatility, and reversion to pubertal pulsatility patterns ( 15 , 16 ). Consistent with clinical observations, the degree of luteinizing hormone (LH) suppression did not correlate reliably with duration of illness or degree of thinness, and return of menses did not demonstrate a simple relationship to weight. Studies over the past few years have sought to identify the regulators of GnRH pulsatility responsible for amenorrhea in women with starvation. Such studies have focused primarily on the roles of leptin and kisspeptin. Low levels of leptin, a 16 kDa adipokine, appear to signal energy unavailability and inhibit normal reproductive function in rodent models. Consistent with this hypothesis, leptin replacement during a 48-h fast prevented the starvation-induced delay in estrous in female mice ( 17 ), and congenital leptin deficiency in humans is complicated by hypogonadatrophic hypogonadism ( 18 ). As would be expected given the low fat mass characteristic of women with anorexia nervosa, amenorrheic women with anorexia nervosa have, on average, lower leptin levels than normal-weight women, and leptin levels strongly correlate with fat mass ( 19 ). However, there is no leptin level cutoff that predicts amenorrhea. There is significant overlap in leptin levels between women with anorexia nervosa with amenorrhea and women of comparable low weight and psychologic characteristics of anorexia nervosa but preserved menstrual function ( 5 ). Although leptin administration in women with anorexia nervosa has not been studied, Welt et al. administered leptin to normal-weight (BMI of 18.8–24.4 kg/m 2 ) women with hypothalamic amenorrhea, which resulted in ovulatory cycles in three of eight women studied ( 20 ), raising the possibility that leptin may play an important pathophysiologic role in the development of hypothalamic amenorrhea in women with hypothalamic amenorrhea. However, a decrease in weight, attributable to a reduction in fat mass, was observed in the leptin-treated women, consistent with an anorexigenic effect of leptin and limiting its therapeutic potential in anorexia nervosa. More recent research has focused on kisspeptin, an endogenous ligand for the kisspeptin receptor, as a putative important regulator of reproductive function in women with functional hypothalamic amenorrhea, though no studies specifically examining its role in women with anorexia nervosa have been published. Acute, but not chronic, administration of kisspeptin has been shown to result in gonadotropin release in women with hypothalamic amenorrhea ( 21 ), and inactivating mutations cause pubertal failure ( 22 , 23 ). Further studies are warranted to determine the pathophysiology of amenorrhea in anorexia nervosa.
Hypogonadotrophic hypogonadism results in relative hypoandrogenemia, in addition to hypoestrogenemia, in women with anorexia nervosa ( 24 ). Sixty percent of testosterone is derived from ovarian sources in healthy women of reproductive age, with the remaining 40% from adrenal precursors ( 25 ). Published data suggest that adrenal androgen precursor secretion is not compromised in women with anorexia nervosa. Therefore, hypogonadotropic hypogonadism appears to be responsible for the hypoandrogenemia observed in such women. In one study, cortrosyn stimulation after dexamethasone suppression stimulated an exuberant cortisol response in women with anorexia nervosa compared with normal-weight controls; however, stimulated dehydroepiandrosterone (DHEA) levels were comparable in the two groups ( 26 ). In a large cross-sectional study, DHEA sulfate (DHEAS) levels were not lower in women with anorexia nervosa compared with healthy, normal-weight controls, except in those receiving oral contraceptives (a binding globulin effect) ( 24 ), though, in contrast, another study reported decreased DHEAS levels in relation to an assay normal range ( 27 ). The effects of androgen deficiency in women with anorexia nervosa are largely unknown. A cross-sectional study showed inverse associations between androgen levels and severity of both depression and anxiety symptoms in women with anorexia nervosa, independent of weight ( 28 ), suggesting that relative androgen deficiency may be a factor contributing to the severity of mood-related symptoms in such patients. Pilot studies administering low-dose testosterone in replacement doses in other adult female populations suggest possible positive mood effects with few side effects ( 29 – 31 ), but it remains to be established whether low-dose androgen administration would be a useful clinical tool for the treatment of mood comorbidities alone or in combination with other agents in women with anorexia nervosa.
Hypothalamic-pituitary-adrenal axis dysregulation
Anorexia nervosa is characterized by hypercortisolemia in many adults ( 32 ) and adolescents ( 33 ). Elevated 24-h urine free cortisol levels ( 34 ), overnight mean serum cortisol levels ( 32 , 35 , 36 ), cortisol response to cortrosyn administration ( 26 ), dexamethasone suppressibility ( 37 ), and midnight salivary cortisol levels ( 38 ) are all commonly, though not universally, elevated in women with anorexia nervosa. Although the clinical manifestations of hypercortisolemia in this cachetic group of patients may appear to be inconsistent with those observed in patients with Cushing's syndrome, with closer examination there are several parallels. In women with anorexia nervosa, higher urine free cortisol levels predict truncal fat accumulation with weight gain ( 39 ), and in adolescent girls, higher overnight cortisol levels predict weight gain ( 40 ). Moreover, overnight blood cortisol levels are inversely associated with bone mineral density and positively associated with severity of depression and anxiety symptoms in women with anorexia nervosa ( 32 ). Therefore, hypercortisolemia may also contribute to the severe bone loss incurred and the highly prevalent psychiatric comorbidities in women with anorexia nervosa.
GH resistance
The fact that serum GH concentrations increase with fasting was first reported in 1963 in Science concurrently with a description of the development of the first GH assay sensitive and specific enough to detect changes of a clinically relevant magnitude ( 41 ). Later it was reported that GH burst frequency, burst mass, and burst duration are higher in women with anorexia nervosa than healthy controls, resulting in a 4-fold higher daily pulsatile GH secretion and 20-fold increase in basal GH secretion ( 42 ). Misra et al. demonstrated that this relative GH elevation extends to adolescents with anorexia nervosa compared with healthy adolescents, who themselves experience endogenous GH secretion in excess of that of healthy adults ( 43 ). However, despite elevated GH levels in patients with anorexia nervosa, IGF-I levels are not increased, as would be expected. To the contrary, chronic starvation causes a state of GH resistance in the liver, and IGF-I levels are low in women ( 44 ) and adolescents ( 43 ) with anorexia nervosa compared with healthy controls. The suppression of IGF-I is not an artifact of binding globulin abnormalities. This was shown by Stoving et al. using a kinase receptor activation assay to measure IGF-I bioactivity, which was found to be low in women with anorexia nervosa ( 45 ). In a recent study, Fazeli et al. investigated whether the state of GH resistance can be overcome with supraphysiologic recombinant human GH administration. In a 12-week randomized, placebo-controlled study administering a mean maximum daily dose of 1.4 ± 0.1 mg/d, serum IGF-I levels did not increase significantly compared with placebo ( 46 ). In addition, fat mass and leptin levels decreased. These data suggest that GH resistance in states of undernutrition is not easily overcome and support the established role of GH as a mediator of lipolysis, independent of IGF-I.
Skeletal dysregulation
Bone loss is a severe and prevalent complication of anorexia nervosa in women, with 90% of young women having a T score of less than −1.0 and 40% less than −2.0 ( 47 ) and is characterized by decreased bone formation and increased resorption ( 44 ). A study of 214 women with anorexia nervosa, mean age 25 yr, demonstrated osteopenia in 52% and osteoporosis in 35%, with fewer than 15% of women having normal bone mineral density at all skeletal sites tested ( 14 ). The annual rate of decline in bone mineral density at the spine and hip is approximately 2.5% in adults with anorexia nervosa ( 48 ). Adolescence is a critical time for bone mass accrual, and studies have shown that adolescent girls with anorexia nervosa do not experience the usual linear increase in bone mass during puberty ( 49 ), resulting in lower bone mineral density at the spine and hip than in healthy adolescents of comparable bone age ( 33 ). This deficit places them at risk of attaining lower-than-normal peak bone mass and may make them more vulnerable to developing osteoporosis and fractures later in life. In recent years, novel imaging techniques have made it possible to assess bone microarchitecture, and with microcomputed tomography techniques, it has been shown that bone volume and trabecular thickness become abnormal early in the course of the disease in adolescent girls, even before decreases in bone mineral density are detectable by conventional dual energy x-ray measurements ( 50 ). Bone microarchitectural parameters, including trabecular thickness and separation, are abnormal in adults with anorexia nervosa as well ( 51 ), and IGF-I levels are strong predictors of abnormal bone microarchitectural parameters ( 51 ). In addition, bone strength in women with anorexia nervosa has been modeled by finite element analysis and found to be lower than in healthy controls ( 52 ) ( Fig. 1 ). The clinical effects of low bone mineral density, abnormal skeletal microarchitecture, and reduced bone strength are an elevated fracture rate reported at 30% ( 14 ) and seven times that expected for age and sex ( 53 ).

Bone microarchitecture is abnormal in women with anorexia nervosa and bone strength is reduced. Shown are flat-panel volume computed tomography images of the distal radius in a woman with anorexia nervosa (A) and a healthy control (B). [Adapted from C. J. Walsh et al. : Women with anorexia nervosa: finite element and trabecular structure analysis by using flat-panel volume CT. Radiology 257:167–174, 2010 ( 52 ), with permission. © Radiological Society of North America.]
Neuroendocrine and nutritional factors are important determinants of bone loss and bone recovery. Normalization of reproductive function is the strongest predictor of skeletal recovery, and recovery of menses predicts an increase in bone mineral density independent of changes in weight ( 48 ). In addition, in a comparison of bone mineral density in 74 women with anorexia nervosa and 42 women with all of the characteristics (psychiatric and weight) of anorexia nervosa, except for amenorrhea, bone mineral density was marked lower in amenorrheic women than eumenorrheic women (mean T score −1.9 vs. −0.9 at the posteroanterior spine) ( 5 ). However, of note, eumenorrheic women with anorexia nervosa also had lower bone mineral density than expected for healthy women of comparable age, suggesting that hypogonadism is an important, but not the only, factor responsible for bone loss in women with anorexia nervosa. However, it is important to consider another interpretation of these data, which is that amenorrhea may simply be a marker of degree of undernutrition and/or degree of global endocrine dysregulation. In addition, bone mineral density is lower in amenorrheic women with anorexia nervosa than in normal-weight women with functional hypothalamic amenorrhea ( 54 ). These data suggest that nutritional factors are important determinants of bone mineral density, possibly due to both direct mechanical effects and effects on neuroendocrine modulators of skeletal metabolism. BMI is an important determinant of bone mineral density in women with anorexia nervosa, with lean body mass the most important component ( 49 ), perhaps due to the anabolic pull of muscle on bone. Recent evidence suggests that enteric and pancreatic peptides, including peptide YY (PYY) ( 55 , 56 ) and amylin ( 57 ), may also play a role in mediating the effects of nutritional intake on bone. Overnight PYY levels are strongly inversely associated with bone mineral density, particularly at the spine, in women with anorexia nervosa ( 56 ), and fasting PYY levels are inversely associated with markers of bone turnover in adolescent girls with anorexia nervosa ( 55 ). Hypercortisolemia is prevalent in anorexia nervosa ( 32 , 36 ) and appears to play a role in the etiopathology of bone loss ( 32 ), consistent with data in patients with hypercortisolemia of other causes, for example Cushing's disease and exogenous glucocorticoid administration. Vitamin D and calcium intake are comparable in women with anorexia nervosa and healthy women of comparable age ( 58 ) and therefore are not likely to be important pathogenetic factors. Likewise, albumin levels, a marker of hypoproteinemia, are not reduced in women with anorexia nervosa and are elevated in a subset of women with anorexia nervosa ( 14 ), possibly from hemoconcentration, but hypoproteinemia has not been ruled out as a potential etiologic factor in the development of bone loss in anorexia nervosa.
An interesting new area of investigation is the effect of starvation on bone marrow fat, which is elevated in women ( 59 ) and adolescents ( 60 ) with anorexia nervosa, and may be causatively related to bone loss, a theory that is supported by the strong inverse association between bone marrow fat and bone mineral density ( 59 ). These human data are consistent with a published report demonstrating elevated bone marrow fat and reduced bone mineral density in calorically restricted mice ( 61 ). Bone and fat cells share a common mesenchymal precursor stem cell within bone marrow, capable of differentiating into adipocytes or osteoblasts. However, it is not understood whether increased bone marrow fat is implicated in the pathogenesis of bone loss in anorexia nervosa or represents default into the fat lineage as a result of impaired osteoblastogenesis. It is also unknown whether fat from this depot secretes adipokines that modulate reproduction or other functions. Studies have demonstrated that GH/IGF-I ( 62 ), leptin ( 63 ), and peroxisomal proliferator-activated receptor-γ agonists ( 64 ) may influence the differentiation pathway. One study found that levels of Pref-1 (preadipocyte factor-1), a member of the epidermal-like growth factor protein family, are higher in women with anorexia nervosa than healthy controls and correlate positively with bone marrow fat ( 65 ).
As anorexia nervosa is frequently chronic, weight recovery is not achievable for many patients, and relapses occur. In addition, bone mineral density recovery to normal does not always follow weight recovery. Therefore, the development of therapies to address this complication is of critical importance. Studies have capitalized on addressing the known neuroendocrine abnormalities associated with the disease, as well as data using therapies proven effective in postmenopausal women. However, estrogen, an effective therapy in postmenopausal women, is ineffective in women with anorexia nervosa. Two randomized, placebo-controlled studies have shown that oral estrogen administration alone is ineffective—at replacement doses ( 66 ) or as oral contraceptives ( 67 )—to increase bone mineral density in adults with anorexia nervosa. These results were unexpected, as one might hypothesize that estrogen would be an ideal therapy in this hypogonadal population, and may reflect the known effects of oral estrogen to suppress IGF-I production by the liver ( 68 ). In support of this hypothesis, a randomized, placebo-controlled study showed that recombinant human IGF-I (rhIGF-I) replacement plus oral contraceptive administration was more effective (2.8% increase in spine bone mineral density over 9 months compared with placebo) than rhIGF-I alone (1.4% increase) or oral contraceptives (no significant increase) alone or than placebo ( 69 ). A recent randomized, placebo-controlled study demonstrated efficacy of risedronate in adults with anorexia nervosa ( 70 ), consistent with an earlier small open-label pilot study ( 71 ). In this study, bone mineral density increased at the posteroanterior lumbar spine by 3%, lateral spine by 4%, and hip by 2% over the 1-yr study period. Of note, the mean bone mineral density of the treated group remained below normal for age; therefore, further studies are warranted to determine how to optimize bone mineral density in women with anorexia nervosa. In addition, although the published data on bisphosphonate administration during pregnancy are reassuring ( 72 , 73 ), there are limited data available, and therefore bisphosphonates should be prescribed with caution in women of reproductive age. Other therapies that have been shown to be effective for postmenopausal bone loss have not been tested in this population. The question of whether bone mass responds positively to exercise in this disease has been raised, and a recent study confirmed that excessive moderate loading exercise has a deleterious effect on bone mineral density in underweight women with anorexia nervosa, in whom exercise is largely discouraged by providers due to the negative effects on energy balance; however, in the same study, a beneficial effect was observed in women who were weight recovered from the illness ( 74 ).
When considering therapies for osteoporosis, adolescent girls with anorexia nervosa need to be approached separately from adults due to the differences in skeletal physiology, and one cannot extrapolate efficacy data from adults to adolescents. Adolescence is a period of time in which there is rapid bone mass accrual, leading to peak bone mass and tonically increased bone turnover. In contrast, in adolescent girls with anorexia nervosa, markers of bone metabolism—both formation and resorption—are suppressed ( 75 ). Whether a physiologic estrogen replacement strategy and/or rhIGF-I replacement would be effective to preserve or increase bone turnover and mass in adolescents with anorexia nervosa is unknown. Clinical trials have not identified any effective therapy for bone loss for this group. One randomized, placebo-controlled study of bisphosphonate therapy in adolescent girls demonstrated increases in bone mineral density compared with baseline values but not compared with placebo ( 76 ). In that study, both groups gained weight, which was the most important determinant of the increases in bone mineral density observed ( 76 ). Similarly, a trial of DHEA therapy in adolescent girls and young adults combined did not show efficacy after controlling for increases in weight that occurred during the treatment period ( 77 ).
Endocrine Recovery with Weight Gain
Weight gain in women with anorexia nervosa is followed by partial recovery of endocrine function. The hypogonadotropic hypogonadism observed in women with anorexia nervosa is thought largely to be functional, and in their original studies characterizing LH pulsatility, Boyar et al. demonstrated reversibility of LH secretory dysregulation with weight gain ( 15 , 16 ). However, subsequent studies have reported that amenorrhea persists in approximately 15% of women despite weight recovery ( 78 ), but the underlying causes are not understood. Persistence of psychologic symptoms characteristic of eating disorders despite weight normalization and lower leptin levels may play a role ( 79 ), and the latter may reflect lack of complete resolution of body composition abnormalities, in particular relatively lower fat mass despite weight gain. GH resistance appears largely to be reversible with weight gain, with a stepwise normalization of IGF-I levels ( 42 , 45 , 80 ), as does hypercortisolemia, though the latter appears to persist in a subset of women who have gained weight to normal ( 37 , 81 – 83 ), consistent with data showing hypercortisolemia in a subset of normal-weight women with functional hypothalamic amenorrhea from overexercise or stress ( 32 ). Longer-term weight normalization may be necessary for resolution of hypercortisolemia in some women with anorexia nervosa ( 81 ). Bone mineral density improves with recovery from anorexia nervosa, but not to normal ( 84 ), highlighting the importance of developing effective therapies for this complication so that women with a history of anorexia nervosa will not experience an increased risk of fractures later in life. Recovery of spontaneous menses appears to be more important for skeletal recovery than weight gain itself, which is inadequate to reverse bone loss in most women ( 48 , 85 ).
Etiology of Anorexia Nervosa
Heritability.
Studies have reported heritability of between 56 and 84% ( 86 – 89 ) for anorexia nervosa, suggesting a probable genetic disposition to the disease. However, as yet, no clear genetic picture has emerged, possibly because heritability is likely multifactorial and complex. Close to 200 genetic-association studies have identified putative genetic candidates implicated in regulatory pathways for appetite, energy, neurotransmission, reward, neuroendocrine, and inflammatory systems. Rask-Andersen et al. recently reviewed these studies and reported that 128 polymorphisms of 43 genes have demonstrated associations, and they identified five genes for which the strongest data have been generated ( 90 ). These included one appetite-regulating gene (Agouti-related protein), two genes that are associated with reward pathways (catechol- O -methyl transferase and opioid receptor δ CNR1) and two genes that are associated with regulation of mood and psychiatric disorders [brain-derived neurotrophic factor (BDNF) and small-conductance calcium-activated potassium channel].
Appetite dysregulation
Recent studies evaluating the regulation of appetite, reward, and psychiatric comorbidities in women with anorexia nervosa have yielded interesting insights into the disease that may provide clues to its pathophysiologic basis. Although women with anorexia nervosa restrict their caloric intake, it is not clear whether they experience normal sensations of hunger, and there is increasing evidence of appetite hormone dysregulation in women with the disorder. Concentrations of a number of orexigenic and anorexigenic hormones, which signal the need and lack of need for nutrient intake, respectively, have been investigated. It has been known for some time that levels of the anorexigenic hormone leptin, secreted by adipocytes after meals, is lower in women with anorexia nervosa compared with lean controls ( 19 , 91 ), and this follows logically from the fact that the source of leptin, adipocytes, is greatly reduced in mass in women with anorexia nervosa. However, elevation of the anorexigenic hormone PYY ( 55 , 92 ), which is secreted by intestinal L cells, is not as simple to explain and appears paradoxical, as it is released in response to food intake, which is reduced in anorexia nervosa. This raises the possibility that PYY or related pathways may be implicated in some way in the etiology of the eating disorder, but this would be entirely speculative. Ghrelin, which is released by oxyntic cells of the stomach, ( 93 – 95 ) has also been found to be elevated in anorexia nervosa, though one recent study reported decreased ghrelin levels in women with a binge/purge subtype ( 96 ). A recent study demonstrated an exaggerated decrease in ghrelin levels with insulin infusion during a euglycemic hyperinsulinemic clamp, which the authors hypothesized might lead to an elevated sensation of satiety ( 97 ). The orexigenic hormone elevation has largely been explained as adaptive, i.e. an “attempt” to stimulate appetite and increase nutritive intake. Whether one possible factor contributing to the development of anorexia nervosa in a subset of women is a resistance to the effects of orexigenic hormones to stimulate appetite and, if so, whether such resistance predisposes women to the disorder or whether this elevation is simply acquired as a result of chronic starvation, is unknown. To address the question of whether anorexia nervosa is associated with ghrelin resistance, Hotta et al. performed a pilot study, administering ghrelin to five subjects, ages 14–35, hospitalized for anorexia nervosa, and reported an increase in hunger and food intake ( 98 ). The results of this study suggest that patients with anorexia nervosa are capable of responding to the orexigenic effects of ghrelin but does not address the question of whether relative ghrelin resistance is a characteristic of anorexia nervosa. This study was small and was not placebo-controlled; therefore, further studies are needed to investigate the putative role of ghrelin and other orexigenic hormones in the pathophysiology of anorexia nervosa. PYY and cortisol have also been shown to be independently and positively associated with degree of eating disorder cognition ( 99 ), further evidence of a possible link between appetite regulation and the etiopathology of anorexia nervosa.
Reward circuitry dysregulation
The question of whether dysregulation of reward circuitry contributes to the pathophysiology of anorexia nervosa has also been raised in the context of a reported anhedonia in response to eating ( 100 ). In a binge/purge rodent model, investigators induced food addiction, including characteristic withdrawal and relapse, as well as cross-tolerance to alcohol and cocaine ( 101 , 102 ). Although these data are more directly applicable to bulimia nervosa than anorexia nervosa, they raise the possibility that dysregulation of reward pathways could play an etiologic role in the development of eating disorders.
Neuromodulator dysregulation
Another possibly important clue to the pathophysiologic basis of anorexia nervosa is the high prevalence of psychiatric comorbidities, including depression, which is observed in 50 to 75% of patients ( 103 , 104 ). In addition, this population is enriched with specific personality traits, including perfectionism, which persists after recovery from eating disorders ( 105 ) and neuroticism, which is a risk factor for the development of anorexia nervosa ( 86 ). Moreover, clinical observations that psychotropic medications that bind serotonin and dopamine receptors result in weight gain have led investigators to consider whether these neurotransmitters might be involved in the pathogenesis of the eating disorder. However, until recently, this line of investigation has proven difficult. For example, in 1984, Kaye et al. reported decreased 5-hydroxyindoleacetic acid, a major metabolite of serotonin, in women with anorexia nervosa, which increased to normal with weight recovery ( 106 ), suggesting a putative pathophysiologic communality with depression. However, the authors could not rule out or control for the possible confounding contribution of dietary tryptophan, of which 5-hydroxyindoleacetic acid is also a metabolite. More recently, BDNF, a peptide that has been implicated in the pathogenesis of major depressive disorder, has been studied. In a mouse model in which BDNF was conditionally knocked out in brain tissue, mice develop hyperphagia and weight gain, as well as anxiety ( 107 ). BDNF levels have also been found to be lower in women with anorexia nervosa compared with controls ( 108 ). Because in this study levels were also lower in normal-weight women with bulimia nervosa, these findings appear to be independent of nutritional status, but whether these findings reflect the cause or effect of an eating disorder and whether peripheral levels reflect brain concentrations remain unknown. The development of sophisticated genetic and imaging techniques may enhance the ability of investigators to determine the role of endogenous psychoactive molecules and their receptors and neural pathways in the development of eating disorders.
Treatment of Anorexia Nervosa
The primary goals of recovery remain weight restoration and psychiatric recovery, and a team approach, which may include a psychiatrist or psychologist, primary care physician, and nutritionist, is the standard of care. Inpatient therapy is required for patients of extremely low weight, suicidality, and/or severe medical or psychologic comorbidities. A number of specific psychologic approaches have been studied, but few randomized, controlled trials have been performed. A review of randomized, controlled anorexia nervosa treatment trials in 2007 concluded that there was little evidence for efficacy of any specific treatment strategy, but that cognitive behavioral therapy may reduce the risk for relapse in women who have achieved weight recovery ( 109 ). A more recent randomized, controlled trial demonstrated improved outcomes with both individual and family-based psychotherapy in adolescent girls, with family-based therapy demonstrating a small advantage in durability of treatment effect ( 110 ). Despite the increasing use of psychotropic medications, clinical trials have mostly been disappointingly negative in demonstrating antidepressant effects on the disease itself ( 111 ) or psychiatric comorbidities ( 112 ) and have been plagued by difficulty retaining study subjects ( 113 ). Similarly, studies of the efficacy of atypical antipsychotics, of which there are few controlled trials, do not provide evidence of efficacy for weight gain in women with anorexia nervosa ( 114 ). Therefore, identification of effective therapies for this disorder and its complications is critical.
Conclusions
Anorexia nervosa is a primary psychiatric disorder complicated by serious endocrine disorders, including hypogonadism, hypercortisolemia, hyponatremia, and severe bone loss, for which there are few effective therapies. Further research will be important to increase our understanding of the etiopathology of the disease and of its complications and to identify effective therapies for anorexia nervosa itself and its attendant consequences.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 DK052625 ; and R01 MH083657 .
Disclosure Summary: The author has no relevant conflicts to declare.
Abbreviations:

COMMENTS
Introduction. Anorexia nervosa is a psychiatric disorder characterized by altered body image, persistent food restriction and low body weight 1.In 2013, the American Psychiatric Association revised the diagnostic criteria for anorexia nervosa by making the weight criteria less stringent and removing the requirement for amenorrhea to be present 1.The formal diagnosis of 'atypical anorexia ...
Summary. Anorexia nervosa (AN) is prevalent in adolescents and young adults, and endocrine changes include hypothalamic amenorrhea, a nutritionally acquired growth hormone resistance with low insulin like growth factor-1 (IGF-1), relative hypercortisolemia, decreases in leptin, insulin, amylin and incretins, and increases in ghrelin, PYY and ...
Anorexia nervosa is a devastating disease with a variety of endocrine manifestations. The effects of starvation are extensive and negatively affect the pituitary gland, thyroid gland, adrenal glands, gonads, and bones. Appetite is modulated by the neuroendocrine system, and characteristic patterns of leptin and ghrelin concentrations have been ...
Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. ... Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss ...
Anorexia Nervosa Hormonal changes. The most extensively studied eating disorder is anorexia nervosa. Typically, hypothalamic amenorrhea is accompanied by low levels of gonadotropins and a profound estrogen deficiency. ... Some reversal of the bone loss occurs with return of menses, but similar to anorexia nervosa, the use of hormone replacement ...
INTRODUCTION. Anorexia nervosa (AN) is a psychiatric illness with devastating consequences. The incidence of AN is currently around 8 per 100 000 persons per year, and the prevalence rate of AN is 0.3% in young females ().The diagnostic criteria for AN include refusal to maintain a weight of at least 85% of that expected for height, extreme fear of gaining weight, disturbed perception of body ...
An important component in the treatment of anorexia nervosa (AN) is the evaluation and management of its endocrine complications, including functional hypogonadotropic hypogonadism and increased fracture risk. The body's adaptive response to chronic starvation results in many endocrine abnormalities, most of which are reversible upon weight restoration. A multidisciplinary team with ...
Anorexia nervosa is prevalent in adolescents and young adults, and endocrine changes include hypothalamic amenorrhoea; a nutritionally acquired growth-hormone resistance leading to low concentrations of insulin-like growth factor-1 (IGF-1); relative hypercortisolaemia; decreases in leptin, insulin, amylin, and incretins; and increases in ghrelin, peptide YY, and adiponectin. These changes in ...
Epub 2014 Apr 2. Anorexia nervosa is prevalent in adolescents and young adults, and endocrine changes include hypothalamic amenorrhoea; a nutritionally acquired growth-hormone resistance leading to low concentrations of insulin-like growth factor-1 (IGF-1); relative hypercortisolaemia; decreases in leptin, insulin, amylin, and incretins; and ...
with hormonal changes in anorexia nervosa.14-17 Although 50% of adults with anorexia nervosa recover after behavioural, psychiatric, and medical therapy,18 about 30% only partly recover, and the remainder are characterised by remission and relapse or chronic disease.19,20 Of major concern is the high risk of suicide,
Anorexia nervosa (AN) is a psychiatric disease associated with notable medical complications and increased mortality. ... Most hormonal changes are thought to represent a response to starvation ...
Abstract. Anorexia nervosa (AN) is a persistent psychiatric disorder that is marked by abnormal reduced weight and amenorrhea, which may be primary or secondary. AN affects multiple endocrine axes such as gonadal, thyroid, and adrenal axis, growth hormone, and insulin-like growth factor-1, adipokines such as leptin, gut peptides like ghrelin ...
Anorexia nervosa (AN) is a common condition in adolescents and young adults associated with hypogonadotropic hypogonadism (manifesting as functional hypothalamic amenorrhea), relative hypercortisolemia, a nutritionally acquired growth hormone resistance with low insulin-like growth factor-1 levels, low levels of leptin and oxytocin, and high levels of ghrelin and peptide YY.
Anorexia nervosa (AN) is characterized by severe undernutrition associated with alterations in multiple endocrine axes, which are primarily adaptive to the state of caloric deprivation. Hormonal changes include growth hormone (GH) resistance with low insulin like growth factor-1 (IGF-1) levels, hypothalamic hypogonadism, relative ...
Those hormonal shifts interact not only with appetite-regulating brain regions but also affect energy expenditure, physical activity, behavior, cognition, as well as rewarding/motivational drive. This narrative review aims to present emerging biological concepts underlying anorexia nervosa.
Thyroid levels: It is not uncommon for T3 levels to be low in people with anorexia nervosa. This manifests as bradycardia (slow heart rate) and hypothermia (low body temperature)—features that serve to preserve energy. Behavioral/psychological symptoms (e.g., anxiety, hyperactivity, impulsivity): Hormones that activate our fight-or-flight ...
Anorexia nervosa is prevalent in adolescents and young adults, and endocrine changes include hypothalamic amenorrhoea; a nutritionally acquired growth-hormone resistance leading to low concentrations of insulin-like growth factor-1 (IGF-1); relative hypercortisolaemia; decreases in leptin, insulin, amylin, and incretins; and increases in ghrelin, peptide YY, and adiponectin.
Anorexia (an-o-REK-see-uh) nervosa — often simply called anorexia — is an eating disorder characterized by an abnormally low body weight, an intense fear of gaining weight and a distorted perception of weight. People with anorexia place a high value on controlling their weight and shape, using extreme efforts that tend to significantly ...
Anorexia nervosa is a complex and deadly psychiatric disorder. It is characterized by a significant degree of both co-occurring psychiatric diseases and widespread physiological changes which affect nearly every organ system. ... Hormonal changes include growth hormone (GH) resistance with low insulin-like growth factor-1 (IGF-1) levels ...
Abstract. Anorexia nervosa is a syndrome of unknown etiology. It is associated with multiple endocrine abnormalities. Hypothalamic monoamines (especially serotonin), neuropeptides (especially neuropeptide Y and cholecystokinin) and leptin are involved in the regulation of human appetite, and in several ways they are changed in anorexia nervosa.
Anorexia Nervosa (AN) is an eating disorder characterized by self-starvation leading to weight loss, undernutrition, and subsequent adaptive typical hormonal changes [1, 2].Some of these changes are responsible for a functional gonadal axis blockade [].Specifically, the combination of weight loss, blunted leptin [], high cortisol [] and high ghrelin plasma levels [6, 7] results in a functional ...
Anorexia nervosa is a syndrome, that is collections of symptoms, which is not defined by its etiology. The severe cases are intractable. The syndrome is associated with multiple, profound endocrine alterations which may be adaptive, reactive or etiologic. Adaptive changes potentially may be inappropriate in clinical settings such as inpatient ...
Anorexia nervosa has two subtypes: binge-purge type and restricting type. Restricting type In restricting type, people severely limit the amount and type of food they eat, but they do not engage ...
Anorexia nervosa is a primary psychiatric disorder with serious endocrine consequences, including dysregulation of the gonadal, adrenal, and GH axes, and severe bone loss. ... Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. ... Bone marrow changes in adolescent girls with anorexia nervosa ...