Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Long-term changes in body composition and their relationships with cardiometabolic risk factors: A population-based cohort study
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing
Affiliation Department of Early Childhood Development, Capital Institute of Pediatrics, Beijing, China
Roles Data curation, Formal analysis
Affiliation Beijing Center for Disease Prevention and Control, Beijing, China
Roles Formal analysis
Affiliation Child Health Big Data Research Center, Capital Institute of Pediatrics, Beijing, China
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
- Zhaoyang Fan,
- Yunping Shi,
- Guimin Huang,
- Dongqing Hou,
- Junting Liu

- Published: May 13, 2021
- https://doi.org/10.1371/journal.pone.0251486
- Peer Review
- Reader Comments
The aim of the present study was to classify the latent body fat trajectories of Chinese adults and their relationships with cardiometabolic risk factors. Data were obtained from the China Health Nutrition Survey for 3,013 participants, who underwent six follow-up visits between 1993 and 2009. Skinfold thickness and other anthropometric indicators were used to estimate body composition. The latent growth model was used to create fat mass to fat-free mass ratio (F2FFMR) trajectory groups. Blood pressure, fasting plasma glucose, total cholesterol, triglycerides, and high- and low-density lipoprotein–cholesterol were measured in venous blood after an overnight fast. Logistic regression was used to explore the relationships of F2FFMR trajectory with cardiometabolic risk factors. In men, four types of F2FFMR trajectory were identified. After adjustment for behavioral and lifestyle factors, age, and weight status, and compared with the Low stability group, the High stability group showed a significant association with diabetes. In women, three types of F2FFMR trajectory were identified. Compared to the Low stability group, the High stability group showed significant associations with diabetes and hypertension after adjustment for the same covariates as in men. Thus, in this long-term study we have identified three F2FFMR trajectory groups in women and four in men. In both sexes, the highly stable F2FFMR is associated with the highest risk of developing diabetes, independent of age and body mass. In addition, in women, it is associated with the highest risk of hypertension, independent of age and body mass.
Citation: Fan Z, Shi Y, Huang G, Hou D, Liu J (2021) Long-term changes in body composition and their relationships with cardiometabolic risk factors: A population-based cohort study. PLoS ONE 16(5): e0251486. https://doi.org/10.1371/journal.pone.0251486
Editor: Y. Zhan, German Centre for Neurodegenerative Diseases Site Munich: Deutsches Zentrum fur Neurodegenerative Erkrankungen Standort Munchen, GERMANY
Received: December 8, 2020; Accepted: April 28, 2021; Published: May 13, 2021
Copyright: © 2021 Fan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The datasets analyzed during the current study are available in the following website: http://www.cpc.unc.edu/projects/china/ .
Funding: Jt Liu received the fund of Beijing Hospitals Authority Youth Program, code: QML20191302( http://www.bjygzx.org.cn/ ). Zy Fan received the fund of National Key Research and Development Program of China, grant No. 2018YFC1002503( https://service.most.gov.cn/index/ . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
1. Introduction
Obesity has become a major health problem [ 1 ], with the prevalence of overweight and obesity in adults reaching 36.9% and 38.0% in men and women, respectively [ 2 ]. Millions of deaths can be ascribed to obesity worldwide [ 3 , 4 ]. Fat mass is a direct indicator for evaluating obesity, and obesity has trajectory effects [ 5 ], and longitudinal mixed-effects and latent growth curve models are commonly used to characterize changes in body mass index (BMI) and their relationships with subsequent outcomes [ 6 – 10 ]. Excessive fat mass has an adverse effect on cardiometabolic risk factors. However, there is a maximum capacity for adipose expansion, and when this is reached, lipids accumulate in other tissues and cause metabolic disease [ 11 ]. In contrast, fat-free mass protects against the development of cardiometabolic risk factors [ 12 ]. Therefore, we aimed to construct a metabolic load-capacity model, in which fat mass is the metabolic load and fat-free mass is the metabolic capacity [ 13 , 14 ].
Percentage fat mass is usually used to assess cardiometabolic status, but this may be inappropriate mathematically; fat mass is the numerator, but is also included in the denominator [ 15 ]. Instead, the fat mass to fat-free mass ratio (F2FFMR) may be a superior indicator of the ability to maintain homoeostasis at the level of the organ or tissue. F2FFMR can be used for the prediction of metabolic risk in population-based studies [ 16 ]. However, it is not clear whether changes in F2FFMR have effects, and how fat mass and fat-free mass ratio changes. To date, some studies [ 17 – 19 ] explored the body composition changes based on small-scale survey and short-term follow-up in different groups of population, few studies have characterized the trajectories of F2FFMR and their associations with the development of cardiometabolic risk factors in adults based large-scale population-based longitudinal study in China.
We hypothesized that an accumulation of body fat over time would be associated with the development of cardiometabolic risk factors. In the present study, we aimed to evaluate the association of body composition trajectory with the prevalence of cardiometabolic risk factors (dyslipidemia, diabetes, and hypertension), to better inform obesity control and prevention.
2.1. Study design
We used data from the China Health and Nutrition Survey (CHNS) to characterize body composition trajectory. Data were accessed from the Carolina Population Center ( http://www.cpc.unc.edu/projects/china ). The CHNS is an ongoing, large-scale, open, longitudinal, household-based survey that is conducted in China [ 20 ]. Nine provinces were selected, and a multi-stage random cluster sampling method stratified by income was used in each province. The first wave of the CHNS was completed in 1993, which was followed by subsequent waves in 1997, 2000, 2004, 2006, and 2009. A detailed description of the survey has been published elsewhere [ 20 ]. This study was approved by the Institutional Review Board of the National Institute for Nutrition and Food Safety, China Center for Disease Control and Prevention, and the University of North Carolina at Chapel Hill. All the participants provided their written informed consent.
2.2. Study population
The study cohort comprised adults aged 18–60 years at baseline in 1993 for whom age, sex, and physical examination data [skinfold thickness, body mass, height, systolic blood pressure (SBP), and diastolic blood pressure (DBP)] were available. Participants who were pregnant at the time of the survey, for whom data were missing or biologically implausible, or who had cancer were excluded. Those for whom data from at least two rounds of the survey were available were included. Ultimately, 3,013 participants were studied, of whom 1,637 were women. A flow chart for the study is shown in Fig 1 .
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0251486.g001
2.3. Measurement and definition of body fat mass
Skinfold thickness, height, and body mass were measured using standard protocols [ 21 ]. Skinfold thickness was measured three times using skinfold calipers over the triceps muscle on the right arm, and the mean value was used in analyses. Height was measured without shoes using a Seca stadiometer (Seca North America East, Hanover, MD, USA), and body mass was measured while wearing lightweight clothing using a calibrated beam balance. BMI was calculated by dividing body mass (in kilograms) by the square of height (in meters). Weight status was defined as BMI ≥24 kg/m 2 for overweight and ≥28 kg/m 2 for obesity, respectively. Body fat mass was estimated using equations that included BMI and skinfold thickness: for men, fat mass percentage (FMP) = (0.742 × BMI) + (0.950 × triceps skinfold) + (0.335 × age) − 20.0; and for women, FMP (%) = (0.730 × BMI) + (0.548 × triceps skinfold) + (0.270 × age) − 5.5 [ 22 ]. Fat mass was calculated as FMP × body mass, and fat-free mass was calculated as body mass − fat mass. F2FFMR was calculated by dividing fat mass by fat-free mass.
2.4. Cardiometabolic risk factors
SBP and DBP were measured three times using the right arm after 10 min of rest in a seated position, using mercury sphygmomanometers with appropriate cuff sizes [ 23 ], and the mean values were used in analyses. Hypertension was defined as SBP/DBP ≥140/90 mm Hg, or the use of antihypertensive drugs, or a self-reported diagnosis of hypertension [ 23 , 24 ]. After an overnight fast, blood sample was collected and biochemical test was completed in 2009. Total cholesterol (TC), triglyceride (TG), and high- and low- density lipoprotein–cholesterol (HDL-C and LDL-C) were measured using the glycerol-phosphate oxidase method on a Hitachi 7600 automated analyzer (Tokyo, Japan). High TC was defined as ≥6.2 mmol/l, high TG as ≥2.3 mmol/l, high LDL-C as ≥4.1 mmol/l, and low HDL-C as <1.0 mmol/l. Dyslipidemia was defined as any of high TC, high TG, high LDL-C, or low HDL-C, according to the guidelines for the prevention and treatment of dyslipidemia in Chinese adults [ 25 ]. Fasting plasma glucose (FPG) was measured using the glucose oxidase-phenol and 4-aminophenazone method (Randox Laboratories Ltd., Crumlin, UK), and diabetes was defined as FPG ≥7.0 mmol/l or the use of anti-diabetic medication.
2.5. Covariates and definitions
Educational level was classified according to attendance at junior high school or below, high or technical school, or college and above. Physical activity was categorized as light, moderate, or heavy. Income was categorized according to individual net income per year as <$3,000, $3,000–$4,999 and ≥$5,000. Marital status was defined as married or single. Living conditions were defined as urban or rural. Smoking was defined as the use of any nicotine-based product in the preceding year, and the participants were classified as smokers, non-smokers, or former smokers. Alcohol consumption was defined using the previous year’s consumption, and participants were classified as drinkers, non-drinkers, and former drinkers. Age and weight status were also adjusted in the model simultaneously.
2.6. Statistical analysis
Body fat trajectory patterns were identified using the group‐based trajectory modeling method [ 26 ] and longitudinal body fat data. Model fitting and parameter estimation were performed using the maximum likelihood method. Specific trajectory patterns were identified using the Bayes information criterion (BIC) in the group‐based trajectory modeling [ 26 , 27 ]. The most appropriate models were considered to be those that permitted the most homogeneous grouping of the individual patterns, selected from among those with low BIC values. The minimum sample size for each trajectory group was 3% of the total cohort. For both men and women, a quadratic model was selected, and four and three trajectory groups were identified, respectively ( Table 1 ). According to the trends in each trajectory group between 1993 and 2009, they were labelled “Low stability”, “High stability”, “Increase and decrease”, and “Increasing” in men; and “Low stability”, “High stability”, and “Increasing” in women ( Fig 2 ).
https://doi.org/10.1371/journal.pone.0251486.g002
https://doi.org/10.1371/journal.pone.0251486.t001
In the “Low stability” groups, F2FFMR remained low and did not vary substantially. In the “High stability” groups, F2FFMR remained high and did not vary substantially. In the “Increase and decrease” group, F2FFMR started low, then increased, before decreasing to a low level again. In the “Increasing” groups, F2FFMR increased, from low to high. Each participant was assigned to one of these groups and their basic characteristics were compared using the chi‐square test for categorical variables and the ANOVA F test for continuous variables. Multinomial logistic regression was used to assess the relationships between the body composition trajectory group and cardiometabolic risk factors, with the Low stability group as the reference. All the analyses were stratified according to sex. In addition, all the socioeconomic, demographic, and lifestyle covariates were included in the final multivariate analysis model. Age and weight status at the final visit were included as covariates in the adjusted analysis. SAS 9.4 (Cary, NC, USA) was used for the data analysis and trajectory analysis was performed using Mplus 8.3 software (Los Angeles, CA, USA). All the statistical tests were two‐sided, and P ≤0.05 was regarded as indicating statistical significance.
A total of 3,013 individuals (1,637 women and 1,376 men) were included in the study. The men were allocated to the following groups: 83.9% Low stability, 3.6% High stability, 6.8% Increase and decrease, and 5.7% Increasing. There were no differences in marital status or alcohol consumption among these groups, but there were differences in age, location, educational level, individual net income, smoking, physical activity, body mass, and FMP. The women were allocated to the following groups: 88.9% Low stability, 7.6% High stability, and 3.5% Increasing. There were no differences in educational level, individual net income, or alcohol consumption among these groups, but there were differences in the other factors (Tables 2 and 3 ).
https://doi.org/10.1371/journal.pone.0251486.t002
https://doi.org/10.1371/journal.pone.0251486.t003
In logistic regression models 1–4, cardiometabolic risk factors were used as dependent variables and trajectory group was the independent variable. The Low stability group was the reference group in each model. In men, high TC and high LDL-C were not associated with F2FFMR trajectory group, regardless of whether they were adjusted for covariates or not. Prior to adjustment for any covariates, the Increase and decrease trajectory group was significantly associated with high TG [crude odds ratio (OR) 1.65, 95% confidence interval (CI) 1.00–2.70)]. When adjusted for age in 2009, the Increase and decrease trajectory group remained significantly associated with high TG (crude OR 1.87, 95% CI 1.13–3.10). However, when further adjusted for body mass in 2009, this association disappeared. The High stability group was significantly associated with low HDL-C (crude OR 2.06, 95% CI 1.00–4.24) prior to adjustment, and after adjustment for age in 2009, the adjusted OR and 95% CI were 2.37 (1.13–4.94). However, after further adjustment for body mass status in 2009, this association disappeared.
The Increase and decrease group was significantly associated with dyslipidemia prior to adjustment (crude OR, 95% CI: 1.64, 1.07–2.53), and after adjustment for age in 2009 (adjusted OR, 95% CI: 1.79, 1.16–2.76). In addition, the High stability group was significantly associated with dyslipidemia after adjustment for age in 2009 (adjusted OR, 95% CI: 1.95, 1.08–3.51). However, after further adjustment for body mass in 2009, this association disappeared. The High stability group and the Increase and decrease group were significantly associated with diabetes after adjustment for age and body mass in 2009 (adjusted OR, 95% CI: 2.68, 1.31–5.51 and 1.90, 1.04–3.46, respectively). After further adjustment for educational level, smoking, alcohol consumption, location, marital status, and physical activity in model 4, the High stability group remained significantly associated (adjusted OR, 95% CI: 2.72, 1.25–5.92), but the association disappeared in the Increase and decrease group. After adjustment for age in 2009, the High stability group and the Increase and decrease group were also significantly associated with hypertension (adjusted OR, 95% CI: 2.48, 1.32–4.68 and 1.75, 1.12–2.72, respectively), but after further adjustment for other covariates, this association disappeared ( Table 4 ).
https://doi.org/10.1371/journal.pone.0251486.t004
In women, prior to adjustment, the High stability group was associated with high TC (crude OR, 95% CI: 2.25, 1.44–3.51). The High stability and Increasing groups were significantly associated with high TG, with crude ORs (95% CIs) of 2.61 (1.75–3.89) and 2.64 (1.49–4.66), respectively. The High stability group was associated with high LDL-C (crude OR, 95% CI: 2.15, 1.39–3.30) and dyslipidemia (crude OR, 95% CI: 2.63, 1.81–3.81). In addition, the High stability and Increasing groups were significantly associated with diabetes (crude OR, 95% CI: 4.52, 2.89–7.08 and 2.44, 1.16–5.11, respectively) and hypertension (crude OR, 95% CI: 4.03, 2.72–5.98 and 2.89, 1.66–5.04, respectively) ( Table 5 ).
https://doi.org/10.1371/journal.pone.0251486.t005
In women, after adjustment for age in 2009, the same associations were identified ( Table 5 ). After further adjustment for body mass in 2009 in model 3, the associations with high TC, high TG, high LDL-C, and low HDL-C disappeared. There was a significant association between the High stability group and dyslipidemia (crude OR, 95% CI: 1.55, 1.03–2.34), but this association disappeared after adjustment for other covariates in model 4. The High stability group was also significantly associated with diabetes and hypertension (adjusted OR, 95% CI: 3.06, 1.54–6.08 and 2.05, 1.09–3.85, respectively) ( Table 5 ).
4. Discussion
In the present study, we have identified four patterns of F2FFMR trajectory in men and three in women using data from a 16-year population-based cohort study. By comparing the risks of dyslipidemia, diabetes, and hypertension among the participants with these different patterns, we determined that the participants in the High stability group were at the highest risks of diabetes and hypertension. Through comparing impact of the high stability group, increasing group, increase and decrease group on diabetes. We speculate the effect of F2FFMR on cardiometabolic risk is likely to accumulate slowly, and high F2FFMR in early life might have an impact on diabetes in later life. Our findings show that the monitoring of F2MMR trajectory may help identify individuals who are at higher risk of diabetes and hypertension.
In the present study, we found dyslipidemia was not statistically associated with F2MMR trajectory after adjustment for age and weight status in men, lifestyle covariates in women. So dyslipidemia might be primarily determined by body mass in men, but by lifestyle in women. The body composition of men and women differs: adipose tissue is more likely to accumulate around the trunk and abdomen of men, but around the hips and thighs of women [ 28 , 29 ]. Study of the F2FFMR indicates that the adverse effects of high fat mass can be offset by the protective effects of high fat-free mass. Specifically, high fat-free mass protects against high TC and high LDL-C [ 30 ]. However, F2FFMR reflects whole-body composition, and does not discriminate between the effects of body fat in differing locations. For example, excess accumulation of fat mass, especially in the upper body, is associated with dyslipidemia in normal-weight individuals [ 31 ]. In addition, visceral fat is a risk factor for dyslipidemia in men, and this effect is independent of the influence of BMI and waist circumference [ 32 ], but might not be a risk factor in women [ 33 ]. BMI is an independent risk factor for hypertension in both men and women, and high fat mass is associated with a higher risk of hypertension, even in non-obese populations [ 34 ]. In contrast, it has also been shown that a reduction in fat-free mass is more strongly associated with the normalization of blood pressure than a reduction in fat mass [ 35 ].
The present findings also suggest that the impact of F2FFMR trajectory on cardiometabolic outcomes is mediated by current body mass. A study of a cohort from birth has also shown that the trajectory of fat mass is associated with the development of cardiometabolic risk factors in adulthood and is affected by BMI in adulthood [ 36 ]. In both men and women, F2FFMR of Hight stability was associated with diabetes in the present study, and this association remained even after adjustment for the covariates and body mass. The fat-to-muscle ratio is associated with blood glucose [ 37 ] and visceral fat mass might predict the risk of prediabetes or diabetes [ 38 ]. Fat-free mass, which mainly consists of muscle, is protective against diabetes, and skeletal muscle plays an important role in the consumption and storage of glucose [ 39 ], the regulation of blood glucose, and the prevention of hyperglycemia [ 40 ]. Thus, people with low muscle mass are at a higher risk of developing type 2 diabetes than those with high muscle mass, and the lower the percentage muscle mass, the higher the risk of developing type 2 diabetes [ 41 , 42 ]. Furthermore, visceral fat has an independent effect on cardiometabolic risk factors, such as abnormal lipid and glucose metabolism [ 30 , 37 ]. However, it was not possible to analyze the effect of visceral fat mass alone in the present study.
In the present study we used data from the CHNS, which is a nationwide study that has been conducted for over 16 years. Therefore, this study is meaningful because it is the first study to use group‐based trajectory modeling method to analyze the change of F2FFMR in Chinese, and the results provide strong evidence for associations between long-term changes in body composition and the development of cardiometabolic risk factors. We first use the F2FFMR as a metabolic load-capacity indicator for study with the cardiometabolic outcome. The latent growth model was used to create the body fat trajectory groups. However, there were some limitations to the study. First, the fat mass and fat-free mass were estimated using a verified model that included BMI and upper arm skinfold thickness, rather than by direct measurement. Therefore, there may be some bias in the body composition data. Second, the trajectory groups, except for the Low stability group, were relatively small. Larger samples are required to verify the association of F2FFMR trajectory with dyslipidemia and other cardiometabolic risk factors. Third, blood pressure was measured once, whereas a clinical diagnosis of hypertension should be made on the basis of three measurements made on three different days. Due to unavailability of data, we could not distinguish between type 1 diabetes and type 2 diabetes in this study. Fourth, F2FFMR trajectory might be affected by differences in ethnicity and eating habits. The present study was of the Chinese adult population; therefore, the findings require confirmation in other populations.
In conclusion, four types of F2FFMR trajectory were identified in men and three in women. High stability trajectory of F2FFMR was associated with the highest risk of developing diabetes in men, and diabetes and hypertension in women, independent of age and current body mass. Our results also suggest that the association between F2FFMR trajectory and dyslipidemia and hypertension in men is mediated by current body mass.
Acknowledgments
This research used data from CHNS. We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center (P2C HD050924, T32 HD007168), the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, R24 HD050924, and R01-HD38700) and the NIH Fogarty International Center (D43 TW009077, D43 TW007709) for support for the CHNS data collection and analysis of files from 1989 to 2015 and future surveys, the China–Japan Friendship Hospital, the Ministry of Health for support for CHNS 2009, the Chinese National Human Genome Center in Shanghai since 2009, and the Beijing Municipal Center for Disease Prevention and Control since 2011. We also thank Mark Cleasby, PhD, from Edanz Group ( https://en-author-services.edanz.com/ ) for editing a draft of this manuscript.
- View Article
- PubMed/NCBI
- Google Scholar
- 26. Nagin D. Group-based modeling of development (Harvard University Press, Cambridge, Mass., London, 2005).
CT-based screening of sarcopenia and its role in cachexia syndrome in pancreatic cancer
Affiliations.
- 1 Department of Diagnostical and Interventional Radiology, Heidelberg University Hospital, Heidelberg, Germany.
- 2 Department of Radiology at Medical Educational and Scientific Center University Hospital, Lomonosov Moscow State University, Moscow, Russia.
- 3 Institute for Diagnostical and Interventional Radiology, Ludwigshafen Clinical Hospital, Ludwigshafen am Rhein, Germany.
- 4 University College Dublin School of Chemistry, Dublin, Ireland.
- 5 Moscow City Clinical Cancer Hospital No. 1, Oncology No. 4, Moscow, Russia.
- PMID: 38271373
- PMCID: PMC10810529
- DOI: 10.1371/journal.pone.0291185
Since computed tomography (CT) is a part of standard diagnostic protocol in pancreatic ductal adenocarcinoma (PDAC), we have evaluated the value of CT for sarcopenia screening in patients with PDAC, intending to expand the diagnostic value of tomographic studies. In our study, we included 177 patients with available CT images. Two groups were formed: Group 1 consisted of 117 patients with PDAC in various locations and stages and Group 2, or the control group, consisted of 60 "nominally healthy" patients with other somatic non-oncological diseases. The body mass index (BMI) was defined as a ratio of patient's weight to the square of their height (kg/m2). CT-based body composition analysis was performed using commercially available software with evaluation of sarcopenia using skeletal muscle index (SMI, cm2/m2). Based on the SMI values, sarcopenia was found in 67.5% of patients (79 out of 117) in the first patient group. It was found more frequently in males (42 out of 56; 75%) than in females (37 out of 61; 60.6%). Additionally, we observed a decrease in muscle mass (hidden sarcopenia) in 79.7% in patients with a normal BMI. Even in overweight patients, sarcopenia was found in 50% (sarcopenic obesity). In patients with reduced BMI sarcopenia was found in all cases (100%). Statistically significant difference of SMI between two groups was revealed for both sexes (p = 0,0001), with no significant difference between groups in BMI. BMI is an inaccurate value for the assessment of body composition as it does not reflect in the details the human body structure. As SMI may correlate with the prognosis, decreased muscle mass- especially "hidden" sarcopenia or sarcopenic obesity- should be reported. The use of CT-based evaluation of sarcopenia and sarcopenic obesity will allow for a better treatment response assessment in patients with cancer cachexia.
Copyright: © 2024 Khristenko et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- Cachexia / diagnostic imaging
- Cachexia / etiology
- Carcinoma, Pancreatic Ductal* / pathology
- Early Detection of Cancer
- Muscle, Skeletal / diagnostic imaging
- Muscle, Skeletal / pathology
- Obesity / pathology
- Pancreatic Neoplasms* / complications
- Pancreatic Neoplasms* / diagnostic imaging
- Retrospective Studies
- Sarcopenia* / diagnosis
- Sarcopenia* / diagnostic imaging
- Tomography, X-Ray Computed
- Wasting Syndrome* / pathology
Grants and funding
- Open access
- Published: 31 March 2024
Very low-calorie ketogenic diet (VLCKD): a therapeutic nutritional tool for acne?
- Ludovica Verde 1 , 2 na1 ,
- Evelyn Frias-Toral 3 na1 ,
- Sara Cacciapuoti 4 na1 ,
- Daniel Simancas-Racines ORCID: orcid.org/0000-0002-3641-1501 5 ,
- Matteo Megna 4 ,
- Giuseppina Caiazzo 6 ,
- Luca Potestio 4 ,
- Maria Maisto 7 ,
- Gian Carlo Tenore 7 ,
- Annamaria Colao 2 , 8 , 9 ,
- Silvia Savastano 2 , 8 ,
- Giovanna Muscogiuri 2 , 8 , 9 na2 &
- Luigi Barrea ORCID: orcid.org/0000-0001-9054-456X 10 , 2 na2
Journal of Translational Medicine volume 22 , Article number: 322 ( 2024 ) Cite this article
Metrics details
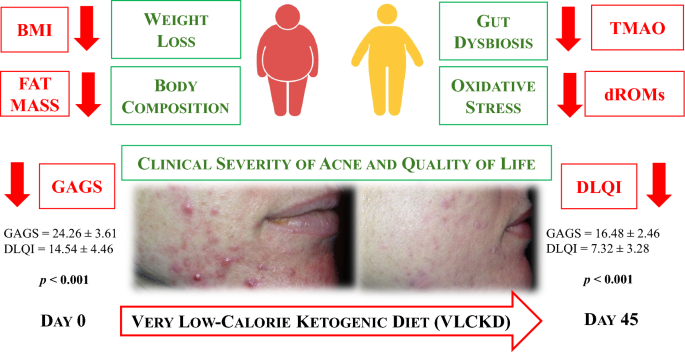
Acne, a chronic inflammatory disease impacting the pilosebaceous unit, is influenced significantly by inflammation and oxidative stress, and is commonly associated with obesity. Similarly, obesity is also associated with increased inflammation and oxidation. The role of diet in acne remains inconclusive, but the very low-calorie ketogenic diet (VLCKD), known for weight loss and generating anti-inflammatory ketone bodies, presents promising potential. Despite this, the effects of VLCKD on acne remain underexplored. This study aimed to investigate the efficacy of a 45-day active phase of VLCKD in reducing the clinical severity of acne in young women with treatment-naïve moderate acne and grade I obesity.
Thirty-one women with treatment-naïve moderate acne, grade I obesity (BMI 30.03–34.65 kg/m 2 ), aged 18–30 years, meeting inclusion/exclusion criteria, and consenting to adhere to VLCKD were recruited. Baseline and post-intervention assessments included anthropometric measurements, body composition, phase angle (PhA), trimethylamine N-oxide (TMAO) levels, and reactive oxygen metabolite derivatives (dROMs) as markers of inflammation, dysbiosis, and oxidative stress, respectively. A comprehensive dermatological examination, incorporating the Global Acne Grading System (GAGS) and the Dermatology Life Quality Index (DLQI), was conducted for all women.
VLCKD resulted in general improvements in anthropometric and body composition parameters. Significantly, there were significant reductions in both the GAGS score (Δ%: − 31.46 ± 9.53, p < 0.001) and the DLQI score (Δ%: − 45.44 ± 24.02, p < 0.001) after the intervention. These improvements coincided with significant decreases in TMAO ( p < 0.001) and dROMs ( p < 0.001) levels and a significant increase in PhA (Δ%: + 8.60 ± 7.40, p < 0.001). Changes in the GAGS score positively correlated with changes in dROMs ( p < 0.001) and negatively with PhA ( p < 0.001) even after adjusting for Δ% FM. Changes in the DLQI score positively correlated with changes in dROMs ( p < 0.001) and negatively with PhA ( p < 0.001) even after adjustment for Δ% FM.
Given the side effects of drugs used for acne, there is an increasing need for safe, tolerable, and low-cost treatments that can be used for acne disease. The 45-day active phase of VLCKD demonstrated notable improvements in acne severity, and these improvements seemed to be attributable to the known antioxidant and anti-inflammatory effects of VLCKD.
Graphical Abstract
Introduction
Acne vulgaris (acne) is a complex, chronic inflammatory skin disease involving the pilosebaceous unit [ 1 ]. The prevalence of acne varies by time and country, and lifestyle may influence it [ 2 , 3 ]. This skin condition affects 70–80% of adolescents and persists into the 20 s and 30 s in about 64% and 43% of affected individuals, respectively [ 4 , 5 ]. In addition, several studies show that acne is more common in adult females when compared to adult males [ 6 , 7 ]. Of interest, Chang J et al. reported a 1.5-fold higher proportion of dermatology visits for acne among women compared to men ages 20–29 years [ 8 ]. This sex difference in dermatological care may be tied to an increased acne severity among adult women as well as an increased impact on quality of life in this population [ 6 , 7 , 8 ]. In this context, acne patients may have significant quality of life (QoL) impairment [ 9 ] and its assessment, as an integral part of acne management in these patients, is recommended by several international guidelines [ 10 , 11 ]. The Dermatology Life Quality Index (DLQI) is the most widely used health-related quality of life questionnaire in dermatology, particularly in studies on acne [ 10 , 12 ].
Acne can result in enduring scarring and hyperpigmentation [ 13 , 14 ], necessitating effective prevention and treatment to mitigate its significant impact on patients' quality of life [ 13 ]. A characteristic feature of acne patients is lesion pleomorphism, where different types of lesions, both inflammatory (such as papules, pustules, and nodules) and non-inflammatory (like comedones), may coexist in the same individual [ 1 ]. The clinical manifestations of acne can vary widely based on factors such as the severity, number, and type of predominant lesions [ 1 ].
The multifaceted pathogenesis of acne is attributed to several factors, including hyperseborrhea, hyperkeratinization of the pilosebaceous duct, colonization by Propionibacterium acnes , and perifollicular inflammation [ 15 ]. Abnormal desquamation of the sebaceous follicle epithelium (comedogenesis), sebaceous gland hyperplasia with seborrhea, increased bacterial colonization, and immunologic and inflammatory elements are the main pathophysiologic factors influencing acne development [ 15 ].
Key players in acne pathophysiology involve complex immunochemical pathways associated with inflammation, encompassing various inflammatory mediators and their target receptors, such as cytokines, defensins, peptidases, sebaceous lipids, and neuropeptides [ 16 ]. Elevated levels of prostaglandin E2 and peroxisome proliferator-activated receptor (PPAR)-γ can contribute to sebaceous gland hyperplasia and excessive sebum production, leading to inflammation and acne lesions [ 17 ]. Propionibacterium acnes also plays a role in triggering the release of pro-inflammatory cytokines [ 17 ].
In addition to increased sebum production and altered keratinization, recent discoveries highlight the microbiome as a third major player in acne development, interacting with the innate immune system [ 18 ]. The intestinal flora's influence on acne is speculated to involve interactions with the mammalian target of rapamycin (mTOR) pathway [ 19 , 20 , 21 ]. Metabolites from the gut microbiota may regulate cell expansion, fat metabolism, and metabolic functions through the mTOR pathway [ 22 ]. The interplay between mTOR and gut microbiota may form a mechanism by which the intestinal flora exacerbates acne, particularly in cases of gut dysbiosis and a disrupted intestinal barrier, creating a positive feedback loop and amplifying host metabolism and inflammation [ 23 ].
Apart from the traditional factors linked to acne, recent findings have established a connection between oxidative stress and the development of this condition [ 24 ]. Notably, strains associated with acne could release porphyrins, leading to an escalation in reactive oxygen species (ROS) formation and initiating an inflammatory response in keratinocytes [ 25 ]. This inflammatory process is linked to an imbalance between oxidants and antioxidants [ 24 ]. The role of ROS in acne vulgaris pathogenesis is significant, influencing the mTOR pathway, PPAR, toll-like receptor (TLR), and the innate immune system, thereby causing inflammation through alterations in the production of various pro-inflammatory cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-8, and IL-1 [ 24 ].
Obesity, a condition characterized by excess body weight and adipose tissue accumulation, has been associated with various inflammatory and metabolic disorders [ 26 ]. However, the link between obesity and acne is not fully understood, but emerging evidence suggests a potential connection through inflammatory and hormonal mechanisms [ 27 ]. Obesity is known to induce a state of chronic low-grade inflammation, marked by increased levels of pro-inflammatory cytokines and adipokines [ 26 ]. This inflammatory state may contribute to the development and exacerbation of acne by influencing the pathways involved in sebum production, follicular hyperkeratinization, and immune responses in the skin [ 27 ]. Additionally, obesity is often associated with insulin resistance and elevated levels of insulin-like growth factor 1 (IGF-1) [ 28 ], both of which have been implicated in the pathogenesis of acne [ 1 ]. Moreover, the gut microbiota, which plays a crucial role in maintaining overall health, can be altered in individuals with obesity [ 29 , 30 ]. The interplay between obesity, gut dysbiosis, and acne may involve complex interactions among inflammation, hormonal regulation, and the immune system [ 31 ]. Very low-calorie ketogenic diet (VLCKD) has been shown to have anti-inflammatory and antioxidant effects, improve insulin sensitivity, and modulate the gut microbiota. Addressing acne through dietary interventions, such as VLCKD, could potentially impact both acne and the associated inflammatory and gut dysbiosis components [ 31 ].
To date, there is a large gap in the scientific literature on the use of VLCKDs for skin diseases. While there is some evidence supporting the use of ketogenic diets in psoriasis [ 32 , 33 ], to our knowledge, no studies to date have evaluated the efficacy of VLCKD in reducing the clinical severity of acne. Thus, considering the existence of inflammation, oxidative stress, and dysbiosis in patients with acne, we suppose that a highly antioxidant and anti-inflammatory dietary therapy such as VLCKD, beyond the well-known weight loss effects, can contribute to improve both oxidation and dysbiosis and, consequently, improve the clinical severity of acne. In this context, the main aim of this study was to evaluate the efficacy of 45 days of active phase of VLCKD in reducing the clinical severity of acne in a group of young women with treatment-naïve moderate acne and grade I obesity.
Materials and methods
Population study.
This study included 31 treatment-naïve women affected by moderate acne attending the outpatient clinic of the Units of Endocrinology and Dermatology of Federico II University Hospital. Ethical approval for the study was obtained from the Local Ethics Committee (reference no. 50/20), and all procedures adhered strictly to the World Medical Association's Code of Ethics, particularly the Declaration of Helsinki, outlining principles for human experimentation. The study's objectives and procedures were clearly communicated to all women, and written informed consent, expressing their willingness to participate, was obtained before their involvement.
At baseline, all women were assessed during the follicular phase of the menstrual cycle, and a comprehensive medical history, including drug usage, was documented. Inclusion criteria encompassed young women of childbearing age (18–30 years) with untreated moderate acne (Global Acne Grading System—GAGS—scores ranging from 19 to 30) and grade I obesity (BMI 30.0–34.9 kg/m 2 ). To enhance sample homogeneity, only non-smoking women with no regular physical activity (less than 30 min of aerobic exercise per day) and meeting specific criteria were included, while those with certain exclusion criteria were omitted:
Age < 18 years and > 30 years;
Women with mild or severe acne;
Women with any other active skin condition (e.g., psoriasis or hidradenitis suppurativa) that might interfere with acne assessment;
Presence of one or more contraindications for VLCKD as per current European Association for the Study of Obesity (EASO) guidelines [ 34 ];
Women with a medical history affecting blood glucose or insulin concentrations, including diabetes types 1 and 2, prediabetes, or insulin resistance (Homeostatic model assessment for insulin resistance > 2.5), and/or taking medications altering blood glucose levels or insulin concentrations;
Women with acne lasting > 6 months or receiving systemic acne treatment for at least 3 months;
Pregnant or lactating women in the past 6 months;
Women with a self-reported recent weight change (> 10% weight change within the last 6 months);
Endocrine disorders affecting body composition or nutritional status, including biochemical hyperandrogenaemia and/or hyperandrogenism, oligomenorrhea due to polycystic ovarian syndrome, or secondary etiologies (according to the Endocrine Society) [ 35 ];
Chronic diseases affecting fluid homeostasis, such as liver or renal chronic diseases, cancer and acute or chronic inflammatory diseases;
Use of drugs impacting body composition, nutrient metabolism, or weight loss;
Dietary regimens in the last three months, including ketogenic diets, vegan or vegetarian diets, or supplementation with antioxidants, vitamins, or minerals;
Women with implanted pacemakers or defibrillators due to the theoretical risk of interference with the bioelectrical impedance analysis (BIA) device activity.
Figure 1 shows a flow chart of included and excluded women.
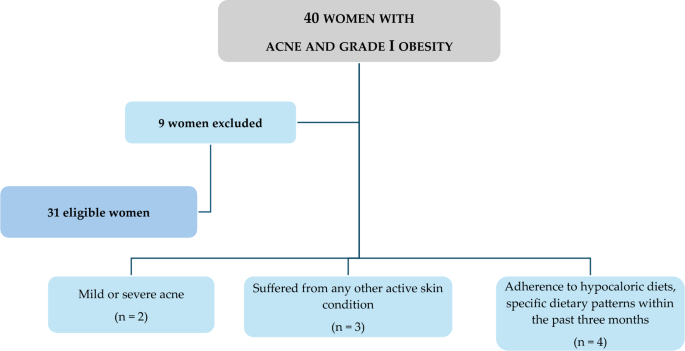
Flow chart of the study participants
Study protocol
The study protocol encompassed a series of five visits (T0-day 0, T1-day 7, T2-day 21, T3-day 35, T4-day 45) over a total span of 45 days (Fig. 2 ). In detail, at baseline (T0), a comprehensive assessment carried out by a team of Endocrinologist, Dermatologist, and Nutritionist was conducted to ascertain the eligibility of patients. Those meeting the criteria for inclusion and exclusion were enrolled in the study and provided their written informed consent. At this point, the Endocrinologist carried out the first medical examination to ascertain the inclusion criteria for the study. Then, the dermatologist performed the clinical acne assessment and confirmed the inclusion criteria for each patient. Finally, the Nutritionist carried out nutritional status assessments (anthropometry and body composition) and drew up the VLCKD dietary therapy. All participants were then given personalized instructions for adhering to the diet. Simultaneously, with the support of nursing staff, blood samples were collected for general biochemical tests, oxidative stress evaluation, and trimethylamine N-oxide (TMAO) levels. Finally, women were advised to maintain the same lifestyle habits.
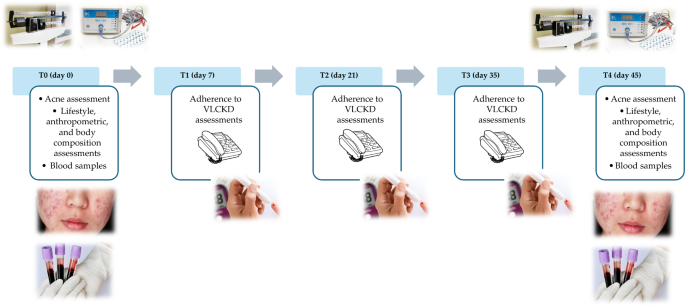
In the subsequent follow-up visits (T1-day 7, T2-day 21, and T3-day 35), a nutritionist carried out a telephone interview to evaluate adherence to the diet and the state of ketosis.
Adherence to the diet was assessed by asking the patient if she was consuming the number of VLCKD replacement meals, if she was drinking at least 2 L of water per day, and if she was respecting the written indications on dietary therapy. Ketosis status was assessed through ketone body measurements extracted from capillary blood samples, and the Nutritionist recorded only whether the patient had ketosis or not (YES/NO).
In all of these follow-up visits, the Nutritionist also documented any changes in physical activity levels or food and drink consumption patterns outlined in the VLCKD protocol.
In the last visit (T4-day 45), a final round of endocrinological, dermatological, and nutritional assessments was conducted. Blood samples were collected once more for the repetition of oxidative stress and TMAO analyses.
Acne severity assessment
Every woman underwent a comprehensive dermatological examination, which included the use of the GAGS, a quantitative scoring system designed to evaluate the severity of acne. Initially developed by Doshi et al. [ 36 ], the GAGS score is calculated by adding six regional subscores. Specifically, each point is determined by multiplying the factor assigned to each region (3 for the chest and upper back, 1 for the chin and nose, and 2 for the forehead and each cheek) by the highest weighted lesion within that region (4 for ≥ one nodule, 3 for ≥ one pustule, 2 for ≥ one papule, and 1 for ≥ one comedone). These regional factors consider the density of pilosebaceous units, their surface area, and distribution. The cumulative local scores yield the global GAGS score, ranging from 0 to 52. The severity of acne was categorized into three GAGS groups: mild (GAGS score 1–18, characterized by several non-inflammatory comedones with fewer inflammatory lesions), moderate (GAGS score from 19 to 30, marked by numerous comedones, papules, and pustules, but without nodules), and severe (GAGS score from 31 to 38, indicating the presence of inflammatory nodules in addition to papules and pustules) [ 32 , 33 ]. A single experienced dermatologist clinically assessed the GAGS score. To prevent rating biases, the dermatologists conducting the evaluations were kept unaware of the study's design [ 36 , 37 ].
Quality of life assessment
Women in the study filled out the DLQI, a questionnaire consisting of 10 items designed to evaluate the overall impact of skin disease on quality of life [ 12 ]. The total score spans from 0 to 30, where 0 signifies no influence of the skin disease on quality of life, and 30 indicates the maximum impact on quality of life. The grading system is as follows: 0–1 denotes no effect on the patient’s life, 2–5 signifies a small effect, 6–10 indicates a moderate effect, 11–20 suggests a very large effect, and 21–30 implies an extremely large impact on the woman’s life [ 12 ].
Anthropometric measurements
Anthropometric assessments were conducted by a certified clinical nutrition specialist, adhering to the International Society for the Advancement of Kinanthropometry (ISAK 2006) guidelines. The measurements were performed in the morning, between 8 and 10 a.m., following an overnight fast. Women, as previously documented [ 38 , 39 ], were attired in light clothing without shoes during the evaluation. Weight was assessed using a calibrated balance beam scale (Seca 711; Seca, Hamburg, Germany), and height was measured with a wall-mounted stadiometer (Seca 711; Seca, Hamburg, Germany). Subsequently, BMI was calculated as weight (kg) divided by height squared (m 2 ).
In accordance with the World Health Organization (WHO)’s criteria, women were categorized as follows: a BMI of 25.0–29.9 kg/m 2 indicated overweight, and a BMI within the range of 30.0–34.9 kg/m 2 denoted grade I obesity [ 40 ]. Waist circumference (WC) was determined following the guidelines of the National Center for Health Statistics. A non-stretchable measuring tape was used at the natural indentation or at a midway level between the lower edge of the rib cage and the iliac crest if no natural indentation was visible. The measurements were recorded to the nearest 0.1 cm.
Body composition
Body composition was evaluated using a BIA phase-sensitive system administered by a certified clinical nutrition specialist with 5 years of expertise in employing the BIA method for body composition assessment (800-µA current at a single frequency of 50 kHz, BIA 101, RJL Akern Bioresearch, Florence, Italy) [ 41 ], in accordance with previously documented procedures [ 39 , 42 , 43 ]. The BIA analysis adhered to the guidelines set by the European Society of Parental and Enteral Nutrition (ESPEN) [ 44 ]. Women were instructed to remove their shoes and socks, and the electrode contact areas (BIATRODES Akern Srl; Florence, Italy) were cleansed with alcohol immediately before placement on the hand and the ipsilateral foot, following the protocol outlined by Kushner [ 45 ]. Phase angle (PhA) was computed using the relationship between resistance (R) and reactance (Xc) based on the formula: PhA (°, degrees) = Xc/R* (180/π).
The BIA data were acquired under strictly standardized conditions, with women refraining from drinking, eating, and exercising for 6 h and abstaining from alcohol consumption within 24 h prior to testing. Women assumed a supine position with their limbs slightly separated from the body. The BIA examination was consistently conducted by the same nutritionist using the identical device to mitigate potential interobserver and interdevice variations. Regular checks of the BIA tool were performed with resistors and capacitors of known values, demonstrating reliability with within-day and between-day measurement variations of < 1.4% for R, < 1.5% for Xc, and < 1.7% for R, < 2.0% for Xc, respectively. The coefficient of variation (CV) for repeated measurements of R and Xc at 50 kHz was assessed in 8 individuals, yielding CVs of 1.3% for R and 1.2% for Xc.
Laboratory parameters
Reactive oxygen metabolites (dROMs) were evaluated as biomarkers indicative of oxidative stress using an automated analyzer (Free Carpe Diem, Diacron International, Grosseto, Italy) and corresponding commercial kits (Diacron International) [ 46 , 47 ]. Specifically, for dROMs assessment, 10 µL of serum was transferred into 1 cm cuvettes containing 1 mL of R2 reagent (acetate buffer, pH 4.8). The resulting mixture was gently mixed, and 10 µL of R1 reagent (a chromogenic mixture comprising aromatic alkyl-amine, A-NH2) was added. After inversion mixing, the samples were read at 546 nm (5 min, 37 °C) using an automated analyzer.
dROMs, which are oxygen metabolites generated by free radical attacks at the expense of biomolecules, were stable and quantifiable. Specifically, the test employed here is based on Fenton's reaction, where, in the presence of iron, dROMs in serum generate alkoxyl (R − O*), and peroxyl (R − OO*) radicals. These radicals, in turn, oxidize an alkyl-substituted aromatic amine, producing a photometrically quantified pink-colored derivative ([A − NH2*] + ) [ 48 , 49 ]. dROMs are considered valuable biomarkers of oxidative stress, with determined ranges as follows: (i) normal: 250–300 Units Carratelli (UCARR), (ii) borderline: 300–320 UCARR, (iii) mild oxidative stress: 321–340 UCARR, (iv) moderate oxidative stress: 341–400 UCARR, (v) high oxidative stress: 401–500 UCARR, and (vi) very high oxidative stress: > 500 UCARR, where 1 UCARR = 0.08 mg H 2 O 2 /dL [ 48 , 49 ].
The reliability of the analysis was assessed by calculating the CV % at both intra- and inter-assay levels for all collected samples, resulting in an estimated CV % below 2.72% for both parameters.
Determination of circulating levels of TMAO
Serum levels of TMAO were measured in samples stored at − 80 °C, a condition demonstrated to maintain TMAO stability for several years in a previous study [ 50 ]. The quantification of circulating TMAO levels followed the method outlined by Beale and Airs [ 50 ], as detailed in our prior research [ 51 , 52 ], with minor adjustments. In summary, serum proteins were precipitated using methanol (serum:methanol, 1:2, v/v); the samples were vortex-mixed for 2 min, centrifuged at 14,000 g for 10 min (4 °C), and the supernatants were collected and subjected to analysis using the High-Performance Liquid Chromatography-Mass Spectrometry (HPLC–MS) method [ 53 ]. The HPLC–MS conditions and method optimization adhered to Beale and Airs [ 54 ]. The HPLC system Jasco Extrema LC-4000 system (Jasco Inc., Easton, MD, USA) was coupled to a single quadrupole mass spectrometer (Advion ExpressIonL CMS, Advion Inc., Ithaca, NY, USA) equipped with an electrospray ionization (ESI) source, operating in positive ion mode. Chromatographic separation utilized a Luna hydrophilic interaction liquid chromatography (HILIC) column (150 × 3 mm, 5 µm particles) along with a guard column, both provided by Phenomenex (Torrance, CA, USA).
The sensitivity of the analytical method was described by the determination of Limit of Detection (LoD) of 2 ng/mL and Limit of Quantification (LoQ) of 6 ng/mL. In order to evaluate the precision of the method used, the CV% at intra- and inter-day level was calculated at three different TMAO levels (0.3, 3, and 13 µM), resulting in a calculated intra-day CV% of 8.12, 1.54, and 1.52 µM and of inter-day CV% of 9.2, 2.2, and 3.3 µM, respectively. Similarly, over the same TMAO levels, the accuracy of the method was calculated by the evaluation of the accuracy (% bias) both intraday and interday, leading to an estimation of % bias ranging from—3.52 to 0.66, indicating of high reliability of the used LC/MS method.
VLCKD intervention
According to EASO guidelines [ 34 ] and the consensus statement from the working group of the Club of the Italian Society of Endocrinology (SIE)-diet therapies in endocrinology and metabolism [ 55 ], VLCKD consists of different phases (active—ketogenic—, re-education—non-ketogenic—, and maintenance). This study evaluated only the active phase, the ketogenic one.
The dietary composition adhered to specific parameters, with a total energy intake of less than 800 kcal per day. This energy was derived from a distribution of 13% from carbohydrates (less than 30 g per day), 43% from protein (1.3 g per kilogram of ideal body weight), and 44% from fat. The ideal body weight (kg) was calculated using the Lorentz equation: ideal body weight = height (cm) − 100 − [(height − 150)/2] [ 56 ]. Throughout VLCKD, meals with high biological value were provided as replacements, and the protein content originated from sources such as whey, soy, eggs, and peas. To ensure nutritional adequacy during the VLCKD, supplementation was introduced. This included B-complex vitamins, vitamins C and E, essential minerals like potassium, sodium, magnesium, and calcium, as well as omega-3 fatty acids. The active phase of VLCKD was collaboratively devised by a Nutritionist and endorsed by an Endocrinologist. The schematic representation of the active phase of VLCKD according to KeNuT multisteps dietary protocol with meal replacements proposed by the Club of the Italian Society of Endocrinology (SIE)—Diet Therapies in Endocrinology and Metabolism are reported in Table S1 (Additional file 1 ).
Statistical analysis
The MedCalc® package (Version 12.3.0, 1993–2012 MedCalc Software bvba—MedCalc Software, Mariakerke, Belgium) and IBM SPSS Statistics Software (PASW Version 21.0, SPSS Inc., Chicago, IL, USA) were employed for the data analysis. The statistical analysis specifically focused on women with measurements at both baseline and after 45 days of the active phase of VLCKD. Results were expressed as mean ± standard deviation (SD) for continuous variables and as a number and percentage (n, %) for categorical variables. The Kolmogorov–Smirnov test was used to assess data distribution, and the paired Student’s t -test was utilized to compare differences between baseline and measurements after 45 days of the active phase of VLCKD. Spearman’s correlation was applied to assess the association between baseline and measurements after 45 days of the VLCKD phase in terms of percentage changes (∆%).
The study population included 31 women with treatment-naïve moderate acne (19 ≥ GAGS ≤ 30, median value 24), grade I obesity (BMI 30.03 to 34.65 kg/m 2 , median value 33.05 kg/m 2 ), aged 18 to 30 years.
Anthropometric characteristics and body composition of the study population at baseline and after 45 days of the active phase of VLCKD are reported in Table 1 . After 45 days of the active phase of VLCKD, in the entire study population, both BMI (Δ%: − 8.08 ± 1.52, p < 0.001) and WC (Δ%: − 7.51 ± 1.67, p < 0.001) were significantly reduced compared to baseline. After 45 days of the active phase of VLCKD, fat mass (FM) (kg and %) (Δ%: − 11.34 ± 4.90 and − 11.34 ± 4.90, both p < 0.001) and fat free mass (FFM) (kg) (Δ%: − 1.66 ± 1.38, p < 0.001) were significantly reduced while FFM (%) (Δ%: + 7.02 ± 2.32, p < 0.001) slightly increased. A significant increase in PhA (Δ%: + 8.60 ± 7.40, p < 0.001) compared to the baseline was also detected.
Parameters of dysbiosis (TMAO) and oxidative stress (dROMs) in the study population at baseline and after 45 days of the active phase of VLCKD are reported in Table 2 . After 45 days of the active phase of VLCKD, in the entire study population, we observed significant reductions in TMAO (Δ%: − 51.97 ± 15.98, p < 0.001) and dROMs (Δ%: − 38.07 ± 18.40, p < 0.001) levels compared to baseline.
The dermatological parameters of the study population at baseline and after 45 days of the active phase of VLCKD are shown in Table 3 . Of note, after 45 days of the active phase of VLCKD, both the GAGS score (Δ%: − 31.46 ± 9.53, p < 0.001), and the DLQI score (Δ%: − 45.44 ± 24.02, p < 0.001) decreased significantly compared to baseline (Fig. 3 ).
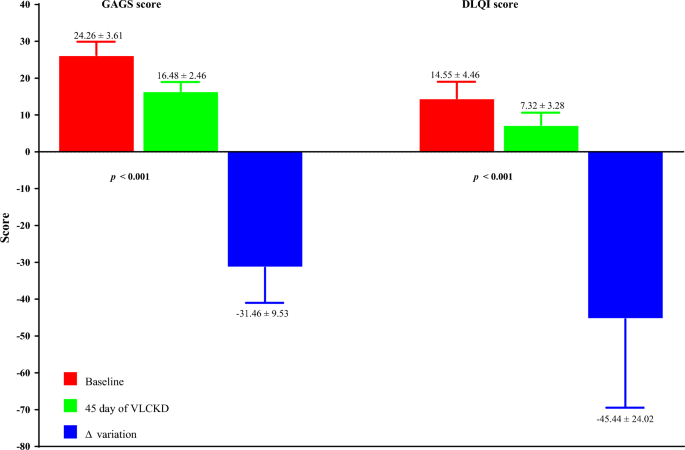
Baseline and post-45-day VLCKD dermatological parameters of women with acne. A p-value in bold type denotes a significant difference (p < 0.05). VLCKD very low-calorie ketogenic diet, Δ% percentage change, GAGS global acne grading system, DLQI dermatology life quality index
Table 4 reports the simple and adjusted correlations among changes in the GAGS score and changes in the study parameters after 45 days of the active phase of VLCKD. Changes in the GAGS score positively correlated with changes in weight ( p = 0.004), BMI ( p = 0.001), WC ( p = 0.012), total body water (TBW) (lt) ( p = 0.001), extracellular water (ECW) (lt) ( p < 0.001), ECW (%) ( p = 0.001), FM (kg) ( p = 0.023), skeletal muscle mass (SMM) ( p = 0.012), TMAO ( p = 0.020), dROMs ( p < 0.001) and DLQI ( p < 0.001) and negatively with R ( p = 0.010), Xc ( p < 0.001), PhA ( p < 0.001), ICW (%) ( p = 0.001), BCM ( p = 0.017), and BCMI ( p = 0.016). Interestingly, the correlations with dROMs ( p < 0.001) (Fig. 4 ) and PhA ( p = 0.005) were maintained even after adjustment for Δ% FM.
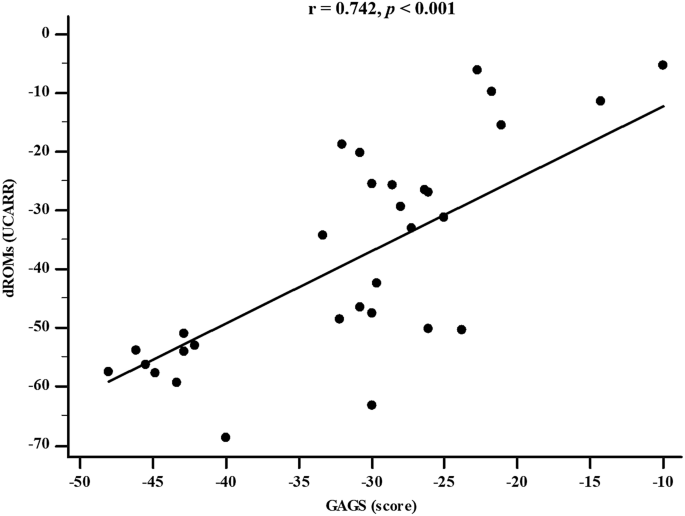
Correlation between GAGS score and dROMs levels after 45 days of active phase of VLCKD (adjusted for Δ% FM). GAGS global acne grading system, dROMs reactive oxygen metabolites
Table 5 reports the simple and adjusted correlations among changes in the DLQI score and changes in the study parameters after 45 days of the active phase of VLCKD. Changes in the DLQI score positively correlated with changes in weight ( p < 0.001), BMI ( p < 0.001), WC ( p = 0.002), ECW (lt) ( p < 0.001), ECW (%) ( p < 0.001), FM (kg) ( p < 0.001), FM (%) ( p < 0.001), TMAO ( p < 0.001), dROMs ( p < 0.001) and GAGS ( p < 0.001) and negatively with Xc ( p < 0.001), PhA ( p < 0.001), ICW (lt) ( p < 0.001), ICW (%) ( p = 0.001), FFM (%) ( p = 0.002), BCM ( p < 0.001) and BCMI ( p < 0.001). Of note, the correlations with dROMs ( p < 0.001) and PhA ( p < 0.001) were maintained even after adjustment for Δ% FM.
In this study, a cohort of 31 women with treatment-naïve moderate acne and grade I obesity underwent the active phase of VLCKD for 45 days. As expected, at the end of the active phase of VLCKD, anthropometric measurements showed significant reductions in both BMI and WC, and, for body composition, FM (kg and %) decreased significantly, while FFM (%) showed a slight increase. In addition, there were also notable increments observed in PhA, and this was consistent with previous research [ 39 , 42 , 43 ]. PhA is a BIA parameter that serves as an indicator of cellular health and the distribution of body fluids. It has been recognized as a prognostic marker for both the incidence of illnesses and the likelihood of mortality in cases of chronic inflammatory conditions [ 57 ]. It’s worth noting that PhA values tend to be diminished in a significant portion of inflammatory disorders, which encompass conditions like psoriasis and hidradenitis suppurativa [ 58 , 59 ].
The results of this study are promising for acne patients. The results of our study represented a novel finding, as they showed a significant reduction in TMAO and dROMs levels after the 45-day active phase of VLCKD in women with acne and obesity. This novel finding not only underscored the potential efficacy of VLCKD in the management of these conditions but also indicated a positive impact on oxidative stress and gut dysbiosis, both potential mechanisms influencing acne severity. Together, in fact, the simultaneous decrease in the GAGS score and DLQI score added an additional level of significance to our results. This dual improvement not only suggests an improvement in acne severity but also highlights a substantial improvement in participants’ overall quality of life. These findings carry significant implications regarding the potential benefits of VLCKD, particularly for patients struggling with acne and obesity, a category of patients particularly exposed to dysbiosis, oxidative stress, and a high risk of cardiovascular diseases [ 60 , 61 , 62 ].
In this scenario, the decline in oxidative stress, as indicated by reduced levels of dROMs, and the amelioration of gut dysbiosis, represented by decreased TMAO levels, induced by VLCKD, may collectively constitute the underlying pathophysiological mechanism associated with the beneficial outcomes of this dietary therapy in mitigating the clinical severity of acne. Presently, there exists a substantial gap in the scientific literature regarding the utilization of VLCKDs in diverse skin disorders [ 63 , 64 ]. Despite some evidence supporting the use of VLCKDs in psoriasis [ 32 ], there is a notable absence of clinical studies, to the best of our knowledge, assessing the effectiveness of VLCKD in treating acne.
Interestingly, gut dysbiosis is implicated in elevating systemic inflammation, which correlates with the onset and clinical severity of acne [ 65 ]. Moreover, studies indicate that regular intake of probiotics, particularly those containing lactobacillus strains, over 12 weeks is associated with a significant reduction in inflammatory acne lesions (30% to 67%) and a concurrent decrease in IGF-1 levels by 32% [ 66 , 67 , 68 , 69 ].
Although limited research has explored the connection between VLCKDs and microbiota, both human and animal studies report positive effects on restructuring bacterial composition and enhancing gut biological function, fostering an increase in anti-inflammatory bacteria [ 70 ]. VLCKDs may influence the gut microbiota through metabolites produced by various bacteria, resulting in improved short-chain fatty acids production, reduced lactate, and increased hydrogen sulfide [ 70 ].
Recent evidence suggests that elevated insulin levels may contribute significantly to acne development through effects on sex hormones, subsequently influencing sebum production and inflammation. VLCKDs are associated with reduced insulin levels, leading to a decline in IGF-1 levels [ 71 ], ultimately triggering an increase in IGFBP-3 levels [ 64 , 72 ]. This reduction in insulin and IGF-1 levels contributes to heightened SHBG levels, leading to decreased androgen production and circulation, even in the skin, correlating with diminished sebum production [ 63 , 64 , 72 ]. The decline in IGF-1 levels, induced by VLCKDs, may attenuate IGF-1 signaling, leading to decreased androgen synthesis and inhibition of the AKT-mTORC1 pathway [ 73 , 74 ]. The subsequent reduction in androgen levels via mTORC2-mediated AKT inactivation [ 75 , 76 ], along with the increased expression of Domain Containing MTOR Interacting Protein (DEPTOR), an inhibitor of mTORC1 and mTORC2 negatively regulated by androgen receptor, further enhances mTORC1 inhibition [ 77 ].
The reduction in sebum quantity and quality hampers the overgrowth of Propionibacterium acnes , thereby improving the skin biofilm [ 76 ]. Lower levels of Propionibacterium acnes -derived lipoteichoic acid and free palmitic acid act via TLR2 to inhibit the activation of the NLRP3 inflammasome, thereby reducing Th17 cell-driven inflammation and inhibiting pro-inflammatory cytokine secretion, including IL-1β and IL-1 release [ 76 ].
Moreover, the anti-inflammatory properties of ketone bodies, extensively discussed in a recent review [ 78 ], are likely beneficial for the inflammatory nature of acne, leading to a reduction in both systemic and local inflammatory processes.
Given these factors, including the improvement of the gut microbiota and the reduction of inflammation and oxidative stress, it is hypothesized that VLCKDs may contribute to diminishing the development and clinical severity of acne.
Limitations of the study were:
The sample was limited, and this may have affected the generalizability of the results. However, we used accurate inclusion and exclusion criteria to increase the value of any results;
Lack of a control group; however, for the short treatment period, a comparison with another diet, such as the Mediterranean diet, would have been ineffective, requiring a longer period for comparable results;
The sample included only women, limiting consideration of the effect of VLCKD on men. However, acne afflicts women more [ 6 , 7 ] and our results could be better applied based on this sex disproportion.
We did not evaluate C-reactive protein, which is often used as a marker of systemic inflammation, and this may affect the completeness of our evaluations. However, it is important to note that some studies suggest that C-reactive protein may not be an ideal marker in acne, as inflammation in this dermatologic condition is considered more localized than systemic [ 79 , 80 ];
A comprehensive analysis of safety and interactions with drugs used to treat acne has not been conducted; in fact, we only recruited treatment-naïve patients. Assessing these interactions will be crucial to ensuring the safety of the treatment;
Further studies, preferably randomized, are needed to compare the efficacy and safety of VLCKD with other dietary therapies available for patients with acne;
Another limitation of this study is that our results refer to a relatively short period of time. It would be interesting to know if these improvements are maintained over time.
We also outline the strengths of the study:
The absence of dropouts is surely a strength of this study;
VLCKD used highly controlled replacement meals, ensuring a strictly monitored caloric and nutritional intake. This contributed to maintaining a highly controlled and standardized diet for all participants;
The patients were followed by a specialized multidisciplinary team that continuously monitored adherence to VLCKD. In detail, we constantly monitored levels of physical activity and diet adherence during the phone calls and follow-up visits;
Stringent inclusion criteria were used. In detail, we applied very stringent inclusion criteria, including only young women of childbearing age, only with treatment-naïve (absence of any treatment for acne) moderate acne, grade I obesity, non-smokers, and those who do not regularly practice physical activity.
The practical implications of incorporating VLCKD into the clinical management of acne disease highlight its potential as a safe, cost-effective, and complementary treatment option, particularly for patients with obesity. This expands the range of treatment options available for individuals struggling with both acne and obesity, offering a potential solution that addresses both conditions simultaneously. However, its successful implementation requires careful consideration of individual patient characteristics, ongoing monitoring, and collaboration among healthcare professionals to ensure safety, efficacy, and long-term sustainability.
With this study, we propose for the first time VLCKD as a possible therapeutic tool for young women with moderate acne and obesity. The results of this study are promising for acne patients. In this context, given the possible side effects of medications used for acne, there is a growing need for safe, tolerable, and low-cost alternative treatments that can be used to reduce the clinical severity of moderate acne in patients with obesity, possibly also as an adjunct to pharmacological therapy for acne disease, since it has been widely demonstrated that VLCKD is tolerable, safe, and effective. Therefore, VLCKD could be used in the repertoire of clinical management of acne disease within a multidisciplinary team that includes the presence of a qualified nutritionist.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Quality of life
Dermatology Life Quality Index
Peroxisome proliferator-activated receptor γ
Mammalian target of rapamycin
Reactive oxygen species
Toll-like receptor
Tumor necrosis factor α
Interleukin
Insulin-like growth factor 1
Very low calorie ketogenic diet
Global Acne Grading Score
Body mass index
European Association for the Study of Obesity
Bioelectrical impedance analysis
Trimethylamine N -oxide
Waist circumference
European Society of Parental and Enteral Nutrition
Phase angle
Coefficient of variation
Reactive oxygen metabolites
Units Carratelli
High-performance liquid chromatography-mass spectrometry
Hydrophilic interaction liquid chromatography
Electrospray ionization
Limit of detention
Limit of quantification
Standard deviation
Fat free mass
Total body water
Extracellular water
Skeletal muscle mass
Domain containing MTOR interacting protein
Zaenglein AL. Acne vulgaris. N Engl J Med. 2018;379(14):1343–52.
Article PubMed Google Scholar
Ballanger F, Baudry P, N’Guyen JM, Khammari A, Dreno B. Heredity: a prognostic factor for acne. Dermatology. 2006;212(2):145–9.
Article CAS PubMed Google Scholar
Di Landro A, Cazzaniga S, Parazzini F, Ingordo V, Cusano F, Atzori L, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67(6):1129–35.
Bagatin E, Timpano DL, Guadanhim LR, Nogueira VM, Terzian LR, Steiner D, et al. Acne vulgaris: prevalence and clinical forms in adolescents from Sao Paulo. Brazil An Bras Dermatol. 2014;89(3):428–35.
Fabbrocini G, Izzo R, Donnarumma M, Marasca C, Monfrecola G. Acne smart club: an educational program for patients with acne. Dermatology. 2014;229(2):136–40.
Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004–2013. J Am Acad Dermatol. 2017;77(3):456-63 e4.
Branisteanu DE, Toader MP, Porumb EA, Serban IL, Pinzariu AC, Branisteanu CI, et al. Adult female acne: clinical and therapeutic particularities (Review). Exp Ther Med. 2022;23(2):151.
Chang J, Nock MR, Cohen JM, Bunick CG. Acne accounts for an almost 2.5-fold higher proportion of dermatology visits among adult females compared to adult males in the United States: a study of the national ambulatory medical care survey from 2002–2016. PLoS ONE. 2023;18(9): e0290763.
Article CAS PubMed PubMed Central Google Scholar
Chernyshov PV, Zouboulis CC, Tomas-Aragones L, Jemec GB, Manolache L, Tzellos T, et al. Quality of life measurement in acne. Position paper of the European Academy of Dermatology and Venereology task forces on quality of life and patient oriented outcomes and acne, rosacea and hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2018;32(2):194–208.
Marron SE, Chernyshov PV, Tomas-Aragones L. Quality-of-life research in acne vulgaris: current status and future directions. Am J Clin Dermatol. 2019;20(4):527–38.
Nast A, Dreno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne—update 2016—short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–8.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6.
Aktan S, Ozmen E, Sanli B. Anxiety, depression, and nature of acne vulgaris in adolescents. Int J Dermatol. 2000;39(5):354–7.
Fabbrocini G, Cacciapuoti S. Evaluation, prevention, and management of acne scars: issues, strategies, and enhanced outcomes. J Drugs Dermatol. 2018;17(12):s44–8.
PubMed Google Scholar
Moon J, Yoon JY, Yang JH, Kwon HH, Min S, Suh DH. Atrophic acne scar: a process from altered metabolism of elastic fibres and collagen fibres based on transforming growth factor-beta1 signalling. Br J Dermatol. 2019;181(6):1226–37.
Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27–35.
PubMed PubMed Central Google Scholar
Zouboulis CC. Endocrinology and immunology of acne: two sides of the same coin. Exp Dermatol. 2020;29(9):840–59.
Balato A, Cacciapuoti S, Di Caprio R, Marasca C, Masara A, Raimondo A, et al. Human microbiome: composition and role in inflammatory skin diseases. Arch Immunol Ther Exp (Warsz). 2019;67(1):1–18.
Jung MJ, Lee J, Shin NR, Kim MS, Hyun DW, Yun JH, et al. Chronic repression of mTOR Complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci Rep. 2016;6:30887.
Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319(5864):777–82.
Sommer F, Adam N, Johansson ME, Xia L, Hansson GC, Backhed F. Altered mucus glycosylation in core 1 O -glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS ONE. 2014;9(1): e85254.
Article PubMed PubMed Central Google Scholar
Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Feng Y, Ralls MW, Xiao W, Miyasaka E, Herman RS, Teitelbaum DH. Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann N Y Acad Sci. 2012;1258:71–7.
Kardeh S, Moein SA, Namazi MR, Kardeh B. Evidence for the important role of oxidative stress in the pathogenesis of acne. Galen Med J. 2019;8: e1291.
Dreno B, Dagnelie MA, Khammari A, Corvec S. The skin microbiome: a new actor in inflammatory acne. Am J Clin Dermatol. 2020;21(Suppl 1):18–24.
Karczewski J, Begier-Krasinska B, Staszewski R, Poplawska E, Gulczynska-Elhadi K, Dobrowolska A. Obesity and the risk of gastrointestinal cancers. Dig Dis Sci. 2019;64(10):2740–9.
Dreno B, Gollnick HP, Kang S, Thiboutot D, Bettoli V, Torres V, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29(Suppl 4):3–11.
Berryman DE, Glad CA, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9(6):346–56.
Muscogiuri G, Verde L, Sulu C, Katsiki N, Hassapidou M, Frias-Toral E, et al. Mediterranean diet and obesity-related disorders: what is the evidence? Curr Obes Rep. 2022;11(4):287–304.
Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639–47.
Barrea L, Cacciapuoti S, Megna M, Verde L, Marasca C, Vono R, et al. The effect of the ketogenic diet on acne: could it be a therapeutic tool? Crit Rev Food Sci Nutr. 2023. https://doi.org/10.1080/10408398.2023.2176813 .
Barrea L, Caprio M, Camajani E, Verde L, Elce A, Frias-Toral E, et al. Clinical and nutritional management of very-low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: a practical guide for the nutritionist. Crit Rev Food Sci Nutr. 2023;63(31):10775–91.
Barrea L, Megna M, Cacciapuoti S, Frias-Toral E, Fabbrocini G, Savastano S, et al. Very low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: an update for dermatologists and nutritionists. Crit Rev Food Sci Nutr. 2022;62(2):398–414.
Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L, et al. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–45.
Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92.
Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36(6):416–8.
Barrea L, Donnarumma M, Cacciapuoti S, Muscogiuri G, De Gregorio L, Blasio C, et al. Phase angle and Mediterranean diet in patients with acne: two easy tools for assessing the clinical severity of disease. J Transl Med. 2021;19(1):171.
Barrea L, Di Somma C, Macchia PE, Falco A, Savanelli MC, Orio F, et al. Influence of nutrition on somatotropic axis: milk consumption in adult individuals with moderate-severe obesity. Clin Nutr. 2017;36(1):293–301.
Barrea L, Muscogiuri G, Aprano S, Vetrani C, de Alteriis G, Varcamonti L, et al. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int J Obes (Lond). 2022;46(9):1591–7.
(WH) WHO. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr. 2010;64(1):2–5.
Barrea L, Verde L, Santangeli P, Luca S, Docimo A, Savastano S, et al. Very low-calorie ketogenic diet (VLCKD): an antihypertensive nutritional approach. J Transl Med. 2023;21(1):128.
Verde L, Barrea L, Docimo A, Savastano S, Colao A, Muscogiuri G. Chronotype as a predictor of weight loss and body composition improvements in women with overweight or obesity undergoing a very low-calorie ketogenic diet (VLCKD). Clin Nutr. 2023;42(7):1106–14.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–53.
Kushner RF. Bioelectrical impedance analysis: a review of principles and applications. J Am Coll Nutr. 1992;11(2):199–209.
Annunziata G, Ciampaglia R, Maisto M, D’Avino M, Caruso D, Tenore GC, et al. Taurisolo(R), a grape pomace polyphenol nutraceutical reducing the levels of serum biomarkers associated with atherosclerosis. Front Cardiovasc Med. 2021;8: 697272.
Martelli A, Flori L, Gorica E, Piragine E, Saviano A, Annunziata G, et al. Vascular effects of the polyphenolic nutraceutical supplement taurisolo((r)): focus on the protection of the endothelial function. Nutrients. 2021;13(5):1540.
Gerardi G, Usberti M, Martini G, Albertini A, Sugherini L, Pompella A, et al. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med. 2002;40(2):104–10.
Lupoli R, Calcaterra I, Annunziata G, Tenore G, Rainone C, Schiavo L, et al. Post-bariatric hypoglycemia is associated with endothelial dysfunction and increased oxidative stress. Biomedicines. 2022;10(4):916.
Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40.
Barrea L, Muscogiuri G, Pugliese G, de Alteriis G, Maisto M, Donnarumma M, et al. Association of trimethylamine N -oxide (TMAO) with the clinical severity of hidradenitis suppurativa (acne inversa). Nutrients. 2021;13(6):1997.
Barrea L, Muscogiuri G, Pugliese G, Graziadio C, Maisto M, Pivari F, et al. Association of the chronotype score with circulating trimethylamine N -Oxide (TMAO) concentrations. Nutrients. 2021;13(5):1671.
Yu W, Xu C, Li G, Hong W, Zhou Z, Xiao C, et al. Simultaneous determination of trimethylamine N-oxide, choline, betaine by UPLC-MS/MS in human plasma: an application in acute stroke patients. J Pharm Biomed Anal. 2018;152:179–87.
Beale R, Airs R. Quantification of glycine betaine, choline and trimethylamine N-oxide in seawater particulates: minimisation of seawater associated ion suppression. Anal Chim Acta. 2016;938:114–22.
Barrea L, Caprio M, Camajani E, Verde L, Perrini S, Cignarelli A, et al. Ketogenic nutritional therapy (KeNuT)-a multi-step dietary model with meal replacements for the management of obesity and its related metabolic disorders: a consensus statement from the working group of the Club of the Italian Society of Endocrinology (SIE)-diet therapies in endocrinology and metabolism. J Endocrinol Invest. 2024;47:487–500.
Lorenz MW, Graf M, Henke C, Hermans M, Ziemann U, Sitzer M, et al. Anthropometric approximation of body weight in unresponsive stroke patients. J Neurol Neurosurg Psychiatry. 2007;78(12):1331–6.
da Silva BR, Orsso CE, Gonzalez MC, Sicchieri JMF, Mialich MS, Jordao AA, et al. Phase angle and cellular health: inflammation and oxidative damage. Rev Endocr Metab Disord. 2023;24(3):543–62.
Barrea L, Fabbrocini G, Annunziata G, Muscogiuri G, Donnarumma M, Marasca C, et al. Role of nutrition and adherence to the mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11(1):57.
Barrea L, Macchia PE, Di Somma C, Napolitano M, Balato A, Falco A, et al. Bioelectrical phase angle and psoriasis: a novel association with psoriasis severity, quality of life and metabolic syndrome. J Transl Med. 2016;14(1):130.
Chopra D, Arens RA, Amornpairoj W, Lowes MA, Tomic-Canic M, Strbo N, et al. Innate immunity and microbial dysbiosis in hidradenitis suppurativa—vicious cycle of chronic inflammation. Front Immunol. 2022;13: 960488.
Lelonek E, Bouazzi D, Jemec GBE, Szepietowski JC. Skin and gut microbiome in hidradenitis suppurativa: a systematic review. Biomedicines. 2023;11(8):2277.
Tzellos T, Zouboulis CC, Gulliver W, Cohen AD, Wolkenstein P, Jemec GB. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015;173(5):1142–55.
Fomin DA, Handfield K. The ketogenic diet and dermatology: a primer on current literature. Cutis. 2020;105(1):40–3.
Paoli A, Grimaldi K, Toniolo L, Canato M, Bianco A, Fratter A. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25(3):111–7.
Sinha S, Lin G, Ferenczi K. The skin microbiome and the gut-skin axis. Clin Dermatol. 2021;39(5):829–39.
Fabbrocini G, Bertona M, Picazo O, Pareja-Galeano H, Monfrecola G, Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes. 2016;7(5):625–30.
Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17(2):114–22.
Kim J, Ko Y, Park YK, Kim NI, Ha WK, Cho Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26(9):902–9.
Kim MJ, Kim KP, Choi E, Yim JH, Choi C, Yun HS, et al. Effects of Lactobacillus plantarum CJLP55 on clinical improvement, skin condition and urine bacterial extracellular vesicles in patients with acne vulgaris: a randomized, double-blind, placebo-controlled study. Nutrients. 2021;13(4):1368.
Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic diet and microbiota: friends or enemies? Genes (Basel). 2019;10(7):534.
Paoli A, Cenci L, Pompei P, Sahin N, Bianco A, Neri M, et al. Effects of two months of very low carbohydrate ketogenic diet on body composition, muscle strength, muscle area, and blood parameters in competitive natural body builders. Nutrients. 2021;13(2):374.
Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138(12):1584–90.
Cong TX, Hao D, Wen X, Li XH, He G, Jiang X. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311(5):337–49.
Gu H, An HJ, Gwon MG, Bae S, Leem J, Lee SJ, et al. Bee venom and its major component melittin attenuated cutibacterium acnes- and IGF-1-induced acne vulgaris via inactivation of Akt/mTOR/srebp signaling pathway. Int J Mol Sci. 2022;23(6):3152.
Leo MS, Sivamani RK. Phytochemical modulation of the Akt/mTOR pathway and its potential use in cutaneous disease. Arch Dermatol Res. 2014;306(10):861–71.
Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36(1):29–40.
Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25(9):545–55.
Barrea L, Caprio M, Watanabe M, Cammarata G, Feraco A, Muscogiuri G, et al. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit Rev Food Sci Nutr. 2022;63:1–17.
Google Scholar
Monib KME, El-Fallah AA, Salem RM. Inflammatory markers in acne vulgaris: saliva as a novel diagnostic fluid. J Cosmet Dermatol. 2022;21(3):1280–5.
Namazi MR, Parhizkar AR, Jowkar F. Serum levels of hypersensitive-C-reactive protein in moderate and severe acne. Indian Dermatol Online J. 2015;6(4):253–7.
Download references
Acknowledgements
Not applicable.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Ludovica Verde, Evelyn Frias-Toral and Sara Cacciapuoti contributed equally to this work as co-first.
Giovanna Muscogiuri and Luigi Barrea contributed equally to this work as co-last.
Authors and Affiliations
Department of Public Health, University of Naples Federico II, Via Sergio Pansini 5, 80131, Naples, Italy
Ludovica Verde
Centro Italiano per la cura e il Benessere del Paziente con Obesità (C.I.B.O), Unità di Endocrinologia, Diabetologia e Andrologia, Dipartimento di Medicina Clinica e Chirurgia, Università degli Studi di Napoli Federico II, Via Sergio Pansini 5, 80131, Naples, Italy
Ludovica Verde, Annamaria Colao, Silvia Savastano, Giovanna Muscogiuri & Luigi Barrea
School of Medicine, Universidad Espíritu Santo, Samborondón, 0901952, Ecuador
Evelyn Frias-Toral
Section of Dermatology-Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy
Sara Cacciapuoti, Matteo Megna & Luca Potestio
Centro de Investigación en Salud Pública y Epidemiología Clínica (CISPEC), Facultad de Ciencias de la Salud Eugenio Espejo, Universidad UTE, Quito, 170129, Ecuador
Daniel Simancas-Racines
Dipartimento di Scienze Biomediche avanzate, Università Degli Studi Di Napoli Federico II, Naples, Italy
Giuseppina Caiazzo
Department of Pharmacy, University of Naples “Federico II”, Naples, Italy
Maria Maisto & Gian Carlo Tenore
Unità di Endocrinologia, Diabetologia e Andrologia, Dipartimento di Medicina Clinica e Chirurgia, Università degli Studi di Napoli Federico II, Via Sergio Pansini 5, 80131, Naples, Italy
Annamaria Colao, Silvia Savastano & Giovanna Muscogiuri
Cattedra Unesco “Educazione Alla Salute E Allo Sviluppo Sostenibile”, University Federico II, Naples, Italy
Annamaria Colao & Giovanna Muscogiuri
Dipartimento di Benessere, Nutrizione e Sport, Università Telematica Pegaso, Centro Direzionale, Via Porzio, Isola F2, 80143, Naples, Italy
Luigi Barrea
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: LB and GM; data curation; LV; formal analysis LB; methodology MM; supervision: AC, SS and GCT; roles/writing—original draft: LV, EFT, SC, DSR, MM, GC and LP; writing—review & editing. LV, GM and LB. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Daniel Simancas-Racines .
Ethics declarations
Ethics approval and consent to participate.
The study was conducted in accordance with the guidelines outlined in the Declaration of Helsinki, which provides ethical principles for medical research involving human subjects. Additionally, the Ethics Committee of the Federico II University of Naples reviewed the study procedures and granted a positive opinion on the study protocol (reference no. 50/20).
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Schematic representation of the active phase of VLCKD according to KeNuT multisteps dietary protocol with meal replacements proposed by the Club of the Italian Society of Endocrinology (SIE)—Diet Therapies in Endocrinology and Metabolism.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Verde, L., Frias-Toral, E., Cacciapuoti, S. et al. Very low-calorie ketogenic diet (VLCKD): a therapeutic nutritional tool for acne?. J Transl Med 22 , 322 (2024). https://doi.org/10.1186/s12967-024-05119-5
Download citation
Received : 28 January 2024
Accepted : 20 March 2024
Published : 31 March 2024
DOI : https://doi.org/10.1186/s12967-024-05119-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Very low-calorie ketogenic diet
- Ketogenic diet
- Inflammation
- Oxidative stress
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10810529

CT-based screening of sarcopenia and its role in cachexia syndrome in pancreatic cancer
Ekaterina khristenko.
1 Department of Diagnostical and Interventional Radiology, Heidelberg University Hospital, Heidelberg, Germany
Valentin Sinitsyn
2 Department of Radiology at Medical Educational and Scientific Center University Hospital, Lomonosov Moscow State University, Moscow, Russia

Tatiana Rieden
3 Institute for Diagnostical and Interventional Radiology, Ludwigshafen Clinical Hospital, Ludwigshafen am Rhein, Germany
Parker Girod
4 University College Dublin School of Chemistry, Dublin, Ireland
Hans-Ulrich Kauczor
Philipp mayer, miriam klauss, vladimir lyadov.
5 Moscow City Clinical Cancer Hospital No. 1, Oncology No. 4, Moscow, Russia
Associated Data
All relevant data are within the manuscript and its Supporting Information files.
Since computed tomography (CT) is a part of standard diagnostic protocol in pancreatic ductal adenocarcinoma (PDAC), we have evaluated the value of CT for sarcopenia screening in patients with PDAC, intending to expand the diagnostic value of tomographic studies. In our study, we included 177 patients with available CT images. Two groups were formed: Group 1 consisted of 117 patients with PDAC in various locations and stages and Group 2, or the control group, consisted of 60 "nominally healthy" patients with other somatic non-oncological diseases. The body mass index (BMI) was defined as a ratio of patient’s weight to the square of their height (kg/m 2 ). CT-based body composition analysis was performed using commercially available software with evaluation of sarcopenia using skeletal muscle index (SMI, cm 2 /m 2 ). Based on the SMI values, sarcopenia was found in 67.5% of patients (79 out of 117) in the first patient group. It was found more frequently in males (42 out of 56; 75%) than in females (37 out of 61; 60.6%). Additionally, we observed a decrease in muscle mass (hidden sarcopenia) in 79.7% in patients with a normal BMI. Even in overweight patients, sarcopenia was found in 50% (sarcopenic obesity). In patients with reduced BMI sarcopenia was found in all cases (100%). Statistically significant difference of SMI between two groups was revealed for both sexes (p = 0,0001), with no significant difference between groups in BMI. BMI is an inaccurate value for the assessment of body composition as it does not reflect in the details the human body structure. As SMI may correlate with the prognosis, decreased muscle mass- especially "hidden" sarcopenia or sarcopenic obesity- should be reported. The use of CT-based evaluation of sarcopenia and sarcopenic obesity will allow for a better treatment response assessment in patients with cancer cachexia.
Introduction
Cachexia syndrome is a common condition in various pathological entities including, cancer, sepsis, chronic heart disease, and chronic obstructive pulmonary disease (COPD) [ 1 ]. Most definitions of cachexia in previous studies commonly described it as a multidimensional syndrome or condition, which include such contributing factors as anorexia, weight loss, chemosensory distortion, inflammation, hypermetabolism, and early satiety. Cachexia led to such outcomes as anaemia, asthenia, psychosocial distress, dyspnoea, dependency upon third persons, toxicity of chemotherapy, and death [ 2 ]. Recently, the number of publications in metabolic imaging in general, and cancer cachexia in particular, increased dramatically. Many of them define muscle depletion, or sarcopenia, as a key factor in the development of cancer cachexia syndrome, thus changing the definition of cachexia.
In the literature, cancer cachexia is described as a multifactor syndrome, which is characterized by an ongoing loss of skeletal muscle mass which can take place with or without simultaneous loss of fat mass. Patients with cancer cachexia syndrome don’t fully respond to conventional nutritional support, which leads to progressive functional impairment. In terms of pathophysiology, this syndrome is defined by a negative protein and energy balance which is caused by a combination of abnormal metabolism and reduced food intake [ 3 ]. The universally accepted diagnostic criteria for cachexia is weight loss of >5% in the last 6 months or weight loss of > 2% in patients already showing depletion according to current bodyweight and height (body-mass index <20 kg/m) or skeletal muscle mass.
Tumors and inflammatory diseases of the pancreas are often accompanied by the development of severe metabolic disorders associated with weight loss, changes in body structure, and, in particular, a decrease in muscle mass (sarcopenia). The revised European Working Group on Sarcopenia in Older People (EWGSOP2) definition of sarcopenia suggests using muscle strength as the primary diagnostic criterium [ 4 – 7 ]. However, the vast majority of studies showing prognostic value of sarcopenia in cancer used CT-based calculations of muscle mass with specific cut-offs [ 8 ]. Despite multiple studies in various cancer type show the importance of CT-based sarcopenia screening [ 8 ], the assessment of body composition in pancreatic cancer patients is still far from being routine which is the underlying reason for our study.
Many studies show that in clinical practice, the analysis of body composition allows estimation of isolated loss of muscle mass (sarcopenia) in cancer patients, thereby suggesting the presence of cancer cachexia and assessing the rate of its progression. Changes in the physical condition of the patients may signal risks of performing invasive procedures and predetermine the overall prognosis [ 9 – 11 ]. Today, the diagnostic imaging studies, such as multi-detector computed tomography (MDCT) and magnetic resonance imaging (MRI), are widely used to estimate the body composition and muscle area and mass [ 12 ].
Since computed tomography (CT) is a part of standard diagnostic protocol in pancreatic ductal adenocarcinoma (PDAC), we have evaluated the value of CT for sarcopenia screening in patients with PDAC, intending to expand the diagnostic value of tomographic studies. Because low reproducibility may be a result of a poor scan level selection, a clear anatomical reference point (skeletal marker, not the soft tissue or organs) and a standardized approach to calculating the "skeletal-muscular index of L3" is important for analyzing body composition [ 13 ].
Ethics approval and consent
Our study was performed according to the Declaration of Helsinki. The local ethical review board of Russian Medical Academy of Continuing Medical Education (RMANPO) issued an ethical approval for the study without further restrictions (from 21 st of October 2014, N8). A written informed consent was obtained from the study participants. All relevant data are within the manuscript and its Supporting Information files.
Patient selection and data collection
We included 177 patients in our study (91 males and 86 females), who underwent diagnostical studies and therapy from March 2009 till April 2014. Group 1 included 117 patients with histologically proven pancreatic cancer (PC) (mean age 64,8±10,5; females– 48%, males 52%). There were 54 (46%) patients with stages I-II (preoperative evaluation) and 63 unresectable stage III-IV (54%) patients. G roup 2 included 60 patients who got a CT of the abdomen because of a clinical suspicion of pancreatic disorder but no proven cancer of the pancreas (mainly chronic pancreatitis). There was no statistically significant difference with Group 1 parameters including age and sex distribution. The patients with resectable PC undergone the surgical treatment und those with unresectable stage–chemotherapy.
The body mass index (BMI) was defined as a ratio of patient’s weight to the square of their height (kg/m 2 ). The reference values were the generally accepted values of 18.6 kg/m 2 -24.9 kg/m 2 , without sex differentiation.
Radiological methods and assessment
In this study, we evaluated unenhanced CT-scans with 1.5 mm slice thickness as they provide sufficient diagnostic information for assessment of body composition. CT examinations were carried out using Somatom Sensation MDCT-scanner (64 rows, Siemens) and Discovery 750 dual energy CT-scanner (GE Healthcare). All studies included unenhanced phase, which was used for the analysis. The technical parameters of the studies were the following: 120 kW tube voltage and 170 mA tube current. During the study, the patients were instructed to hold their breath for 9–12 seconds. The range of radiation dose was from 4 to 25 mSv (cumulative dose). For postprocessing, we used the Slice-O-Matic body composition analysis software by TomoVision (Montreal, Canada).
Evaluation of muscle mass (sarcopenia estimation) was performed by one radiologist (EK, 10 years of experience in abdominal imaging) in all the cases. The mean time for the analysis of each patient for the manual assessment was also evaluated.
For the differentiated estimation of the body structure, the muscle tissue area (cm 2 ) was determined by two consecutive axial slices performed at the body level of the third lumbar vertebrae. We selected all the striated muscles which included m. psoas major, m. quadratus lumborum, m. erector spinae, m. obliquus internus abdominis, m. obliquus externus abdominis, m. rectus abdominis, and m. transversus abdominis on each of the two following axial CT slices. Then, the sum of the muscle areas for each slice was calculated automatically, followed by the calculation of the arithmetic mean. The ratio of the obtained skeletal muscle area at the L3 level to the patient’s height squared was the "skeletal-muscular index of L3" (SMI, cm 2 /m 2 ). The cut-off values of SMI for sarcopenia were accepted according to the recommendations of the leading research team in this field by V. Baracos [ 1 , 12 , 14 – 18 ]. SMI values, equal or less than 52.4 cm 2 /m 2 for males and 38.5 cm 2 /m 2 for females, correspond to CT-based sarcopenia defined as a state in which the percentage of muscle mass is two or more standard deviations lower than its average values in healthy adults of the same age and sex [ 3 , 19 , 20 ]. Fat tissue area was also calculated- separate for the visceral fat area (VFA) and for the subcutaneous fat area (SFA).
Statistical analysis
Data management was carried out by SAS software release 9.4 (SAS Institute, Cary, North Carolina, USA) and statistical analysis was made using IMB SPSS software, version 24 (IBM Corp.). Quantitative variables presented as medians. Mann–Whitney U test was performed to compare continuous parameters between groups. For categorial parameters, absolute numbers are shown. Two-sided p-values were computed, the differences were considered statistically significant at a P-value of 0.05 or less.
Based on the SMI values, CT-based sarcopenia was found in 67.5% (79 out of 117) of the examined patients in the first patient group with pancreatic ductal adenocarcinoma. In the same patient group, CT-based sarcopenia was more frequently found in males (42 out of 56; 75%) than in females (37 out of 61; 60.6%) although, in both groups sarcopenic patients prevailed ( Fig 1 ). We compared the SMI values with the BMI values as the detection of decreased muscle mass in normal weight and overweight patients, so called “hidden sarcopenia”, is of great clinical importance. The results of this comparison are shown in Table 1 .

Table 2 and Fig 2 show the ratios between BMI and SMI in patients with PDAC (Group 1). In patients with normal BMI, we observed a decrease in muscle mass (hidden sarcopenia) in 79.7% of cases. Even in overweight patients (increased BMI), CT-based sarcopenia was found in 50% of cases. Reduced BMI was found in 6 patients with PC and CT-based sarcopenia was found in all cases (100%). It is noteworthy that CT-based sarcopenia was found more frequently in patients with cancer as the BMI value decreases. However, there was no reliable correlation between these two indicators (p> 0.05).

Comparative sex-specific indicators are shown in Table 3 . We detected statistically significant SMI differences between males and females in the group of patients with pancreatic cancer (Group 1), with questionable differences in BMI values.
In the second group, the mean SMI value was 57.61±3.9 cm 2 /m 2 for males and 43.14±3.2 cm 2 /m 2 for females. Signs of CT-based sarcopenia with an evident weight loss (SMI 37.3 cm 2 /m 2 ; BMI 15.9 kg/m 2 ) were revealed only in one patient from this group—a 43-year-old man with chronic pancreatitis and alcohol abuse.
Fig 3 presents a comparative analysis of the skeletal-muscular index in the first and second group. The statistically significant difference between two groups was revealed for both males and females (p = 0.0001). A different distribution of fat in male and female patients was observed, however, without a statistically significant difference in BMI. Fig 4 shows CT-studies in two different patients (male and female) with the same body mass index (BMI = 24.6 kg/m 2 ), but with different types of fat distribution. A similar distribution of fatty tissue was observed in 64.3% of the males and 73.2% of females examined.

Axial segmented tomograms of two different patients with the same BMI, demonstration obesity of two different types with different distribution of fatty tissue: A—visceral obesity in men due to excess visceral fat tissue; B—subcutaneous obesity in women due to excess subcutaneous fat tissue.
The results of the gender analysis, accounting for SMI, did not reveal a correlation of BMI with the body structure in patients with PDAC. At the same time, the types of fat distribution in men and women were different.
Fig 5 shows the axial slices of two different patients with a healthy BMI value (22.1 kg/m 2 ). In the first patient, both BMI and SMI corresponded to normal values whereas in the second patient, the BMI also corresponded to normal values, but sarcopenia was observed. This is explained by the large area of adipose tissue and reduced muscle tissue area.

A and B: a patient without sarcopenia, both BMI and SMI are within the normal range, 22.1 kg/m 2 and SMI 53.5 cm 2 /m 2 , respectively. A—initial CT-scan on the level of L3 vertebral body; B—segmented CT scan; the area of muscle tissue was 179.2 cm 2 ; the total area of fatty tissue was 102.5.2 cm 2 .BA C and D: a patient with sarcopenia, BMI was normal with 22.1 kg/m 2 , SMI was decreased with 36.2 cm 2 /m 2 .DC A—initial CT image, performed at the L3 vertebral body level; B—segmented CT tomogram; the area of muscular tissue was 122.1 cm 2 ; the total area of fatty tissue was 232.2 cm 2 .
As clinical example of “hidden” sarcopenia, we would like to present the following observation: male 49-year-old patient with pain in the epigastric area, weakness, loss of appetite, and weight loss (8 kg) within 2 previous months. A pancreatic ductal adenocarcinoma in pancreatic body was diagnosed and pathohistologically verified. Standard diagnostical CT protocol was extended with an assessment of BMI and SMI ( Fig 6 ). By objectively excess weight (increased BMI: 27.7 kg/m 2 , normal values of 18.5 kg/m 2 - 25 kg/m 2 ), a significant reduction of SMI to 30.3 cm 2 /m 2 (at normal values of >52.4 cm 2 /m 2 ) was detected.

BMI was increased with 27.7 kg/m 2 , SMI was decreased with 30.3 cm 2 /m 2 .DC A—initial CT image; B—segmented CT scan. The area of muscle tissue was 87.5 cm 2 , the total area of fat tissue—481.8 cm 2 .
The average time spent by a radiologist on CT image processing to calculate the "skeletal-muscular index" was 10±3 minutes.
Sarcopenia was a very common condition in the first group of patients with pancreatic ductal adenocarcinoma (67.5%). Our results correspond to recent results of Chan W.Y. and Chok, who found sarcopenia in up to 65% of patients with PDAC and its association with poor survival outcomes after surgical treatment and chemotherapy [ 21 ]. In our group of PDAC patients, sarcopenia was present in patients with normal and with increased BMI. Thus, the results of our study allow us to consider BMI an inaccurate value for the assessment of body composition, as it does not reflect in the details of the human body structure.
Recently obtained evidence on the leading role of sarcopenia in the development of cachexia is of great clinical importance and allows a new look at many clinical problems. For example, today, the main criterion for the effectiveness of nutritional support is the increase in body weight. Meanwhile, in some cases, stabilization or increase in body weight may be due to an increase in the adipose tissue or edema with the background of increasing sarcopenia. Undoubtedly, the use of sectional imaging as CT and MRI allows a better treatment response assessment in cachexia.
Another important clinical aspect is the impact of sarcopenia on the toxicity of chemotherapy. Differences in toxicity can be attributed mainly to the heterogeneous composition of the patient’s body, which is vital for calculating the dosage of the chemotherapy drug. In this situation, the use of CT or MRI for the analysis of body composition seems to be reasonable since it does not require any additional examinations other than a priori used in the treatment of cancer patients.
Sarcopenic obesity is a clinical and functional condition which is defined by the coexistence of extended fat mass and sarcopenia. Nowadays, different definitions of sarcopenic obesity coexist and the diagnostic criteria and cut-offs are not established by consensus [ 22 ]. In our group of patients with pancreatic ductal adenocarcinoma, we reported sarcopenic obesity in patients with increased BMI and the presence of sarcopenia which was found in 56.4% of patients. Detection of this condition in patients with potentially resectable PDAC is important as it was shown to have higher morbidity and mortality risks and increased risk of developing such postoperative complication as postoperative pancreatic fistula [ 23 , 24 ].
There is also impact of sarcopenic obesity on survival rate in patients undergoing chemotherapy to consider. In the study by Dalal et al. [ 25 ], patients with unresectable locally advanced PDAC were treated with bevacizumab in combination with capecitabine and radiation. The association of an increased loss in SMI of more than 3.8% and poorer survival rates were found (p = 0.02). In patients with sarcopenic obesity, the effect on survival rate was especially evident. According to the results of study by Kays et al. with retrospective design [ 26 ], 6 out of 53 patients with advanced PDAC, who received FOLFIRINOX had sarcopenic obesity. PDAC patients had a significantly shorter median overall survival compared with the other patients that were included in the study (10.4 months vs 16.1 months; p = 0.04). Similar results were shown in other groups of patients, for example in patients with lung and colon cancer. Prado et al . found [ 20 ] that sarcopenia was present in 15% of obese patients with lung and colon cancer. The mean survival in patients with sarcopenia was significantly lower than in patients without sarcopenia (1 and 21 months, respectively; p<0.001). In multifactor analysis, sarcopenic obesity had the most influence on the survival rate.
Sarcopenia has a clinical prevalence not only in chronic conditions and oncologic patients, but in acute conditions as well. Yoon et al. [ 27 ] reported a strong correlation of high visceral fat with low skeletal muscle volume with severity of acute pancreatitis. Visceral fat-to-muscle ratio had a stronger correlation with severity of acute pancreatitis than body weight or BMI. We also found BMI incapable of accurately assessing the body composition as well as the changes in body compositions within the time period completed.
One of the points of the study was the investigation of the length of time spent by a radiologist when calculating SMI. The average time spent by a radiologist on CT image processing to calculate the "skeletal-muscular index" was 10±3 minutes. At the same time, there was a tendency for this time to decrease from 10 to 5 minutes within one month as experience accumulated. Assessment of the patient’s body composition does not carry radiation exposure, as there is no need for additional scanning. Detection of sarcopenia in patients with PC requires only retrospective image analysis, which certainly increases the capabilities of the MDCT.
Recently, Paris MT et al. showed that it is possible to perform an automated body composition analysis using neural networks [ 28 ] where network segmentation took approximately 350 milliseconds/scan using modern computing hardware. The network showed excellent ability to analyze diverse body composition phenotypes and clinical cohorts which can create feasible opportunities to advance our capacity to predict health outcomes in clinical populations.
Since the SMI may be related to the clinical prognosis, it is advisable to report the presence or absence of sarcopenia and the changes in the patient’s body structure during prospective observation. The information about possible metabolic disorders reflected in the body structure is beneficial for physicians in the context of treatment planning.
The routine assessment of body composition with CT and MRI, and sarcopenia in particular, should be integrated in the diagnostic reports in the same way as densitometry in the diagnosis of osteoporosis.
A differentiated approach to assessing body structure allows for a more accurate understanding of the clinical status of patients and the effectiveness of treatment. As skeletal muscle index may correlate with the prognosis, a radiologist should report decreased muscle mass (especially "hidden" sarcopenia or sarcopenic obesity) and the changes in the patients’ body structure. Even today this assessment in the daily clinical practice is far from being routine. Additionally, the use of CT will allow a better treatment response assessment in cachexia. The information about possible metabolic disorders reflected in the body structure is very useful for physicians in the context of treatment planning.
The most important aspect is the influence of sarcopenia on the toxicity of chemotherapy. Differences in toxicity can be explained mainly by the heterogeneous composition of the patients’ bodies, including patients with the same mass and body surface area. In this situation, the use of MDCT for the analysis of body composition is justified since it does not require any additional scanning as it is widely used in the primary diagnostic and treatment assessment of oncologic patients.
Assessment of sarcopenia and sarcopenic obesity should be integrated in clinical management of pancreatic ductal adenocarcinoma. All oncological patients that receive diagnostic CT studies can and should be screened for sarcopenia. However, until recently, this analysis required special software and medical personnel with sufficient skills. Today the emerging role of neural networks in assessment of body composition shows promising results and allows a cut in time of radiological assessment dramatically.
Supporting information
Funding statement.
The author(s) received no specific funding for this work.
Data Availability
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 21 March 2024
Body odor samples from infants and post-pubertal children differ in their volatile profiles
- Diana Owsienko ORCID: orcid.org/0000-0001-6617-7579 1 ,
- Lisa Goppelt 1 ,
- Katharina Hierl 2 ,
- Laura Schäfer 2 ,
- Ilona Croy 2 , 3 &
- Helene M. Loos ORCID: orcid.org/0000-0002-9112-5735 1 , 4
Communications Chemistry volume 7 , Article number: 53 ( 2024 ) Cite this article
415k Accesses
451 Altmetric
Metrics details
- Bioanalytical chemistry
- Chemical ecology
- Mass spectrometry
Body odors change during development, and this change influences the interpersonal communication between parents and their children. The molecular basis for this chemical communication has not been elucidated yet. Here, we show by combining instrumental and sensory analyses that the qualitative odorant composition of body odor samples is similar in infants (0-3 years) and post-pubertal children (14-18 years). The post-pubertal samples are characterized by higher odor dilution factors for carboxylic acids and by the presence of 5α-androst-16-en-3-one and 5α-androst-16-en-3α-ol. In addition to the olfaction-guided approach, the compounds 6-methylhept-5-en-2-one (6MHO), geranyl acetone (GA) and squalene (SQ) were quantified. Both age groups have similar concentrations of 6MHO and GA, whereas post-pubertal children tend to have higher concentration of SQ. In conclusion, sexual maturation coincides with changes to body odor chemical composition. Whether those changes explain differences in parental olfactory perception needs to be determined in future studies with model odors.
Similar content being viewed by others

Pervasive environmental chemicals impair oligodendrocyte development
Erin F. Cohn, Benjamin L. L. Clayton, … Paul J. Tesar

The hidden fitness of the male zebra finch courtship song
Danyal Alam, Fayha Zia & Todd F. Roberts
A physicochemical-sensing electronic skin for stress response monitoring
Changhao Xu, Yu Song, … Wei Gao
Introduction
Chemosensory information conveyed by body odors (BOs) can play a role in social relationships, whether between friends 1 , 2 , partners 3 , 4 , or within families 5 , 6 . BOs contribute differently to interpersonal communication between parents and their offspring at different stages of development. Already shortly after birth, infants learn to recognize their mother´s individual odor 7 , 8 , 9 , 10 and parents are able to identify their own infant’s BO, which is preferred over the smell of other infants 11 , 12 , 13 . BOs of infants are pleasant and rewarding to mothers 14 , 15 , and, as such, probably facilitate parental affection. In contrast, BOs of pubertal children are rated as less pleasant and parents are unable to identify their own child during this developmental stage 12 , 16 , 17 . Some studies even show a parental aversion towards the BO of their opposite sex pubertal children, which may serve to prevent inbreeding 5 , 12 . BO composition thus changes during the stages of development. Chemosensory cues indicating a child’s developmental status 18 are still unknown but can be expected to be related to the onset of physiological changes during puberty, notably the activation of apocrine and apoeccrine sweat glands in the axillary, perineal, genital and anogenital regions 19 , 20 and increased sebum secretion 20 , 21 , 22 . For these reasons, sweat and sebum can be hypothesized to differ qualitatively and quantitatively in line with a child’s developmental status.
So far, only a few researchers have studied the composition of children´s BO. In a pilot study, Uebi et al. 23 tentatively identified 31 compounds in samples obtained from infants’ heads, using MonoTrap silica beads for sampling of volatiles, followed by thermodesorption-comprehensive gas chromatography-time of flight-mass spectrometry (TD-GCxGC-ToF-MS) analysis. Whereas the proportion of several aldehydes (e.g., hexanal, heptanal, octanal, nonanal) did not differ between the analyzed samples, the proportions of the corresponding carboxylic acids (e.g., hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid) were higher in samples obtained from 2–3 day-old infants ( n = 3) compared to those of 1 h-old infants ( n = 2). The reason may be oxidation processes that occur at birth but not in utero . Further, Lam et al. 24 investigated the microbial basis of malodor production in pre-pubertal (5-9 years) and post-pubertal (15-18 years) children. The microbiome of the underarm, neck and head was sampled before and after exercise. For this purpose, sport activities, e.g., treadmill, indoor cycling, aerobics, and basketball, were performed for a duration of 4–5 h, including intervals of physical exercise of 10–15 min followed by 10–15 min breaks. Odor intensity and odor quality of the different body sites were rated by a professional perfumer, before and after exercise. Post-pubertal underarm odor was rated as having a higher intensity than underarm odor of pre-pubertal children (corresponding to findings by Schäfer et al. 17 ). Pre-pubertal BOs were mainly described as sour while post-pubertals’ BOs were mainly described as sour/sulfurous. Subsequent gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O) experiments with pooled and incubated sweat samples of both age groups were conducted to identify malodor-associated volatiles. In general, similar GC-MS and GC-O patterns were found for pre-pubertal and post-pubertal children, and the odorants acetic acid and 3-methylbutanoic acid were more abundant in the post-pubertal samples. These two odorants seemed to be the main contributors to the sour BO. Staphylococcus species were primarily involved in the production of these odorants from precursors in sweat, whereas Corynebacterium species had a lower impact on malodor in that study.
In sum, previous studies have shown developmental differences in children’s BOs. Principles of axillary malodor formation in adults are well-known 25 , 26 , 27 , 28 . However, no one has yet directly compared the chemical composition of BOs from infants and post-pubertal children. To study changes in BOs over this more extended period of development, we characterized the BO profiles of infants (0-3 years) and post-pubertal children (14-18 years) by performing direct contact sampling with cotton pads in the axillary region, solvent extraction and subsequent GC-O and GC-MS analysis. In the first part of the study, samples were analyzed using GC-O. Results revealed that the qualitative composition of odorants was similar in both age groups, mainly dominated by aldehydes and carboxylic acids. In the post-pubertal samples, higher odor dilution factors (OD) of carboxylic acids and 5α-androst-16-en-3-one as well as 5α-androst-16-en-3α-ol were determined. In the second part of the study, the target compounds 6-methylhept-5-en-2-one (6MHO), geranyl acetone (GA), and squalene (SQ) were selected based on a pilot study and quantified by GC-MS. Concentrations of 6MHO and GA were similar for both age groups, SQ tended to be higher in post-pubertal samples. In conclusion, developmental changes go along with an altered BO composition.
We used two independent approaches to characterize the composition of the BO samples. The first approach (‘Identification of odor-active compounds’) aimed to identify odor-active compounds in the BO samples by GC-O and estimate their potential contribution to BO by applying OEDA (for the significance and the limitations of this approach, see Grosch (2001) 29 ). After tentative identification by GC-O, subsequent GC-MS analysis was conducted to complete identification by recording mass spectra, if possible (see Table 1 ). GC-O allows detection of trace compounds with low odor thresholds, which can be below LOD of instrumental detection systems, and is therefore essential in odor analysis. The second approach (‘Quantification of target compounds’) aimed to quantify target compounds by GC-MS independently of the first approach, which were selected based on outcomes of pilot study 1 (see Supplementary Note 1 online) indicating a potential difference between age groups. Please see the subsections ‘BO donors’ and ‘BO sampling procedure’ of the Material and Methods section for a study overview.
Identification of odor-active compounds
In total, 42 odor-active compounds were detected in at least two out of three BO sample pools per age group. Out of these, 16 odorants were tentatively identified based on their retention index (RI) and odor quality in comparison with reference compounds, and 15 odorants were identified by additional comparison with mass spectra. Lastly, 11 compounds remained unidentified. Table 1 lists the (tentatively) identified odor-active compounds with their respective average OD factors (of the three sampling pools per age group) in the BO samples and in the room samples. The table also shows which of these substances were also detected in unexposed cotton pads, perfume-free shower gel and detergent. Among the detected odor-active compounds, aldehydes represented the largest class of substances, including octanal (soapy, citrus-like), nonanal (soapy, citrus-like), decanal (soapy), ( E )-non-2-enal (fatty, cardboard-like), undecanal (soapy, citrus-like, coriander-like), dodecanal (soapy, coriander-like), (2 E ,4 E )-nona-2,4-dienal (fatty, nutty), (2 E ,4 E )-deca-2,4-dienal (fatty, deep-fried), and ( E )-4,5-epoxy-( E )-2-decenal (metallic). These aldehydes were on average between OD 1 and OD 277, and no definite trend between age groups was observed. The second largest group of odorants consisted of carboxylic acids: 2-methylheptanoic acid (fruity, dried plum-like), dodecanoic acid (wax-like, soapy), and myristoleic acid (earthy, green/grassy, green bell pepper-like), all of which were exclusively detected in the BO samples (OD factor range: OD 1 to OD 171). Further, 3-methylbutanoic acid (cheesy), octanoic acid (musty, coriander-like, fatty), and 4-ethyloctanoic acid (goat-like) had higher OD factors in the BO samples than in the room samples (OD 1 to OD 384). All of these carboxylic acids were detected with higher OD factors in the post-pubertal samples compared to the infant samples. In the group of ketones, oct-1-en-3-one (mushroom-like), raspberry ketone (raspberry-like), α-isomethylionone (flowery, soapy), β-ionone (flowery, violet-like), and the monoterpene ketone geranyl acetone (soapy) were present within a range of OD 2 to OD 429. Among these, α-isomethylionone was perceived with a higher average OD factor in the infant BO samples compared to the post-pubertal BO samples. Whereas for the alcohols polysantol (sandalwood-like), sandranol (sandalwood-like), and the monoterpene alcohol linalool (flowery), ranging between OD 3 and OD 203, no obvious difference between age groups emerged, an unknown odor (sandalwood-like, perfume-like) and the sesquiterpene alcohol patchouli alcohol (earthy) were detected with a higher average OD factor in the post-pubertal BO samples. The lactones γ-undecalactone (peach-like, soapy), γ-dodecalactone (peach-like, flowery), and sotolone (celery-like) as well as the phenol-derivate p -cresol (fecal, horse stable-like) and benzaldehyde-derivate vanillin (vanilla-like), perceived in a range of OD 1 to OD 256, revealed no further trends between age groups. Last, for the two age groups, samples differed with regard to the odor-active steroids 5α-androst-16-en-3-one (sweaty, urinal, musk-like) and 5α-androst-16-en-3α-ol (sandalwood-like, musk-like), since these were exclusively detected in the post-pubertal samples.
Quantification of target compounds
In infant BO samples, the average concentrations (±standard error), calculated from the three pools, of 6-methylhept-5-en-2-one (6MHO), geranyl acetone (GA), and squalene (SQ) were 2.7 ± 0.1 µg ml −1 , 33.2 ± 20.1 µg ml −1 , and 8.4 × 10 3 ± 2.6 × 10 3 µg ml −1 , respectively. In the corresponding room samples, 6MHO and GA occurred in concentrations of 0.6 ± 0.1 µg ml −1 and 18.9 ± 6.2 µg ml −1 , respectively. Regarding the samples obtained from post-pubertal children, the average concentrations of 6MHO, GA, and SQ in the distillates were 3.5 ± 1.2 µg ml −1 , 36.6 ± 8.5 µg ml −1 , and 7.7 × 10 4 ± 1.5 × 10 4 µg ml −1 , respectively. In the room samples, 0.5 ± 0.1 µg ml −1 of 6MHO and 22.4 ± 1.7 µg ml −1 of GA were detected. For both age groups, SQ was detected > LOD in the room samples, however in considerably lower abundance than in the BO samples, thus the ratio of SQ to the internal standard was far beyond the calibration range. The results are graphically displayed in Fig. 1 and summarized in Supplementary Note 2 Table S3 online.
Target compounds were quantified in the distillates of pooled body odor (BO) and room samples of infants (0–3 years) and post-pubertal children (14–18 years). In total, per age group three pools were analyzed including samples of six children each. Concentrations of ( a ) 6MHO, ( b ) GA, and ( c ) SQ in the BO and room samples of infants and post-pubertal children (see also Supplementary Note 2 Table S3 online); concentration ratios 6MHO/SQ and GA/SQ in BO samples are depicted in d and e . Note that SQ was out of calibration range in the room samples, due to considerably lower abundance than in the BO samples. For results of statistical comparison, see main text.
Comparing the absolute concentrations of 6MHO and GA between age groups, no significant difference emerged (exact Mann–Whitney U -test: U = 5.500, p = 0.70 and U = 6.000, p = 0.70, respectively). For SQ, the absolute concentration tended to be higher with strong effect in post-pubertal children compared to infant BO samples ( r = 0.8; Mann–Whitney U -test: U = 9.000, p = 0.10).
Regarding comparison of BO and room samples (independent of age groups), concentration of 6MHO was significantly higher with strong effect in BO than in room samples ( r = 0.8; Mann–Whitney U-test: U = 0.000, p = 0.002). For GA, comparison of BO and room samples revealed no significance (Mann–Whitney U -test: U = 13.000, p = 0.485).
Since 6MHO and GA are formed by oxidative degradation of SQ 30 , 31 , the ratios 6MHO/SQ and GA/SQ were calculated and averaged across the three sampling pools. For infants, average concentration ratios (±standard error) of 3.8 × 10 −4 ± 1.2 × 10 −4 and 6.8 × 10 −3 ± 5.4 × 10 −3 for 6MHO and GA, respectively, were determined. For post-pubertal children, the concentration ratio for 6MHO was 4.8 × 10 −5 ± 1.6 × 10 −5 and for GA 5.4 × 10 −4 ± 1.7 × 10 −4 . The ratios of the three sampling pools are depicted in Fig. 1 . Both ratios (6MHO/SQ, GA/SQ) tended to be higher in infant BO samples compared to post-pubertal BO samples with a strong effect ( r = 0.8, 6MHO/SQ: Mann–Whitney U -test: U = 0.000, p = 0.10; GA/SQ: Mann–Whitney U -test: U = 0.000, p = 0.10).
In sum, the results show that 6MHO concentrations were higher in BO samples (independent of age group) than in room samples. Average concentrations of 6MHO and GA in BO samples were similar for both age groups, whereas SQ tended to be higher in post-pubertal BO samples. The 6MHO/SQ ratio and GA/SQ ratio both tended to be higher in infant BO samples than in post-pubertal BO samples.
In the present study BO samples of infants and post-pubertal children were comparatively analyzed by GC-O and GC-MS. Samples of both age groups were qualitatively similar in odorant composition, their odor-active compounds being mainly dominated by aldehydes and carboxylic acids. Nevertheless, two volatile steroids, 5α-androst-16-en-3-one and 5α-androst-16-en-3α-ol, were exclusive to the post-pubertal children. These odor-active steroids are well-known constituents of axillary odor and originate from apocrine sweat through microbial action 26 , 27 , 32 , 33 , 34 , 35 , 36 , 37 . They may contribute, together with other substances, to an altered quality of BO odor in post-pubertal children that became evident in our previous research 12 , 17 . In the present study, mass spectra could not be obtained for both steroids due to their low concentrations. Identification therefore remains tentative, based on retention indices and odor qualities on two different analytical columns. In previous work a recovery rate of 7% was determined for chemical analysis of 5α-androst-16-en-3-one when cotton pads were used as sampling material 38 , pointing to relatively high losses during sample workup. Nonetheless, despite the low recovery rate, both steroids could be clearly identified during GC-O analysis. The 2D-GC-MS system was used to enrich the eluting fraction that included both steroids; however, concentrations remained < LOD. This example shows the higher sensitivity of GC-O analysis for detecting trace compounds with low odor thresholds. To identify 5α-androst-16-en-3-one and 5α-androst-16-en-3α-ol through GC-MS, alternative analysis techniques may be tried in future studies 32 , 39 .
Besides the qualitative differences in odor-active compounds, carboxylic acids seemed to make a greater contribution to the overall BO of post-pubertal children compared to infants, as indicated by higher OD factors. Our results support the finding of Lam et al. 24 that 3-methylbutanoic acid is more abundant in post-pubertal samples. However, it was not among the volatiles with the highest OD factors in the present study. Similarly, acetic acid was not detected in the present study, though previously described as the main influence on children´s BO by Lam et al. 24 Among further carboxylic acids identified by Lam et al. 24 , only octanoic acid was additionally detected in the present study as having higher OD factors in the post-pubertal samples. Zeng and colleagues (1991) 33 also report carboxylic acids as part of axillary odor. In line with the results obtained here, 2-methylheptanoic acid, octanoic acid and 4-ethyloctanoic acid were detected in their studies (in samples from men: Zeng et al. (1991) 33 , in samples from women: Zeng et al. (1996) 35 ). In both studies (1991 and 1996) axillary secretions were sampled with cotton pads, however in the 1996 study of Zeng et al. 35 , apocrine secretion of female donors was sampled, additionally. In that study, only octanoic acid was identified, but not 4-ethyloctanoic acid and 2-methylheptanoic acid, perhaps because sebaceous glands may also be involved in secretion of the carboxylic acid precursors. Natsch et al. 40 reported that 4-ethyloctanoic acid is secreted as a glutamine conjugate by axillary glands. However, the type of gland was not further specified. For future studies, it would be of interest to separately investigate the composition of children´s sebum and secretions of different sweat glands. In addition, it would be of interest to study BOs from other body parts. Here, we focused on axillary odor, but also other regions of the human body emit odor-active compounds and could thus contribute to social communication.
Approximately 15–30% of human sebum consists of free fatty acids 41 , 42 and especially reaction of unsaturated fatty acids with ozone in ambient air leads to the formation of aldehydes as prominent volatiles 30 , 43 , 44 . This class of substances was also predominantly detected in our samples. Further oxidation may lead to the formation of their respective carboxylic acids as final products 44 . Considering these oxidative processes, it can be assumed that the more sebum is secreted, the higher is the abundance not only of long- but also of short- and medium-chained carboxylic acids. Further, not only the amount of initially secreted fatty acids, but also the exposure duration of these skin lipids to ambient air can be an influencing factor leading to a higher amount of carboxylic acids. The latter assumption of aging skin lipids can be strengthened by the results obtained by Uebi et al. 23 , who observed elevated amounts of carboxylic acids in the samples of 2–3 day old infants compared to 1 h-old infants, whereas respective aldehydes remained consistent. This observation and assumptions are also in line with our results, as a trend of increasing OD factors for carboxylic acids appeared in the post-pubertal samples compared to the infants, though not for corresponding aldehydes. For future studies, these GC-O results should be confirmed by quantitative analysis.
Exogenous compounds used as perfuming agents in cosmetic products or detergents were detected in the BO samples, e.g., linalool, α-isomethylionone, polysantol, β-ionone, sandranol, patchouli alcohol (European Commission database for information on cosmetic substances and ingredients (CosIng) 45 ). Exogenous compounds in the human volatilome can hardly be avoided even with the hygienic and dietary protocols employed in the present study. Persistence of such perfuming odorants on human skin is demonstrated here. In addition, analysis of the perfume-free shower gel and detergent, which were provided to the participants, revealed the presence of odor-active compounds in these products. For those odorants, it is difficult to evaluate whether their origin was only in the cosmetic products or whether they also naturally occur on human skin. Another aspect to be considered is that nearly all of the detected compounds were perceived in the BO samples and also in the room samples. Of the (tentatively) identified compounds, only 2-methylheptanoic acid, myristoleic acid, 5α-androst-16-en-3-one, and 5α-androst-16-en-3α-ol were detected in the BO samples, exclusively. This finding indicates that most of the detected compounds are highly volatile, influence indoor air, and easily adhere to textiles (like cotton) when humans are residing in a room (see Weschler (2016) 44 for a review). For some odorants, higher OD factors were determined in the room sample compared to the BO sample, confirming the omnipresence of some BO components in indoor air 46 . Besides being emitted by persons present in the rooms, such compounds can also originate from sebum residues such as SQ and skin lipids that remain on indoor surfaces and can be oxidized to volatile compounds, such as 6MHO, GA and aldehydes 44 , 46 . However, further exogenous origin is also conceivable, e.g., from furniture itself or other objects present in the room, or from the sampling material itself. In future studies, quantification should be carried out in room samples but also in unworn cotton pads not exposed to room air to further evaluate baseline levels of these compounds in the sampling material. Analysis of unworn cotton pads also led to detection of odor-active compounds, although pre-treated with solvent. Direct contact sampling with cotton textiles is often used in BO studies, especially for sensory evaluations 3 , 12 , 17 , 47 , 48 . As this research was closely linked to our previous work 12 , 17 , we decided to use the same BO sampling method in the present study. In future studies sampling of volatiles from indoor air should be controlled for when this sample material is used. For instance, for sensory evaluations, a blank or control textile could be presented to the rater to gain a first impression of the odor intensity of the sampling medium itself. In addition, future analytical studies should consider complementary or alternative methods, e.g., comparing the results of direct contact textile sampling with headspace sampling of skin volatile emissions. In addition, odor reconstitution models representing the odor of infants and post-pubertal children should be prepared and sensorially compared with real BO samples by a trained panel.
Unsaturated or hydroxylated branched fatty acids and sulfanylalkanols commonly described as typical constituents of axillary odors were not detected in our study, though their occurrence could be expected for the samples of post-pubertal children, as precursors for these compounds are secreted by apocrine sweat glands 26 , 28 , 34 , 35 , 49 . A possible reason for this observation is that sampling was conducted over night while sleeping and therefore no sufficient amount of these precursors might have been secreted. However, Lam et al. 24 reported that 3-methyl-2-hexenoic acid and 3-hydroxy-3-methylhexanoic acid were not detected, as well, or only in minimal amounts, although sweat sampling was performed after exercise. A further possible explanation is that the here used sampling and extraction method might not enable detection of these compounds. For instance, it is known that cotton as sampling material is not suited for detection of trace amounts of certain compounds, e.g., sulfury components 38 , 50 . Determining recovery rates for these compounds could help clarify this issue in future studies.
In the second independent approach of our study, the target compounds 6MHO, GA and SQ were quantified via GC-MS based on pilot study 1, see Supplementary Note 1 online. SQ tended to occur in a higher amount (factor 9 higher) in the distillates of BO samples obtained from the post-pubertal compared to the younger age group of infants. Because SQ is one of the main components of human sebum (up to 12%) 42 our finding may be due to androgenic stimulation of sebaceous glands during puberty 20 . Additionally, the percentage of SQ in sebum is higher in adults than in children (9.3–10.2% vs. 6.3%) 51 . In contact with ozone (present in ambient air) SQ is oxidized, leading to the formation of 6MHO and GA, inter alia 30 , 31 , 52 . Additionally, 6MHO and GA might originate from enzymatic activity on the skin. The relative concentrations of 6MHO and GA in relation to SQ, considered to be their precursor, tended to be higher in the infant BO samples than in the post-pubertal BO samples (factor 8 and factor 13 for 6MHO and GA, respectively). This finding may be explained by four different hypotheses. First, the amount of sweat on the skin may be an influencing factor for ozone-mediated reactions. Post-pubertal skin may be covered to a greater extent with aqueous sweat since apocrine and apoeccrine glands are additionally contributing to the overall amount of sweat and further sweating rate increases with maturation 53 , 54 . A higher humidity above the skin surface may lead to lower relative concentrations of SQ ozonolysis products (6MHO and GA). Relative humidity can affect squalene ozonolysis in several ways due to competing reactions of ozone with water vapor and shift of volatile reaction products 31 . Due to the complexity of squalene ozonolysis, and because we are not aware of any study comparing the amount of sweat in the axilla of infants vs the axilla of post-pubertals, further research is needed for clarification. Second, different degrees of skin bacterial colonization (or more generally different enzymatic activity on the skin) may lead to different degrees of SQ degradation products. Bacterial metabolization of SQ on human skin has not yet been investigated, to our knowledge. To prove bacterial action in SQ degradation, incubation of SQ with axillary skin bacteria should be conducted and oxidation intermediates of SQ investigated using LC-MS. Third, skin lipid composition may influence the oxidation rate of SQ. The preferred positions for ozone reactions are carbon-carbon double bonds. Because SQ has six double bonds and is distributed all over the human body, it is the most important compound for ozone reactions 44 . Unsaturated fatty acids are next in importance 44 and their relative amounts change with aging 22 . Hence, SQ may be oxidized to a lesser extent if the proportion of unsaturated fatty acids in skin lipids is higher. This question may be resolved through experiments with different ratios of unsaturated fatty acids to SQ, with incubation at the same ozone concentrations. Additionally, further precursors of 6MHO and GA might be present and thereby influence their concentrations. Fourth, sebum production is higher in post-pubertal children than in infants, yet this higher level does not coincide with higher concentration of oxidation products, as detected here, e.g., because oxidation mainly occurs on the surface area or because of ad-/absorption equilibria of the textile sampling material.
During GC-O analysis, 6MHO ( fruity ) was not detected, whereas GA ( soapy ) was identified with intermediate OD factors. From these results, it appears that both compounds have a minor influence on BO. GC-O does not account for mixture effects, and the sampling technique may for some reason have discriminated against 6MHO and/or GA. Certain work-up losses occur for volatiles when extracted from cotton pads. For 6MHO the recovery rate was 37.9% when cotton pads were used 38 . To further evaluate the potential role of 6MHO and GA in BO perception, one should extract volatiles directly from the headspace above the skin and compare the concentrations to threshold concentrations in air. The thresholds in water (6MHO: 0.75 µg ml −1 , GA: 0.43 µg ml −1 ) could be used in future studies to estimate concentrations in sweat that lead to odor detection of these compounds, but since the composition of sweat and sebum is more complex, additional matrix effects may occur.
Infant BO samples are rated to be more pleasant than BO samples from post-pubertal children (see also our pilot study 2, Supplementary Note 3 online), perhaps because the rather unpleasant smelling steroids are absent, but this awaits clarification by experiments with odor models. For future experiments it would also be of interest to test the physiological impact of 6MHO and GA when perceived by participants in sensory studies, for instance to observe whether they have a direct impact on the perception of children’s BO by parents.
Quantification data showed that 6MHO and GA were also detected in the room samples. When the standard errors of the BO samples and the room samples are compared, there is more variation within the BO samples, perhaps because of interpersonal variation in sebum secretion and seasonal variations. That variation may have two causes: i) higher ozone concentrations during warm and dry weather 55 with higher SQ degradation; and ii) higher sebum and SQ secretion, respectively, as more sebum is secreted during warm temperatures 56 . Fruekilde et al. 52 investigated ubiquitous occurrence of 6MHO and GA. According to their experiments the formation of 6MHO and GA can be traced back to the reaction of ozone with epicuticular waxes of leaves, which contain sesquiterpenoids (nerolidol; farnesol) and triterpenes (SQ) as major precursors. Additionally, they demonstrated formation of these compounds from skin lipids. In our sampling setup, cotton pads were carefully handled with gloves and not with bare fingers, e.g., during sewing or when they were cut out of the T-shirts/bodies. A human presence leads to higher concentrations of 6MHO, GA and to further increases in SQ degradation products in room air and also residues of SQ on indoor surfaces are further oxidized (reviewed by Weschler (2016) 44 , Coffaro & Weschler (2022) 31 ). Therefore, 6MHO and GA may have also been present in the room samples.
Materials and methods
The following chemicals were used: sodium sulfate, dichloromethane (DCM) (VWR International GmbH, Darmstadt, Germany) - prior to use, DCM was freshly distilled; alkanes C 6 -C 34 , nonanal (purity: 95%) (Fluka, Steinheim, Germany); octanal (n.a.), oct-1-en-3-one (50%), ( E )-non-2-enal (97%), undecanal (97%), dodecanoic acid (99,5%), dodecanal (n.a.), (2 E ,4 E )-deca-2,4-dienal (85%), 3-methylbutanoic acid (99%), 4-ethyl octanoic acid (98%), 4-methylphenol (in the following: p -cresol; > 98%), octanoic acid (98%), 4-(4-hydroxyphenyl)butan-2-one (in the following: raspberry ketone; 99%), 5-heptyloxolan-2-one (in the following: γ-undecalactone; 98%), (2 E ,4 E )-nona-2,4-dienal (85%), 4-hydroxy-2,3-dimethyl-2H-furan-5-one (in the following: sotolone; 97%) (Aldrich, Steinheim, Germany); 6-methylhept-5-en-2-one (>98%), ( E )-3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-one (in the following: α-isomethylionone; ≥95%), 6,10-dimethylundeca-5,9-dien-2-one (in the following: geranyl acetone; > 97%), 3,7-dimethylocta-1,6-dien-3-ol (in the following: linalool; 97%) (Sigma Aldrich, Steinheim, Germany); decanal (>98%), (3 R ,5 S ,8 R ,9 S ,10 S ,13 R ,14 S )-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol (in the following: 5α-androst-16-en-3α-ol; n.a.), (5 S ,8 R ,9 S ,10 S ,13 R ,14 S )-10,13-dimethyl-1,2,4,5,6,7,8,9,11,12,14,15-dodecahydrocyclopenta[a]phenanthren-3-one (in the following: 5α-androst-16-en-3-one; n.a.), ( Z )-tetradec-9-enoic acid (in the following: myristoleic acid; > 99%) (Sigma, Steinheim, Germany); ( E )-2-ethyl-4-(2,2,3-trimethylcyclopent-3-en-1-yl)but-2-en-1-ol (in the following: sandranol; n.a.), ( E )-3,3-dimethyl-5-(2,2,3-trimethylcyclopent-3-en-1-yl)pent-4-en-2-ol (in the following: polysantol; n.a.) (kindly provided by Symrise AG, Holzminden, Germany); ( E )-3-[(2 S ,3 S )-3-pentyloxiran-2-yl]prop-2-enal (in the following: ( E )-4,5-epoxy-( E )-2-decenal; 97%) (AromaLab GmbH, Martinsried, Germany); 4-hydroxy-3-methoxybenzaldehyde (in the following: vanillin; 99%), 2-methylheptanoic acid (98%) (ABCR, Karlsruhe, Deutschland); 5-octyloxolan-2-one (in the following: γ-dodecalacton; 97%) (SAFC, Steinheim, Germany); (1 R ,3 R ,6 S ,7 S ,8 S )-2,2,6,8-tetramethyltricyclo[5.3.1.03,8]undecan-3-ol (in the following: patchouli alcohol; > 98%) (Biozol, Eching, Germany); n-triacontane (>98%) (Alfa Aesar by Thermo Fisher Scientific, Kandel, Germany).
The following isotopically labeled standards were ordered: 6-methylhept-5-en-2-one d6, (2 E )-3,7-dimethylocta-2,6-dien-1-ol d2 (in the following: geraniol d2; AromaLAB GmbH, Martinsried, Germany).
BOs were sampled from 18 healthy infants (9 girls, 9 boys; age (M ± SD): 1.3 ± 0.8 years) and 18 healthy post-pubertal children (9 girls, 9 boys; age (M ± SD): 15.5 ± 1.4 years). All children were of Caucasian ethnicity. As sampling took place during the Corona pandemic, all participants, or their respective parents, were queried for COVID-19 symptoms and carried out an olfactory test at home to minimize the potential infection risk mediated by the participants. Recruitment took place during July/August 2021, December 2021/January 2022, and April/May 2022 (resulting in three sampling pools), whereby each time 6 participants (3 girls, 3 boys) per age group were recruited (Fig. 2 ).
Cotton T-shirts with pre-treated and sewed-in cotton pads were used for body odor (BO) sampling of infants (0–3 years) and post-pubertal children (14–18 years). Additionally, a second T-shirt was placed in the room for air sampling during the BO sampling period (overnight). In total, three pools with six children each were recruited. For final analysis, cotton pads of BO and room samples were pooled, respectively, solvent extracted and analyzed by GC-MS and GC-O.
Written informed consent was provided by the children´s parents and – in case of post-pubertal children – additionally by the children themselves prior to the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Ethics Committee of the Dresden University of Technology (EK: 501122018).
Cotton test fabric for sampling BO
Cotton test fabric (100%), following DIN 53919/ISO 2267, was purchased from wfk Testgewebe GmbH (Brüggen-Bracht, Germany). The fabric was delivered cut into 10 cm×10 cm patches. To achieve analytical cleanness, a pretreatment of the cotton test fabric with DCM (3 × 30 ml) was conducted according to Alves Soares et al. 38 .
BO sampling procedure
For BO sampling pre-treated cotton patches were sewed in the left and right axillary region of 100% cotton T-Shirts and body suits (Fruit of the Loom Ltd, Bowling Green, KY, USA), which had been washed with perfume-free detergent (Denkmit Vollwaschmittel Ultra Sensitive, dm-drogerie markt GmbH & Co. KG, Karlsruhe, Germany). Participants received two of these prepared T-shirts/body suits. One T-shirt/body suit was worn by the BO donors for one night (BO sample). The other one was placed inside the room where the BO donors were sleeping, to sample volatile compounds which were present in the room air (room sample; Fig. 2 ). The post-pubertal children were instructed to follow a dietary and hygienic protocol during the 48 h before sampling – in case of the infants, the parents were instructed to ensure that the protocol was followed. Regarding diet, consumption of strongly spiced food, onions, garlic, leek and similar vegetables, asparagus, cabbage, and alcohol should be avoided. Regarding hygiene, perfumed hygienic products and deodorant should not be used. Clothes and bedlinen should be washed with perfume-free detergent (see above). Before the experimental night post-pubertal children were instructed to shower with perfume-free shower gel (Eubos Basic Care Liquid Washing Emulsion, Dr. Hobein (Nachf.) GmbH, Meckenheim, Germany). Analogously, parents were instructed to wash their infants. Perfume-free detergent and shower gel were handed out in an experimental kit. To record the sleeping situation, and the hygienic and dietary protocol, a questionnaire was filled out. After sampling was conducted, the two garments were placed in separate zip lock bags and returned to the experimenter within 8 h and frozen at -20 °C for a maximum of 62 days at the University Hospital Carl Gustav Carus, Dresden, Germany. For chemical analysis the samples were transported on dry ice to the Chair of Aroma and Smell Research, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, where they were stored at -80 °C for a maximum of 15 days. To evaluate the impact of different transport conditions on the olfactory properties of BO samples, we performed a pilot test with BOs of a total of 16 participants (see Supplementary Note 3 online). Transport on dry ice was chosen due to its practicality.
Isolation of volatiles from BO and room samples, and from perfume-free shower gel and detergent
The two sewed-in cotton patches were cut from each T-shirt/body suit. Samples from six participants per age group were pooled for extraction (12 patches in total). Overall, this resulted in three pools per age group. Room samples were pooled analogously (12 patches per pool). See Fig. 2 for sample pooling overview. For extraction of the volatiles 300 ml of DCM were added to the pooled cotton patches and for quantification purpose the (isotopically labeled) internal standards (see Supplementary Note 2 Table S1 online) were added. After stirring for 30 min at room temperature, the solvent was decanted and thereafter used for solvent-assisted flavor evaporation (SAFE) 57 , performed at 50 °C. The distillate was thawed at room temperature and dried with anhydrous sodium sulfate. In a last step, the distillate was concentrated to a volume of 100 µl by means of Vigreux distillation and microdistillation by Bemelmans 58 . Perfume-free detergent (0.11 g) and shower gel (0.14 g), which were provided to the participants for washing clothes and bedlinen and for showering, respectively, were extracted with 30 ml DCM and the extract was then distilled and concentrated analogously. The detergent was also used for washing T-shirt/body suits before handing them over to the participants.
Gas chromatography-olfactometry
Measurements were conducted with a Trace GC Ultra (Thermo Fisher Scientific Inc., Waltham, USA) equipped with a DB-FFAP or DB-5 (30 m × 0.32 mm, film thickness of 0.25 µm, J&W Scientific, Agilent Technology, Santa Clara, CA, USA) as capillary column. The helium carrier gas flow rate was set at 2.5 ml min −1 . After separation on the capillary column the gas flow was split 1:1 and directed to an olfactory detection port (ODP; 270 °C) and a flame ionization detector (FID; 250 °C). For recording of the chromatogram an electric writer Servogor 120 (BBC Goerz Metrawatt, Nürnberg, Germany) was used. Distillates were injected (2 µl) in cold-on-column mode. The initial oven temperature was set at 40 °C for 2 min, followed by a heating ramp of 10 °C min −1 to a final temperature of 240 °C with a hold time of 10 min and 300 °C with 5 min for DB-FFAP and DB-5, respectively.
Odor extract dilution analysis (OEDA) was performed by preparing dilutions 1:1 v/v with DCM. Odor dilution (OD) factors were determined at the dilution stage where the odor impression was last perceived. The diluted distillates were analyzed on a DB-FFAP column.
Gas chromatography-mass spectrometry
GC-MS analysis was performed with a 7890 A GC equipped with a 5975 C MSD (Agilent, Santa Clara, CA, USA). For injection, a multipurpose autosampler MPS2 and CIS4 injection system (Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany) were used. The injection was conducted in cold-on column mode with a volume of 1 µl. For GC separation a DB-FFAP and DB-5 column (30 m × 0.25 mm, film thickness of 0.25 µm, J&W Scientific, Agilent Technology, Santa Clara, CA, USA) were used. Helium carrier gas was set in constant flow at 1.0 ml min −1 . The oven temperature started at 40 °C for 2 min, followed by a ramp of 8 °C min −1 to a final temperature of 240 °C for 10 min (FFAP) or 300 °C for 5 min (DB-5). Mass spectra were recorded with electron ionization (EI) at 70 eV in TIC (40-400 m/z) as well as SIM mode, see Supplementary Note 2 Table S1 online.
Two-dimensional (heart cut) gas chromatography-mass spectrometry/olfactometry (2D-GC-MS/O)
The 2D-GC-MS/O analysis was performed with a system consisting of two 7890B GCs in combination with a 5977B MSD (Agilent Technologies, Santa Clara, California, USA). The sample was injected (1 µl) using a multipurpose autosampler MPS2 and a CIS4 injection system (Gerstel GmbH & Co.KG, Mülheim an der Ruhr, Germany) in cold-on column mode. The first GC was equipped with a multi-column switching system MCS2 and a cryo-trap system CTS1 connecting both GCs (Gerstel GmbH & Co.KG, Mülheim an der Ruhr, Germany). For the first oven a DB-FFAP column (30 m × 0.32 mm, film thickness of 0.25 µm, J&W Scientific, Agilent Technology, Santa Clara, CA, USA) and for the second oven a DB-5 column (30 m × 0.25 mm, film thickness of 0.25 µm, J&W Scientific, Agilent Technology, Santa Clara, CA, USA) was installed. Helium was used as a carrier gas (flow rate: 7.8 ml min −1 ). In the first oven the gas flow was split to a FID (250 °C) and ODP (280 °C). In addition, the effluent was led to the cryo trap during the cut interval and afterwards to the second oven, which was connected with the MSD as well as an ODP. The initial temperature program for the ovens was set at 40 °C for 2 min, followed by a heating ramp of 8 °C min −1 and a final temperature of 240 °C for 5 min (FFAP) or 300 °C for 5 min (DB-5). Mass spectra were recorded in EI mode at 70 eV in the range of 40–400 m/z.
Identification of volatiles
In the first step, volatile and odor-active compounds in the distillates were tentatively identified by GC-O by comparison of retention indices (RIs) on both capillary columns (FFAP and DB-5) and odor impression of respective analytical standards, see Table 1 letter a. For calculation of RIs according to Kovats (1958) 59 a homologous series of alkanes in the range of C6 to C34 was injected. If available, the mass spectrum was compared with the one of a standard, integrated into an in-house developed database established with AMDIS (Version 2.72, National Institute for Standards and Technology, Gaithersburg, USA) for identification of the compound, see Table 1 letter b.
Determination of odor detection threshold of target compounds
Odor detection thresholds in distilled water were determined for 6MHO and GA by a trained panel (4 female, 2 male, M ( ± SD): 26.8 ± 2.7 years, age range: 23–30 years), applying triangle tests. For more information regarding the training of the panel, see Supplementary Note 3 online. For both compounds, six triangle tests were prepared, each consisting of two blank solutions (distilled water) and one target solution (odorant in water), which were filled into glass beakers (WECK Mini-Sturzglas, 140 mL, 60 mm diameter, J. Weck GmbH & Co. KG, Wehr-Öflingen, Germany). The concentration of the target solutions ranged from 0.25 to 60.86 µg ml −1 for 6MHO and from 0.02 to 20.00 µg ml −1 for GA. For each triangle test, the panelist sniffed the three beakers and indicated whether he/she detected a difference (forced choice decision). Individual thresholds were calculated by the geometric mean of the highest non-detected concentration and the lowest detected concentration after which all further tests were correctly detected. The average odor threshold for the panel was calculated by the geometric mean of all individual thresholds.
The compounds 6-methylhept-5-en-2-one, geranyl acetone and squalene were quantified using 6-methylhept-5-en-2-one d6, geraniol d2 and triacontane as standards. These compounds were selected for quantification based on prior pilot tests (see Supplementary Note 1 online). Calibration solutions were prepared with target compound/internal standard ratios of approximately 5:1, 3:1. 1:1, 1:3, 1:5. If necessary, further ratios were prepared and measured. GC-MS measurements were conducted in selected ion monitoring (SIM) mode. The masses selected for each analyte and respective standard are listed in Supplementary Note 2 Table S1 online. For the calibration curves all coefficients of determination were above 0.99 (see Supplementary Note 2 Table S2 online). For limit of detection (LOD) and limit of quantification (LOQ) a signal-to-noise ratio of 3 and 10, respectively, was considered.
Statistical analysis
Data were analyzed with IBM SPSS Statistics 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). Mann-Whitney-U tests were conducted to compare results obtained for different age groups. The exact significance [2*(one-sided significance)] was reported.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Olsson, S. B., Barnard, J. & Turri, L. Olfaction and identification of unrelated individuals: examination of the mysteries of human odor recognition. J. Chem. Ecol. 32 , 1635–1645 (2006).
Article CAS PubMed Google Scholar
Ravreby, I., Snitz, K. & Sobel, N. There is chemistry in social chemistry. Sci. Adv. 8 , eabn0154 (2022).
Article PubMed Central PubMed Google Scholar
Wedekind, C., Seebeck, T., Bettens, F. & Paepke, A. J. MHC-dependent mate preferences in humans. Proc. R. Soc. Lond. B. Biol. Sci. 260 , 245–249 (1995).
Article ADS CAS Google Scholar
Mahmut, M. K., Stevenson, R. J. & Stephen, I. Do women love their partner’s smell? Exploring women’s preferences for and identification of male partner and non-partner body odor. Physiol. Behav. 210 , 112517 (2019).
Weisfeld, G. E., Czilli, T., Phillips, K. A., Gall, J. A. & Lichtman, C. M. Possible olfaction-based mechanisms in human kin recognition and inbreeding avoidance. J. Exp. Child Psychol. 85 , 279–295 (2003).
Article PubMed Google Scholar
Ferdenzi, C., Schaal, B. & Roberts, S. C. Family scents: developmental changes in the perception of kin body odor? J. Chem. Ecol. 36 , 847–854 (2010).
Russell, M. J. Human olfactory communication. Nature 260 , 520–522 (1976).
Article ADS CAS PubMed Google Scholar
Cernoch, J. M. & Porter, R. H. Recognition of maternal axillary odors by infants. Child Dev . 56 , 1593–1598 (1985).
Porter, R. H., Makin, J. W., Davis, L. B. & Christensen, K. M. Breast-fed infants respond to olfactory cues from their own mother and unfamiliar lactating females. Infant Behav. Dev. 15 , 85–93 (1992).
Article Google Scholar
Porter, R. H. & Winberg, J. Unique salience of maternal breast odors for newborn infants. Neurosci. Biobehav. Rev. 23 , 439–449 (1999).
Porter, R. H., Cernoch, J. M. & McLaughlin, F. J. Maternal recognition of neonates through olfactory cues. Physiol. Behav. 30 , 151–154 (1983).
Schäfer, L., Sorokowska, A., Sauter, J., Schmidt, A. H. & Croy, I. Body odours as a chemosignal in the mother–child relationship: new insights based on an human leucocyte antigen-genotyped family cohort. Philos. Trans. R. Soc. B 375 , 20190266 (2020).
Croy, I., Mohr, T., Weidner, K., Hummel, T. & Junge-Hoffmeister, J. Mother-child bonding is associated with the maternal perception of the child’s body odor. Physiol. Behav. 198 , 151–157 (2019).
Lundström, J. N. et al. Maternal status regulates cortical responses to the body odor of newborns. Front. Psychol . 4 , 597 (2013).
Schäfer, L., Hummel, T. & Croy, I. The design matters: how to detect neural correlates of baby body odors. Front. Neurol. 9 , 1182 (2019).
Croy, I., Frackowiak, T., Hummel, T. & Sorokowska, A. Babies smell wonderful to their parents, teenagers do not: an exploratory questionnaire study on children’s age and personal odor ratings in a polish sample. Chemosens. Percept. 10 , 81–87 (2017).
Schäfer, L., Sorokowska, A., Weidner, K. & Croy, I. Children’s body odors: hints to the development status. Front. Psychol. 11 , 320 (2020).
Dorn, L. D., Dahl, R. E., Woodward, H. R. & Biro, F. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl. Dev. Sci. 10 , 30–56 (2006).
Wilke, K., Martin, A., Terstegen, L. & Biel, S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 29 , 169–179 (2007).
Zouboulis, C., Chen, W.-C., Thornton, M., Qin, K. & Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 39 , 85–95 (2007).
Pochi, P. E., Strauss, J. S. & Downing, D. T. Age-related changes in sebaceous gland activity. J. Invest. Dermatol. 73 , 108–111 (1979).
Nazzaro-Porro, M., Passi, S., Boniforti, L. & Belsito, F. Effects of aging on fatty acids in skin surface lipids. J. Invest. Dermatol. 73 , 112–117 (1979).
Uebi, T. et al. Sampling, identification and sensory evaluation of odors of a newborn baby’s head and amniotic fluid. Sci. Rep. 9 , 1–11 (2019).
Article CAS Google Scholar
Lam, T. H. et al. Understanding the microbial basis of body odor in pre-pubescent children and teenagers. Microbiome 6 , 1–14 (2018).
Hasegawa, Y., Yabuki, M. & Matsukane, M. Identification of new odoriferous compounds in human axillary sweat. Chem. Biodivers. 1 , 2042–2050 (2004).
Natsch, A., Gfeller, H., Gygax, P., Schmid, J. & Acuna, G. A specific bacterial aminoacylase cleaves odorant precursors secreted in the human axilla. J. Biol. Chem. 278 , 5718–5727 (2003).
Natsch, A., Schmid, J. & Flachsmann, F. Identification of odoriferous sulfanylalkanols in human axilla secretions and their formation through cleavage of cysteine precursors by a C–S lyase isolated from axilla bacteria. Chem. Biodivers. 1 , 1058–1072 (2004).
Starkenmann, C., Niclass, Y., Troccaz, M. & Clark, A. J. Identification of the precursor of (S)‐3‐methyl‐3‐sulfanylhexan‐1‐ol, the sulfury malodour of human axilla sweat. Chem. Biodivers. 2 , 705–716 (2005).
Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 26 , 533–545 (2001).
Wisthaler, A. & Weschler, C. J. Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proc. Natl Acad. Sci. USA 107 , 6568–6575 (2010).
Article ADS CAS PubMed Central PubMed Google Scholar
Coffaro, B. & Weisel, C. P. Reactions and products of squalene and ozone: a review. Environ. Sci. Technol. 56 , 7396–7411 (2022).
Gower, D., Holland, K., Mallet, A., Rennie, P. & Watkins, W. Comparison of 16-androstene steroid concentrations in sterile apocrine sweat and axillary secretions: interconversions of 16-androstenes by the axillary microflora—a mechanism for axillary odour production in man? J. Steroid Biochem. Mol. Biol. 48 , 409–418 (1994).
Zeng, X.-N. et al. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 17 , 1469–1492 (1991).
Zeng, X.-N. et al. An investigation of human apocrine gland secretion for axillary odor precursors. J. Chem. Ecol. 18 , 1039–1055 (1992).
Article MathSciNet CAS PubMed Google Scholar
Zeng, X.-N., Leyden, J. J., Spielman, A. I. & Preti, G. Analysis of characteristic human female axillary odors: qualitative comparison to males. J. Chem. Ecol. 22 , 237–257 (1996).
Troccaz, M., Starkenmann, C., Niclass, Y., van de Waal, M. & Clark, A. J. 3‐Methyl‐3‐sulfanylhexan‐1‐ol as a major descriptor for the human axilla‐sweat odour profile. Chem. Biodivers. 1 , 1022–1035 (2004).
Starkenmann, C., Mayenzet, F., Brauchli, R. & Troccaz, M. 5α‐Androst‐16‐en‐3α‐ol β‐d‐glucuronide, precursor of 5α‐androst‐16‐en‐3α‐ol in human sweat. Chem. Biodivers. 10 , 2197–2208 (2013).
Alves Soares, T., Owsienko, D., Haertl, T. & Loos, H. M. Recovery rates of selected body odor substances in different textiles applying various work-up and storage conditions measured by gas chromatography-mass spectrometry. Anal. Chim. Acta 1252 , 341067 (2023).
Hartmann, C. et al. Development of an analytical approach for identification and quantification of 5-α-androst-16-en-3-one in human milk. Steroids 78 , 156–160 (2013).
Natsch, A., Derrer, S., Flachsmann, F. & Schmid, J. A broad diversity of volatile carboxylic acids, released by a bacterial aminoacylase from axilla secretions, as candidate molecules for the determination of human‐body odor type. Chem. Biodivers. 3 , 1–20 (2006).
Picardo, M., Ottaviani, M., Camera, E. & Mastrofrancesco, A. Sebaceous gland lipids. Dermatoendocrinol 1 , 68–71 (2009).
Article CAS PubMed Central PubMed Google Scholar
Nicolaides, N. Skin Lipids: their biochemical uniqueness: unlike internal organs, the skin biosynthesizes and excretes unusual fat soluble substances. Science 186 , 19–26 (1974).
Frankel, E. Volatile lipid oxidation products. Prog. Lipid Res. 22 , 1–33 (1983).
Weschler, C. J. Roles of the human occupant in indoor chemistry. Indoor Air 26 , 6–24 (2016).
CosIng - Cosmetics Ingredients. E uropean Commission database for information on cosmetic substances and ingredients , https://ec.europa.eu/growth/tools-databases/cosing/ (2022).
Liu, Y. et al. Observing ozone chemistry in an occupied residence. Proc. Natl Acad. Sci. USA 118 , e2018140118 (2021).
Wedekind, C. & Füri, S. Body odour preferences in men and women: do they aim for specific MHC combinations or simply heterozygosity? Proc. R. Soc. Lond. B. Biol. Sci. 264 , 1471–1479 (1997).
Fialová, J., Sorokowska, A., Roberts, S. C., Kubicová, L. & Havlíček, J. Human body odour composites are not perceived more positively than the individual samples. Iperception 9 , 2041669518766367 (2018).
PubMed Central PubMed Google Scholar
Troccaz, M. et al. Gender-specific differences between the concentrations of nonvolatile (R)/(S)-3-methyl-3-sulfanylhexan-1-Ol and (R)/(S)-3-hydroxy-3-methyl-hexanoic acid odor precursors in axillary secretions. Chem. Senses 34 , 203–210 (2009).
Starkenmann, C. Springer Handbook of Odor (ed Buettner A) 121-122 (Springer International Publishing, 2017).
Boughton, B., MacKenna, R., Wheatley, V. & Wormall, A. Studies of sebum. 8. Observations on the squalene and cholesterol content and the possible functions of squalene in human sebum. Biochem. J. 66 , 32 (1957).
Fruekilde, P., Hjorth, J., Jensen, N., Kotzias, D. & Larsen, B. Ozonolysis at vegetation surfaces: a source of acetone, 4-oxopentanal, 6-methyl-5-hepten-2-one, and geranyl acetone in the troposphere. Atmos. Environ. 32 , 1893–1902 (1998).
Baker, L. B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 6 , 211–259 (2019).
Sinclair, W. H., Crowe, M. J., Spinks, W. L. & Leicht, A. S. Pre-pubertal children and exercise in hot and humid environments: A brief review. J. Sports Sci. Med. 6 , 385 (2007).
Wells, B. et al. Improved estimation of trends in US ozone concentrations adjusted for interannual variability in meteorological conditions. Atmos. Environ. 248 , 118234 (2021).
Qiu, H. et al. Influence of season on some skin properties: winter vs. summer, as experienced by 354 Shanghaiese women of various ages. Int. J. Cosmet. Sci. 33 , 377 (2011).
Engel, W., Bahr, W. & Schieberle, P. Solvent assisted flavour evaporation–a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 209 , 237–241 (1999).
Bemelmans, J. M. H. in Progress in Flavour Research: Proceedings of the 2nd Weurman Flavour Research Symposium . (eds D. G. Land & H. E. Nursten) 79-98 (Applied Science Publisher, 1979).
Kovats, E. Gaschromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 41 , 1915–1932 (1958).
Download references
Acknowledgements
We would like to thank the parents and their children for participating in this study; Miriam Kron for the recruitment of participants for the second sampling pool; Jessica Bayat for the preparation of the triangle tests. This research was supported by the German Research Foundation (grant numbers BU 1351/24-1 and CR 479/11-1) and by the European Union (project number 101046369).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Chair of Aroma and Smell Research, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
Diana Owsienko, Lisa Goppelt & Helene M. Loos
Department of Psychotherapy and Psychosomatics, Technical University of Dresden, Dresden, Germany
Katharina Hierl, Laura Schäfer & Ilona Croy
Department of Clinical Psychology, Friedrich-Schiller-University of Jena, Jena, Germany
Fraunhofer Institute for Process Engineering and Packaging IVV, Freising, Germany
Helene M. Loos
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: D.O., L.S., I.C. and H.M.L.; Methodology: D.O., L.S., I.C. and H.M.L.; Investigation: D.O., L.G. and K.H.; Formal analysis: D.O. and K.H.; Writing—original draft preparation: D.O.; Writing – review and editing: D.O., H.M.L., L.G., K.H., L.S. and I.C.; Supervision: D.O., H.M.L., L.S. and I.C.; Funding acquisition: H.M.L. and I.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Helene M. Loos .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Owsienko, D., Goppelt, L., Hierl, K. et al. Body odor samples from infants and post-pubertal children differ in their volatile profiles. Commun Chem 7 , 53 (2024). https://doi.org/10.1038/s42004-024-01131-4
Download citation
Received : 27 September 2023
Accepted : 20 February 2024
Published : 21 March 2024
DOI : https://doi.org/10.1038/s42004-024-01131-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Body composition assessment enhances the diagnosis of obesity and the monitoring of responses to obesity treatment programs, which is highly relevant to the management of obesity-related chronic diseases. ... doi: 10.1371/journal.pone.0160105. [PMC free article] [Google Scholar] 58. Lukaski H.C., Siders W.A. Validity and accuracy of regional ...
This simple two-compartment (2C) model of the human body has become the mainstay of body composition assessment in clinical nutrition. A little later, in 1940s and 1950s, the 2C model was ...
Human Body Composition. 2nd ed, edited by SB Heymsfield, TG Lohman, ZM Wang, and SB Going, 2005, 536 pages, hardcover, $89. Human Kinetics, Champaign, IL. This new edition of Human Body Composition is a complete reference text and is divided into 5 parts: Part I: The Science of Body Composition Research, Part II: Body Composition Measurement ...
The body composition rule area organizes the more than 30 main body com- ponents into five distinct levels of increasing complexity: atomic, molecular, cellular, tissue system, and whole body (77).
Advances in body composition assessment enable a detailed body composition analyses and the respective organization at different levels. Sports-related professionals are interested in ...
Maintaining a healthy body composition is a cornerstone of chronic disease management and prevention. An excessive accumulation of body fat (i.e., adiposity) is associated with a higher risk of ...
The aim of the present study was to classify the latent body fat trajectories of Chinese adults and their relationships with cardiometabolic risk factors. Data were obtained from the China Health Nutrition Survey for 3,013 participants, who underwent six follow-up visits between 1993 and 2009. Skinfold thickness and other anthropometric indicators were used to estimate body composition.
One critical but underexplored area of obesity research is on body composition and mortality. Previous studies using BMI have acknowledged the major limitation of BMI being an imperfect measure of adiposity because it cannot discriminate fat mass and lean body mass. 5 Thus, understanding body composition may provide new insights into the relationship between obesity and mortality.
In our study, body fat in men and women were 18.33 and 19.82. The body fat percentage in men and women were 25.74% and 34.01%. Visceral fat area in men and women were 91.98 and 77 cm 2. And, with the increase of age, body fat, body fat percentages and visceral fat area also increased, both in men and in women.
The assessment of body composition is a very important element for describing the growth and development of the human body, from birth to old age, and for understanding the origins of health and disease. ... If you have access to journal content via a personal subscription, university, library, employer or society, select from the options below:
Body composition can also be separated into 2 compartments (e.g., FFM and FM) using BodPod, skinfold calipers, bioelectrical impedance, underwater weighing, and ultrasound techniques. Air-displacement plethysmography (BodPod) is an apparatus that estimates body composition based on the inverse relationship between volume and pressure.
Ageing and changes in body composition: the importance of valid measurements. P. Deurenberg, M. Deurenberg-Yap, in Food for the Ageing Population, 2009 9.1 Introduction. Body composition has long interested mankind. Centuries ago the Greeks dissected human cadavers to get an insight into the structure and build of the body, and drawings from the Middle Ages of gross muscle structure are still ...
CT-based body composition analysis was performed using commercially available software with evaluation of sarcopenia using skeletal muscle index (SMI, cm2/m2). Based on the SMI values, sarcopenia was found in 67.5% of patients (79 out of 117) in the first patient group. It was found more frequently in males (42 out of 56; 75%) than in females ...
Body composition. Body composition was evaluated using a BIA phase-sensitive system administered by a certified clinical nutrition specialist with 5 years of expertise in employing the BIA method for body composition assessment (800-µA current at a single frequency of 50 kHz, BIA 101, RJL Akern Bioresearch, Florence, Italy) , in accordance ...
Exercise energy expenditure of a normal-weight adult with a PAL of 1.75 is 3-4 MJ per day and 20-30 MJ per week [ 6 ]. Thus, the minimum intervention interval of exercise studies included in ...
Mar 25, 2024. Pioneering human clinical trials on ketogenic diet in non-elite CrossFit athletes for my master's thesis yielded great results. Since 2016, I've immersed myself in the low-carb keto ...
Lard (LD) and Basa fish offal oil (BFO) have similar fatty acid profiles, both containing high contents of saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA). The present study aimed to investigate the efficacy of partial or complete replacement of marine fish oil (MFO, herring oil) by LD or BFO in the diets of tiger puffer. The control diet contained 49.1% crude protein and 9. ...
The relationship between body composition and cancer survival has been investigated for decades, as noted by a recent review and meta-analysis that included results published over 30 years ago [ 1 ...
The University of Idaho (U of I, or UIdaho) is a public land-grant research university in Moscow, Idaho.It is the state's land-grant and primary research university, and the lead university in the Idaho Space Grant Consortium.The University of Idaho was the state's sole university for 71 years, until 1963.Its College of Law, established in 1909, was first accredited by the American Bar ...
The simplest approach in body composition is the 2C model, dividing body weight into fat mass (FM) and fat-free mass (FFM). The anhydrous FM is the chemically extractable fat with an assumed density of 0.9007 g/cm 3, whereas the FFM is assumed to have a density of 1.1000 g/cm 3 and water content of 73.72 per cent 7.
Measurements of near-surface methane (CH4) mixing ratio and its stable isotope 13C were carried out from January 2018 to December 2020 at the A.M. Obukhov Institute of Atmospheric Physics (IAP) research site in the center of Moscow city. The data show moderate interannual variations in monthly mean CH4 with maximum values being observed predominantly in winter (2.05-2.10 ppmv on average ...
The body mass index (BMI) was defined as a ratio of patient's weight to the square of their height (kg/m 2). CT-based body composition analysis was performed using commercially available software with evaluation of sarcopenia using skeletal muscle index (SMI, cm 2 /m 2). Based on the SMI values, sarcopenia was found in 67.5% of patients (79 ...
Here, we show by combining instrumental and sensory analyses that the qualitative odorant composition of body odor samples is similar in infants (0-3 years) and post-pubertal children (14-18 years).