Essay on AIDS for Students and Children
500+ words essay on aids.
Acquired Immune Deficiency Syndrome or better known as AIDS is a life-threatening disease. It is one of the most dreaded diseases of the 20 th century. AIDS is caused by HIV or Human Immunodeficiency Virus, which attacks the immune system of the human body. It has, so far, ended more than twenty-nine million lives all over the world. Since its discovery, AIDS has spread around the world like a wildfire. It is due to the continuous efforts of the Government and non-government organizations; AIDS awareness has been spread to the masses.


AIDS – Causes and Spread
The cause of AIDS is primarily HIV or the Human Immunodeficiency Virus. This virus replicates itself into the human body by inserting a copy of its DNA into the human host cells. Due to such property and capability of the virus, it is also known as a retrovirus. The host cells in which the HIV resides are the WBCs (White Blood Cells) that are the part of the Human Immune system.
HIV destroys the WBCs and weakens the human immune system. The weakening of the immune system affects an individual’s ability to fight diseases in time. For example, a cut or a wound takes much more time to heal or the blood to clot. In some cases, the wound never heals.
HIV majorly transmits in one of the three ways – Blood, Pre-natal and Sexual transmission. Transfusion of HIV through blood has been very common during the initial time of its spread. But nowadays all the developed and developing countries have stringent measures to check the blood for infection before transfusing. Usage of shared needles also transmits HIV from an infected person to a healthy individual.
As part of sexual transmission, HIV transfers through body fluids while performing sexual activity. HIV can easily be spread from an infected person to a healthy person if they perform unprotective sexual intercourse through oral, genital or rectal parts.
Pre-natal transmission implies that an HIV infected mother can easily pass the virus to her child during pregnancy, breastfeeding or even during delivery of the baby.
AIDS – Symptoms
Since HIV attacks and infects the WBCs of the human body, it lowers the overall immune system of the human body and resulting in the infected individual, vulnerable to any other disease or minor infection. The incubation period for AIDS is much longer as compared to other diseases. It takes around 0-12 years for the symptoms to appear promptly.
Few of the common symptoms of AIDS include fever , fatigue, loss of weight, dysentery, swollen nodes, yeast infection, and herpes zoster. Due to weakened immunity, the infectious person falls prey to some of the uncommon infections namely persistent fever, night sweating, skin rashes, lesions in mouth and more.
Get the huge list of more than 500 Essay Topics and Ideas
AIDS – Treatment, and Prevention
Till date, no treatment or cure is available for curing AIDS, and as a result, it is a life-threatening disease. As a practice by medical practitioners, the best way to curb its spread is antiretroviral therapy or ART. It is a drug therapy which prevents HIV from replicating and hence slows down its progress. It is always advisable to start the treatment at the earliest to minimize the damage to the immune system. But again, it is just a measure and doesn’t guarantee the cure of AIDS.
AIDS prevention lies in the process of curbing its spread. One should regularly and routinely get tested for HIV. It is important for an individual to know his/her own and partner’s HIV status, before performing any sexual intercourse activity. One should always practice safe sex. Use of condoms by males during sexual intercourse is a must and also one should restrict oneself on the number of partners he/she is having sex with.
One should not addict himself/herself to banned substances and drugs. One should keep away from the non-sterilized needles or razors. Multiple awareness drives by the UN, local government bodies and various nonprofit organizations have reduced the risk of spread by making the people aware of the AIDS – spread and prevention.
Life for an individual becomes hell after being tested positive for AIDS. It is not only the disease but also the social stigma and discrimination, felling of being not loved and being hated acts as a slow poison. We need to instill the belief among them, through our love and care, that the HIV positive patients can still lead a long and healthy life.
Though AIDS is a disease, which cannot be cured or eradicated from society, the only solution to AIDS lies in its prevention and awareness. We must have our regular and periodical health checkup so that we don’t fall prey to such deadly diseases. We must also encourage and educate others to do the same. With the widespread awareness about the disease, much fewer adults and children are dying of AIDS. The only way to fight the AIDS disease is through creating awareness.
Customize your course in 30 seconds
Which class are you in.

- Travelling Essay
- Picnic Essay
- Our Country Essay
- My Parents Essay
- Essay on Favourite Personality
- Essay on Memorable Day of My Life
- Essay on Knowledge is Power
- Essay on Gurpurab
- Essay on My Favourite Season
- Essay on Types of Sports
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Expository Essay on HIV AIDS
Jump ahead to:
Here you have Expository essay on HIV AIDS, Learn how to write an Essay by this example.
Introduction
One of the most feared diseases of the twentieth century is HIV. The Human Immunodeficiency Virus (HIV) causes AIDS by destroying the human immune system. It has claimed the lives of more than 29% of the world’s population. AIDS has spread like wildfire over the world since its discovery. The maximum population knows about this dangerous disease, AIDS, thanks to the efforts of the government and non-governmental organizations.
AIDS – Causes and Spread
The Human Immunodeficiency Virus, also known as HIV, is the primary cause of AIDS. This virus replicates itself in the human body by injecting a copy of its DNA into the human host cells. The virus is also known as a retrovirus because of its property and capabilities. WBCs (White Blood Cells), which are part of the human immune system, are the host cells in which HIV lives.
HIV destroys WBCs, and the human immune system is weakened as a result. The immune system’s deterioration impacts a person’s ability to combat diseases quickly. A cut or a wound, for example, takes much longer to heal or the blood to clot. The injury may never recover in rare circumstances.
The direct transmission of HIV is through one of three routes: blood, prenatal, or sexual transmission. During the early stages of HIV transmission, blood transfusions were extremely popular. However, in today’s world, all developed and developing countries have vital processes in place to ensure that blood is clear of the virus before it is transfused. Sharing needles can also transmit HIV from one infected person to another.
HIV can transmit through body fluids during sexual activity as part of sexual transmission. HIV can quickly spread from an infected person to a healthy person through oral, genital, or rectal areas if they engage in unprotective sexual intercourse.
Prenatal transmission means that an HIV-positive woman can easily transmit the virus to her kid during pregnancy, breastfeeding, or even childbirth.
AIDS – Symptoms
HIV reduces the general immune system of the human body by attacking and infecting the WBCs, leaving the infected individual exposed to any other sickness or minor infection. In comparison to other diseases, AIDS has a substantially longer incubation time. The symptoms occur gradually over 0-12 years.
Fever, exhaustion, and weight loss are frequent AIDS symptoms, including diarrhea, enlarged nodes, yeast infection, and herpes zoster. Because of their decreased immunity, the infectious individual is susceptible to various unusual diseases, including persistent fever, nocturnal sweating, skin rashes, oral sores, and more.
AIDS – Treatment and Prevention
There is currently no treatment or cure for AIDS, making it a life-threatening condition. According to medical professionals, antiretroviral therapy, or ART, is the best approach to stop it from spreading. It is a drug therapy that prevents HIV from replicating and thus slows the progression of the disease. To minimize immune system damage, it is always best to begin treatment as soon as possible. However, it is only a precaution and does not guarantee that it will prevent you from AIDS.
The process of halting the spread of AIDS is AIDS prevention. Before engaging in any sexual activity, an individual should be aware of his or her own HIV status and that of his or her partner. It is imperative to practice safe sex at all times. Males should always use condoms during sexual encounters, and they should limit the number of individuals with whom they have sex.
It is not advisable to become addicted to illegal substances or narcotics. Multiple public awareness campaigns led by the United Nations, local governments, and nonprofit organizations have reduced the danger of AIDS spreading by raising a general understanding of the disease’s spread and prevention.
AIDS prevention is the method of preventing the spread of AIDS. It is mandatory to perform a regular check of AIDS. An individual should be aware of his or her own HIV status and that of his or her partner before engaging in any sexual activity. Males should always wear condoms during sexual interactions and keep the number of people they have intercourse with to a minimum.
Addiction to illegal substances or narcotics is not a good idea. Needles and razors that haven’t been sterilized should be avoided at all costs. Multiple public awareness efforts conducted by the UN, local governments, and nonprofit organizations have helped minimize the risk of AIDS spreading by increasing public awareness of the disease’s spread and prevention.
Download Expository Essay on HIV AIDS
If you want to Download the PDF of the Expository Essay on HIV AIDS you can simply click o the given link.
- Expository essay on Depression | 600-700 words | Free PDF
- Essay on Prize Distribution Function in School
- Expository essay on Examination Malpractices | 700-800 words | Free PDF
4 thoughts on “Expository Essay on HIV AIDS in 700-750 words for 6-12, Free Pdf,”
Thanks for this reading blogs, If you have any related query from this blog then you can comment below this blog.
We hope that you found it informative and helpful. If not, please feel free to contact us anytime so that we can help remedy the situation. Thank you again for your time, and we look forward to hearing from you soon!
Yes thank you for supporting me
Thank you for taking the time to read our blog! We hope that you found it helpful. If so, please leave a comment or rating below, and share this blog with your friends on social media. Thank you again for reading!
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Talk to our experts
1800-120-456-456
- Essay on AIDS

HIV (human immunodeficiency virus) is an infection that causes cells in the body that help it fight infections, making a person more susceptible to other infections and diseases. Interaction with certain bodily secretions of an HIV-positive individual, most commonly during unprotected intercourse (sex without the use of a condom or HIV treatment to prevent or treat HIV), or sharing injection drug equipment spreads the virus.
If HIV is not treated, it can progress to AIDS (acquired immunodeficiency syndrome). HIV cannot be eradicated by the human body, and there is no effective HIV cure. As a result, whether you have HIV, you will have it for the rest of your life.
Long and Short AIDS Essay in English
There are many diseases causing microorganisms, like bacteria, viruses, fungi etc. The symptoms of the diseases depend on the type of microorganism that is spreading it. It can vary from mild to severe. AIDS which stands for Acquired Immunodeficiency Syndrome is a viral disease that is rampant in growth. It was only in the last century that this viral disease has proved to be lethal and fatal, taking away about twenty million lives globally. The awareness about the disease and the virus causing it which is HIV or Human Immunodeficiency Virus is more now compared to earlier. In this HIV AIDS essay, we can go through the important information about it and burst some myths.
Below are different ways to write an AIDS essay in English. The essay on HIV AIDS can be of 2 formats, a long essay on HIV AIDS or a short AIDS essay.
Short Essay on Aids
This AIDS essay is a brief one and will cover the important notes about the disease and the ways one can prevent it.
The way of occurrence of this disease is in the name itself, AIDS stands for Acquired Immunodeficiency Syndrome. The disease is acquired via the virus which is called Human Immunodeficiency Virus. It is not an auto-immune disease in the early stages of infection where the immune system in the body fights off infection to protect the body from diseases that go against itself. The virus enters from an outside source and destroys the efficiency of our immune system.
AIDS is transmitted through contact. The contact with infected blood of the HIV OR AIDS patient in any form can easily transfer this viral disease. It can also be transmitted through contact with semen or vaginal fluids of the infected person. This occurs in the case when one is sexually exposed to a person with HIV.
HIV once enters the body, invades and conquers the immune system making the body susceptible to other diseases. It is then very easy for the simple flu or cold infection to be severe as the immune system is no longer fit to fight it.
When detected in the early period can be battled with, but more often than not people assume the symptoms to not be AIDS so it spreads and kills the individual. To be protected when having sex and not sharing any form of toiletries with others is the way to prevent and keep this deadly virus at bay.
Long Essay on AIDS
This is the long format of an essay on HIV AIDS where its workings, causes and effects and remedies are discussed.
There are some diseases that have been borne by the living in this world which has created a ruckus in human history and the struggle to find a permanent cure still exists. AIDS is one such disease. Acquired Immunodeficiency Syndrome is the name of the disease which is also shortened as AIDS.
It has since only the 20 th century affected the human race and many people lost their lives, more than 20 million of them. The virus that aids in the transmission of this disease is Human Immunodeficiency Virus or also called HIV. Due to the same property of immunodeficiency, it is referred to as HIV/AIDS.
Since it affects the immune system severely, the cells and the workings of it in our body must be clearly understood. The immune system’s role in the body is that of a soldier wherein it identifies any sort of anomalies that enters or infiltrates the body and prepares antibodies against it. And kills them in order to prevent infection that has the probability of causing a harmful disease.
Since the cells of the immune system have already created the antibodies, the cell memory is activated when the entry occurs again and the immune system fights and destroys such foreign and harmful matter.
What Happens when HIV Enters the Body?
When a person is infected with the Human immunodeficiency virus, it directly attacks the immune system making the cells weak and incapable of creating antibodies for this particular virus. As they become weak their function to perform the task of defending against other microorganism entrants is also weakened.
When the fighter in our bodies becomes weak, we are more likely to fall ill. The illness can be a simple flu or an allergy and our body cannot fight any further. The symptoms once infected will start to appear within the first two weeks. The symptoms are very flu-like for instance, one will be more tired than usual and fatigue will be more frequent and regular. Other symptoms include sore throat and fever. The risk of opportunistic infections like tuberculosis and herpes also increases. Some people however remain asymptomatic even for longer periods after being infected with the virus.
Cause of HIV/AIDS
The main and only cause of this dreadful disease is the contact through blood, semen, pre-seminal fluid, vaginal fluids, rectal fluids and breast milk. The semen and vaginal fluids are transferred through sex and rectal fluids through anal sex. When people have multiple partners, and they have unprotected sex the transmission is highly likely. The contact through blood can also be via the unhygienic practice of sharing an infected person’s razors, blades. Even unsterilized syringes while taking drugs or even a tattoo parlor where they use unsterilized machines on the body can transmit the virus easily. The transmission means are endless so one must proceed with utmost caution to keep themselves safe either way.
What is the Life Expectancy for the Patients Carrying HIV or AIDs with Them?
Many factors can affect the life expectancy of people living with HIV. Depending on these factors there are many differences in the outcomes between people, and other factors. The factors on which life expectancy depend are:
Access to effective HIV treatment and quality health care.
Start HIV treatment as soon as possible after HIV infection, before your CD4 cell count drops to a low level. The sooner you are diagnosed and start HIV treatment, the better your long-term chances are.
Having serious HIV-related illnesses in the past. This may occur before HIV is diagnosed and/or before HIV treatment is started. These diseases have a detrimental effect on life expectancy.
Results one year after starting HIV treatment. Studies show that life expectancy is better for people who respond well within a year of starting treatment than people who do not respond. In particular, people with a CD4 count of at least 350 and an undetectable viral load during the year have a much better chance long-term.
Year of Diagnosis - HIV treatment and medical care have improved over the years. People who have been diagnosed in recent years are expected to live longer than people who were diagnosed long ago.
Heart diseases, liver diseases, cancer and other health conditions are more likely to be the cause of death than HIV or AIDs.
Injecting drug use - Life expectancy is short for people with HIV who inject drugs, due to drug overdose and viral infections.
Social and Economic Conditions - there are significant differences in life expectancy depending on where you grew up, your income, education, social status and more.
Gender – Men are supposed to live for a shorter period of time than women.
Genetics - you may have certain conditions if close relatives have.
Mental and Emotional Well-being - high levels of stress are associated with reduced life expectancy.
Lifestyle - longevity for people who eat a balanced diet, are physically active, maintain a healthy weight, avoid alcohol abuse or use drugs, and stay in touch with the community. Avoiding smoking is very important in life.
There are a few myths surrounding this disease. It is believed earlier that AIDS can spread even through contact or touch without any exchange of fluids. Like through a hug or just by being near the infected person. That myth has been debunked and it is absolutely untrue. One can freely hug an AIDS patient without worry.
The other one was when kissing, there is an exchange of saliva which is also a fluid and AIDS can spread through kissing, which also proved to be untrue. And HIV always means AIDS that is fatal was another rumor or myth, and this myth is proven wrong where many people have lived longer with HIV by medication and taking care of their health.
There is no permanent cure yet for treating HIV/AIDS, so it is our responsibility to look out for ourselves. The way one can first prevent themselves from being infected is by getting vaccinated. It is important to get tested in your adult life if you have multiple sexual partners and also get your partner tested for the same. The other way is being monogamous. The most used form of prevention is having protected and safe sex and using condoms that creates a barrier for transmission. Do check for sterilized needles in case you decide to get a tattoo or injected. Lessen the use of alcohol and drugs as that is anyway weakening and altering the immune system.
According to the estimates of the Indian government 2.40 million Indians are living with HIV wherein, the infected ones fall in the age group of 15-49, and 39 %of them that is 9,30,00 of them are women. The numbers are alarming and the rate of increase is not slowing down anytime soon. We as a country must break the traditions and conversations about sex should be open and safe. It is high time we lose our lives to this disease which can be prevented.

FAQs on Essay on AIDS
1. Is AIDS an Autoimmune Disease?
In the early stages of HIV infection that leads to AIDS, the immune system only weakens so it is not an auto-immune disease. But during the later and final stages, the workings of the immune system are similar to that of an auto-immune system where it works against itself. And in such cases, the body of the individual is susceptible to many more diseases. AIDS, a disease found in immune deficiency disorder, is caused by HIV and weakens the human immune system. Autoimmune diseases, on the other hand, are where the immune system turns, attacking healthy cells.
2. Does one die from HIV Infection?
The HIV infection results in many symptoms that make the body weaker day by day. But some do not even suffer those symptoms and they may live longer than the ones showing severe symptoms. In any case, it is important to take medications that are prescribed to reduce the severity of symptoms and live a little longer. The best way is to keep healthy and lead an active lifestyle as much as possible. Although the death toll from AIDS has dropped dramatically around the world, this situation increases the risk of contracting a fatal disease — potentially leading to death. No treatment or cure is present for HIV.
3. What method was adopted by the hospitals to report HIV or AIDs cases?
The doctors took the active initiative for the reporting and diagnosis of HIV or AIDs cases all over the world. The methods that all the French hospital wards were known for, for their role in controlling HIV infection, were asked to report the 2000 deaths among HIV-positive adults. The causes of death were recorded using a standard questionnaire. The Mortality 2000 study was launched to explain the distribution of the leading causes of death of HIV-positive people at the national level in France in the year 2000.
4. What is the way of determining the root cause of death in AIDs patients?
Following the International Classification of Diseases, 10th Revision (ICD-10) to death, the information contained in the questionnaire was used to determine the single cause of death. The causes of AIDS were categorized as one cause of death, followed by definitions of AIDS-related diseases. If a standard questionnaire was lost, summarized quarter notices were used to determine the underlying cause of death, if possible. Determination of the AIDs cases was set to the most important things in the list, which was done from the abstracted quarterly notifications from the questionnaires.
5. Is Vedantu a reliable website for knowing about AIDs disease?
Vedantu is the most reliable website for referring to information about AIDs disease. Being one of the most dangerous diseases in the world with no proper treatment or cure, the world's physicians are still under pressure to decipher the way to save a person from this disease. The Vedantu website contains authentic or updated information about this disease and thus the readers and viewers can rely on this source of information for perfect knowledge about the disease and its prevention also.

Essay on HIV AIDs Awareness
Students are often asked to write an essay on HIV AIDs Awareness in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on HIV AIDs Awareness
Understanding hiv and aids.
HIV stands for Human Immunodeficiency Virus. It attacks our body’s defense system. AIDS, or Acquired Immunodeficiency Syndrome, is the condition caused by HIV. It makes people very sick because their bodies can’t fight off illnesses well.
How HIV Spreads
HIV is passed from one person to another through blood, sharing needles, and from mother to baby during birth or breastfeeding. It’s also spread through sex without protection, like condoms.
Preventing HIV
Using new needles and safe sex practices, like condoms, can prevent HIV. Also, medicines can help mothers with HIV not pass the virus to their babies.
Living with HIV
People with HIV can live long, healthy lives with proper medicine. It’s important to get tested and start treatment early.
Spreading Awareness
Talking openly and learning more about HIV can help stop false beliefs and stop the virus from spreading. Schools and communities play a big role in this.
250 Words Essay on HIV AIDs Awareness
HIV stands for Human Immunodeficiency Virus. It attacks our body’s defense system, making it hard to fight off sickness. AIDS, which is Acquired Immune Deficiency Syndrome, happens when HIV has damaged the immune system a lot. People with AIDS can get very sick from infections that don’t usually make healthy people ill.
The Importance of Awareness
Knowing about HIV and AIDS is very important. It helps people learn how to protect themselves and others from getting the virus. Awareness also means understanding that people with HIV need support and should not be treated badly.
HIV can be passed from one person to another through blood, during sex, or from a mother to her baby during pregnancy, birth, or breastfeeding. It is not spread by touching, hugging, or sharing food.
Prevention is Key
Preventing HIV is better than trying to treat it. This means not sharing needles, using protection during sex, and getting tested if you think you might have been exposed to HIV. There are also medicines that can lower the risk of getting HIV.
Getting Tested
Getting tested for HIV is simple and can be private. If a test shows someone has HIV, it’s not the end of the world. With today’s medicines, people with HIV can live long and healthy lives.
Support and Respect
People with HIV deserve to be treated with kindness and respect. Being aware of HIV and AIDS means also fighting against wrong ideas and standing by those who have the virus. This way, we can all help stop HIV from spreading and support those living with it.
500 Words Essay on HIV AIDs Awareness
Understanding hiv/aids.
AIDS, which stands for Acquired Immune Deficiency Syndrome, is a serious health issue caused by the virus called HIV, or Human Immunodeficiency Virus. This virus attacks our body’s defense system, making it hard for the body to fight off diseases. People can get HIV from infected blood, sharing needles, or through unsafe sex. It’s also possible for a mother to pass it to her baby during pregnancy, birth, or breastfeeding.
Why Awareness is Important
Knowing about HIV/AIDS is very important because it helps prevent the spread of the disease. People who are aware are more careful and can protect themselves and others. They know the importance of safe practices, like using new needles for medicines and not sharing them. They also understand why it’s important to have safe sex, using protection to stop the virus from spreading.
One of the key parts of awareness is getting tested for HIV. Tests are the only way to know for sure if someone has the virus. Early testing means that if a person does have HIV, they can start treatment sooner. This helps them live a longer, healthier life and reduces the chance of passing the virus to someone else.
Treatments for HIV/AIDS
There is no cure for HIV/AIDS, but there are medicines called antiretroviral therapy (ART) that help control the virus. These medicines help people with HIV live longer, healthier lives and lower the chance of spreading the virus. Knowing about these treatments is a big part of awareness because it encourages people with HIV to get the help they need.
Support and Acceptance
People with HIV/AIDS often face tough times because others might not understand the disease. They can be treated unfairly or feel alone. HIV/AIDS awareness includes teaching people to be kind and supportive. When everyone understands the disease better, they can help those affected by HIV/AIDS feel accepted and not alone.
Education and Prevention
Teaching kids and adults about HIV/AIDS is a powerful way to stop the disease from spreading. Schools and community groups can give out information on how to stay safe and healthy. They can also explain that HIV is not spread by touching, hugging, or being friends with someone who has the virus.
Global Efforts
Countries around the world are working together to stop HIV/AIDS. They share information, support research for better treatments, and help people get the care they need. It’s a global fight, and awareness is a tool that everyone can use to join in.
HIV/AIDS awareness is about understanding the disease, knowing how to prevent it, and supporting those who have it. It’s about getting tested and starting treatment if needed. Most of all, it’s about kindness and working together to stop the spread of HIV/AIDS. When everyone knows more, they can do more to help themselves and others.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Pop Culture
- Essay on Poor And Rich Lifestyle
- Essay on Polygamy Is Better Than Monogamy
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- How to write an expository essay
How to Write an Expository Essay | Structure, Tips & Examples
Published on July 14, 2020 by Jack Caulfield . Revised on July 23, 2023.
“Expository” means “intended to explain or describe something.” An expository essay provides a clear, focused explanation of a particular topic, process, or set of ideas. It doesn’t set out to prove a point, just to give a balanced view of its subject matter.
Expository essays are usually short assignments intended to test your composition skills or your understanding of a subject. They tend to involve less research and original arguments than argumentative essays .
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
When should you write an expository essay, how to approach an expository essay, introducing your essay, writing the body paragraphs, concluding your essay, other interesting articles, frequently asked questions about expository essays.
In school and university, you might have to write expository essays as in-class exercises, exam questions, or coursework assignments.
Sometimes it won’t be directly stated that the assignment is an expository essay, but there are certain keywords that imply expository writing is required. Consider the prompts below.
The word “explain” here is the clue: An essay responding to this prompt should provide an explanation of this historical process—not necessarily an original argument about it.
Sometimes you’ll be asked to define a particular term or concept. This means more than just copying down the dictionary definition; you’ll be expected to explore different ideas surrounding the term, as this prompt emphasizes.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

An expository essay should take an objective approach: It isn’t about your personal opinions or experiences. Instead, your goal is to provide an informative and balanced explanation of your topic. Avoid using the first or second person (“I” or “you”).
The structure of your expository essay will vary according to the scope of your assignment and the demands of your topic. It’s worthwhile to plan out your structure before you start, using an essay outline .
A common structure for a short expository essay consists of five paragraphs: An introduction, three body paragraphs, and a conclusion.
Like all essays, an expository essay begins with an introduction . This serves to hook the reader’s interest, briefly introduce your topic, and provide a thesis statement summarizing what you’re going to say about it.
Hover over different parts of the example below to see how a typical introduction works.
In many ways, the invention of the printing press marked the end of the Middle Ages. The medieval period in Europe is often remembered as a time of intellectual and political stagnation. Prior to the Renaissance, the average person had very limited access to books and was unlikely to be literate. The invention of the printing press in the 15th century allowed for much less restricted circulation of information in Europe, paving the way for the Reformation.
The body of your essay is where you cover your topic in depth. It often consists of three paragraphs, but may be more for a longer essay. This is where you present the details of the process, idea or topic you’re explaining.
It’s important to make sure each paragraph covers its own clearly defined topic, introduced with a topic sentence . Different topics (all related to the overall subject matter of the essay) should be presented in a logical order, with clear transitions between paragraphs.
Hover over different parts of the example paragraph below to see how a body paragraph is constructed.
The invention of the printing press in 1440 changed this situation dramatically. Johannes Gutenberg, who had worked as a goldsmith, used his knowledge of metals in the design of the press. He made his type from an alloy of lead, tin, and antimony, whose durability allowed for the reliable production of high-quality books. This new technology allowed texts to be reproduced and disseminated on a much larger scale than was previously possible. The Gutenberg Bible appeared in the 1450s, and a large number of printing presses sprang up across the continent in the following decades. Gutenberg’s invention rapidly transformed cultural production in Europe; among other things, it would lead to the Protestant Reformation.
Here's why students love Scribbr's proofreading services
Discover proofreading & editing
The conclusion of an expository essay serves to summarize the topic under discussion. It should not present any new information or evidence, but should instead focus on reinforcing the points made so far. Essentially, your conclusion is there to round off the essay in an engaging way.
Hover over different parts of the example below to see how a conclusion works.
The invention of the printing press was important not only in terms of its immediate cultural and economic effects, but also in terms of its major impact on politics and religion across Europe. In the century following the invention of the printing press, the relatively stationary intellectual atmosphere of the Middle Ages gave way to the social upheavals of the Reformation and the Renaissance. A single technological innovation had contributed to the total reshaping of the continent.
If you want to know more about AI tools , college essays , or fallacies make sure to check out some of our other articles with explanations and examples or go directly to our tools!
- Ad hominem fallacy
- Post hoc fallacy
- Appeal to authority fallacy
- False cause fallacy
- Sunk cost fallacy
College essays
- Choosing Essay Topic
- Write a College Essay
- Write a Diversity Essay
- College Essay Format & Structure
- Comparing and Contrasting in an Essay
(AI) Tools
- Grammar Checker
- Paraphrasing Tool
- Text Summarizer
- AI Detector
- Plagiarism Checker
- Citation Generator
An expository essay is a broad form that varies in length according to the scope of the assignment.
Expository essays are often assigned as a writing exercise or as part of an exam, in which case a five-paragraph essay of around 800 words may be appropriate.
You’ll usually be given guidelines regarding length; if you’re not sure, ask.
An expository essay is a common assignment in high-school and university composition classes. It might be assigned as coursework, in class, or as part of an exam.
Sometimes you might not be told explicitly to write an expository essay. Look out for prompts containing keywords like “explain” and “define.” An expository essay is usually the right response to these prompts.
An argumentative essay tends to be a longer essay involving independent research, and aims to make an original argument about a topic. Its thesis statement makes a contentious claim that must be supported in an objective, evidence-based way.
An expository essay also aims to be objective, but it doesn’t have to make an original argument. Rather, it aims to explain something (e.g., a process or idea) in a clear, concise way. Expository essays are often shorter assignments and rely less on research.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Caulfield, J. (2023, July 23). How to Write an Expository Essay | Structure, Tips & Examples. Scribbr. Retrieved April 4, 2024, from https://www.scribbr.com/academic-essay/expository-essay/
Is this article helpful?

Jack Caulfield
Other students also liked, academic paragraph structure | step-by-step guide & examples, how to write topic sentences | 4 steps, examples & purpose, how to write an argumentative essay | examples & tips, unlimited academic ai-proofreading.
✔ Document error-free in 5minutes ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
HIV/AIDS Definition, Prevention and Treatment Essay
HIV is the short form of human immunodeficiency virus. When the person is infected with HIV, it is more likely that the acquired immunodeficiency syndrome (AIDS) will develop. HIV is a terrible condition because it cannot be cured. Once one has HIV, he or she will have to live with it for the rest of life. The virus infects T cells or CD4 cells. These cells are essential for the efficient functioning of the human immune system.
HIV destroys these cells and makes the human body vulnerable to various kinds of diseases. An infected individual can live up to ten years until HIV develops into AIDS. When the final stage occurs, the human immune system is too weak to resist even the slightest illnesses. As a result, one can die of the usual disease. The most common way of HIV/AIDS transition is the unprotected sexual contact. There is no cure for HIV. However, particular medicines can prolong the life of infected people ( HIV Basics n.d.).
Nowadays, AIDS is regarded as the most urgent issue on the global level. The number of HIV-infected people is immense in South Africa. The virus is the public health threat. For instance, more than thirteen thousand residents died of AIDS in the U.S. in 2012. More than one million people died of AIDS in the world in 2014 ( Basic Statistics n.d.). These rates prove the fact that HIV is the plague of the twenty-first century. Public health safety should be of primary concern.
HIV and AIDS rapid distribution resulted in million deaths of people. This epidemic also has changed the society drastically. The statistics showed that HIV was more often diagnosed in men who had sex with men. Due to this fact, people all over the world became extremely prejudiced against homosexual communities. Almost eighty countries in the world consider homosexuality illegal. Individuals who display the belonging to some of the GLBT communities can be even punished. Fowler (2014) writes that in some countries being a gay is like being a Jew in Nazis Germany.
In my opinion, the government should react timely and adequately to such issues as HIV/AIDS. President Barack Obama established the National HIV/AIDS Strategy in 2010. This program was the first comprehensive step towards fighting the problem ( HIV/AIDS: Moving Forward n.d.).
The strategy was aimed at increasing the level of population’s awareness concerning the ways of HIV transmission. The results show that the level of HIV-infected people decreases every year in America ( HIV in the United States: At a Glance n.d.). This situation can be explained by the fact that Obama’s administration improved the situation in term of public health safety.
Numerous institutions aim at providing the best health care and prevention services. The roles of medical professional and public health expert in HIV/AIDS treatment differ. According to Gebbie, Rosenstock, and Hernandez (2003), public health professionals aim at improving the health condition on the level of population.
They can work in schools or organizations. The task of the public health professional is to educate citizens, make as many people as possible aware of potential threats. Medical professional works with the individual. The medical nurse takes care of the particular patient and makes every effort to improve his or her health condition or prevent the risk. That is the primary difference between responsibilities of the public health professional and the medical professional.
Basic Statistics . (n.d.). Web.
Fowler, N. (2014). HIV Remains Global Health Problem, Thanks to Ignorance and Prejudice . Web.
Gebbie, K., Rosenstock, L., & Hernandez, L. (2003). Who Will Keep the Public Healthy? Washington, D. C., USA: The National Academies Press.
HIV/AIDS: Moving Forward . (n.d.) Web.
HIV Basics . (n.d.) Web.
HIV in the United States: At a Glance (n.d.) Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 23). HIV/AIDS Definition, Prevention and Treatment. https://ivypanda.com/essays/hivaids-definition-prevention-and-treatment/
"HIV/AIDS Definition, Prevention and Treatment." IvyPanda , 23 Feb. 2024, ivypanda.com/essays/hivaids-definition-prevention-and-treatment/.
IvyPanda . (2024) 'HIV/AIDS Definition, Prevention and Treatment'. 23 February.
IvyPanda . 2024. "HIV/AIDS Definition, Prevention and Treatment." February 23, 2024. https://ivypanda.com/essays/hivaids-definition-prevention-and-treatment/.
1. IvyPanda . "HIV/AIDS Definition, Prevention and Treatment." February 23, 2024. https://ivypanda.com/essays/hivaids-definition-prevention-and-treatment/.
Bibliography
IvyPanda . "HIV/AIDS Definition, Prevention and Treatment." February 23, 2024. https://ivypanda.com/essays/hivaids-definition-prevention-and-treatment/.
- Pneumonia Infection & Risk of Mortality in HIV-Infected Children
- Community Health Paper: Human Immunodeficiency Virus
- About Human Immunodeficiency Virus
- Human Immunodeficiency Virus Research in Nursing
- Tuberculosis and Human Immunodeficiency Coinfection
- Acquired Immunodeficiency Syndrome in Uganda
- HIV (Human Immunodeficiency Virus) Prevention
- Acquired Immunodeficiency Syndrome Pathophysiology
- Ancillary Services for HIV/AIDS Patients
- Center for Disease Control and HIV Prevention Goals
- Allergic Rhinitis: A Critical Discussion
- Tregs in Allergies and Autoimmune Diseases
- The Impact of Medical Biotechnology on Society: Vaccines
- B-Cells Role in the Immune System
- B-Cells and Anti Bodies
Essay About HIV/AIDS
Introduction.
Human Immunodeficiency Virus, abbreviated as HIV, attacks the body’s immune system, and if left untreated, it can cause AIDS (Acquired Immune Deficiency Syndrome). HIV is a retroviral disease transmitted through unprotected sexual activity, from mother to child, blood transfusion, contact with infected body fluids, or hypodermic needles (Melhuish & Lewthwaite, 2018). The disease originated from a zoonotic animal, a chimpanzee in Central Africa. The virus version in chimpanzees, Simian Immunodeficiency Virus, is thought to have been passed to humans during their hunting activities way back in 1800. The disease has further been spread across Africa over the decades and eventually into other parts of the world. Its existence in the United States occurred between the mid to late 1970s.
Disease Manifestation
HIV weakens the immune system through infection and destruction of the CD4+ T cells, leading to immunodeficiency at the later stages of the disease. The virus adheres to the CD4+ protein on its surface and other cells to gain entry into the body ( Melhuish & Lewthwaite, 2018 ). Other coreceptors such as CCR5 and CXCR4 are essential in enabling the virus to gain complete access and cause infection to the body cells. HIV infection undergoes three stages: acute illness, chronic infection, and acquired immunodeficiency syndrome (AIDS) (Velloza et al., 2020). The first stage usually develops between 2 to 4 weeks after initial exposure. The stage often goes unrecognized because of the occasionally mild and nonspecific symptoms. Some of the clinical manifestations observed in the first stage include typical rushes distributed on the face and trunk, although they may also appear in the palms and soles. Oral and genital mucocutaneous ulceration is also another clinical manifestation that can be experienced during the first stage. In this stage, gastrointestinal manifestation, facial nerve palsy, acute encephalopathy, and many other clinical symptoms may participate.
In the second stage of infection, the virus continues to multiply but at low levels. Infected individuals who are in this stage may not have any alarming symptoms. The stage can last for up to 10 to 15 years, although it may move so fast in some individuals c. AIDs infection occurs in the third stage. The infection may be manifested by symptoms such as rapid loss of weight, recurring fever, extreme tiredness, prolonged swelling of the lymph glands in the groin, armpits, or neck, sores in the mouth, diarrhea that lasts for more than a week, or memory loss and other neurologic disorder (Nasuuna et al., 2018). When infected individuals are not treated, they may develop severe diseases such as serious bacterial infections, cryptococcal meningitis, tuberculosis, and cancers like Kaposi’s sarcoma and lymphomas.
Diagnosis and Treatment
HIV diagnosis can be made by a rapid diagnostic test that provides results on the same day. Individuals may also test themselves using an HIV self-test kit, although a confirmatory test has to be done later on by a qualified health professional (Mayo Clinic, 2020). The diagnostic test works by detecting antibodies produced by a person as part of their immune response to fight the virus. When the results turn out positive, immediate treatment should be done to manage the virus (Mayo Clinic, 2020). A combination of three or more antiretroviral drugs (ARVs) or antiretroviral therapy (ART) may suppress the symptoms and viral replication within an individual hence allowing recovery of the immune system and regain the ability to protect the body from opportunistic infections.
The public health measures of HIV prevention can be divided into three categories; primary, secondary, and tertiary prevention. Primary prevention measures protect an individual from acquiring HIV infection. It involves strategies such as abstaining from sex, not sharing needles and sharp objects and using condoms when engaging in sexual activities. Prevention medicines such as PrEP (pre-exposure prophylaxis) and PEP (post-exposure prophylaxis) may also be used to protect yourself from the infection (Mayo Clinic, 2020). Secondary HIV prevention involves measures that should be directed to infected individuals to prevent transmission to negative people (Mayo Clinic, 2020). Strategies used in secondary prevention entails giving health education to those who are infected, supporting ART adherence efforts, providing ongoing risk assessment regarding substance use and sexual behavior, encouraging infected individuals to disclose their HIV status to their sexual and drug use partners, prescribing condoms for positive individuals and providing counseling to them (Mayo Clinic, 2020). Tertiary prevention measures ensure the improved treatment to reduce the impact of HIV/AIDS disease and promote recovery. A tertiary prevention strategy aims at reducing complications that may be caused by HIV infection.
Surveillance measures
Local surveillance of HIV may be carried out using various reporting tools to fill HIV infection cases and later submitted to the local health departments for further analysis. The Centre for Disease Control and Prevention (CDC) plays a big role in collecting, analyzing, and disseminating data for national surveillance on HIV/AIDS. The CDC’s National surveillance system monitors HIV trends in the U.S (CDC, 2020). Moreover, the World Health organization can conduct international surveillance of HIV/AIDS, which surveys on HIV sentinel, STDs, and behavior.
Prevalence and Incidence
According to WHO (2020), the global prevalence of HIV is estimated to be over 37.7 million people, including 1.7 million children. The percentage prevalence in adults is 0.7%. Additionally, the incidence of HIV infection was 1.5 million (WHO, 2020). Most people living with HIV live in low and middle-income countries, with East and Southern Africa being the most affected region globally. In 2020, there were 670,000 new cases which amounted to 20.6 million infected individuals in East and Southern Africa.
Interesting facts
According to the WHO, some of the current interesting facts about HIV/AIDS is that it has claimed over 36.3 million people since its emergence; hence, it is still a major public health concern (WHO, 2021). Additionally, over 37.7 million were HIV positive in 2020, whereby 25.4 million were in the WHO African region. WHO also reports that over 680 thousand individuals succumbed to HIV-related infections, and over 1.5 million people acquired HIV/AIDS.
CDC. (2020, June 19). Tracking AIDS Trends . Centers for Disease Control and Prevention. https://www.cdc.gov/hiv/statistics/surveillance/index.html
Eisinger, R. W., & Fauci, A. S. (2018). Ending the HIV/AIDS pandemic. Emerging infectious diseases , 24 (3), 413.
Mayo Clinic. (2020, February 13). HIV/AIDS – Symptoms and causes . https://www.mayoclinic.org/diseases-conditions/hiv-aids/symptoms-causes/syc-20373524
Melhuish, A., & Lewthwaite, P. (2018). Natural history of HIV and AIDS. Medicine , 46 (6), 356-361.
Nasuuna, E., Kigozi, J., Babirye, L., Muganzi, A., Sewankambo, N. K., & Nakanjako, D. (2018). Low HIV viral suppression rates following the intensive adherence counseling (IAC) program for children and adolescents with viral failure in public health facilities in Uganda. BMC Public Health , 18 (1), 1-9.
Velloza, J., Kemp, C. G., Aunon, F. M., Ramaiya, M. K., Creegan, E., & Simoni, J. M. (2020). Alcohol use and antiretroviral therapy non-adherence among adults living with HIV/AIDS in Sub-Saharan Africa: a systematic review and meta-analysis. AIDS and Behavior , 24 (6), 1727-1742.
WHO. (2021, June 9). HIV/AIDS . WHO | World Health Organization. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
Cite This Work
To export a reference to this article please select a referencing style below:
Related Essays
Advancements in human genomics, emergence of the chicago school of architecture, the american health care act of 2017, interdisciplinary plan proposal, professional nursing: empowering healthcare through skill, compassion, and leadership, technology in healthcare, popular essay topics.
- American Dream
- Artificial Intelligence
- Black Lives Matter
- Bullying Essay
- Career Goals Essay
- Causes of the Civil War
- Child Abusing
- Civil Rights Movement
- Community Service
- Cultural Identity
- Cyber Bullying
- Death Penalty
- Depression Essay
- Domestic Violence
- Freedom of Speech
- Global Warming
- Gun Control
- Human Trafficking
- I Believe Essay
- Immigration
- Importance of Education
- Israel and Palestine Conflict
- Leadership Essay
- Legalizing Marijuanas
- Mental Health
- National Honor Society
- Police Brutality
- Pollution Essay
- Racism Essay
- Romeo and Juliet
- Same Sex Marriages
- Social Media
- The Great Gatsby
- The Yellow Wallpaper
- Time Management
- To Kill a Mockingbird
- Violent Video Games
- What Makes You Unique
- Why I Want to Be a Nurse
- Send us an e-mail
Your Article Library
Essay on hiv/aids: signs, symptoms and prevention.
ADVERTISEMENTS:
Essay on HIV/AIDS: Signs, Symptoms and Prevention!
Human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS) is a disease of the human immune system caused by the human immunodeficiency virus (HIV). During the initial infection a person may experience a brief period of influenza-like illness.
This is typically followed by a prolonged period without symptoms. As the illness progresses it interferes more and more with the immune system, making people much more likely to get infections, including opportunistic infections, and tumors that do not usually affect people with working immune systems.
Genetic research indicates that HIV originated in west-central Africa during the early twentieth century. AIDS was first recognized by the Centres for Disease Control and Prevention (CDC) in 1981 and its cause, HIV infection was identified in the early part of the decade.
Since its discovery, AIDS has caused nearly 30 million deaths (as of 2009). As of 2010, approximately 34 million people have contracted HIV globally. AIDS is considered a pandemic —a disease outbreak which is present over a large area and is actively spreading.
Origin of HIV/AIDS:
1. Through African Monkey To human.
2. Through Vaccine Programme
(a) Polio, small pox vaccine from monkey’s kidney-Africa.
(b) Hepatitis-B viral vaccine-Los Angles and New York
HIV/AIDS has had a great impact on society, both as an illness and as a source of discrimination. The disease also has significant economic impacts. There are many misconceptions about HIV/AIDS such as the belief that it can be transmitted by casual non-sexual contact. The disease has also become subject to many controversies involving religion.
Signs and Symptoms :
There are three main stages of HIV infection:
Acute infection, clinical latency and AIDS.
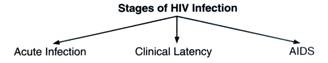
The initial period following the contraction of HIV is called acute HIV, primary HIV or acute retroviral syndrome. Many individuals develop an influenza like illness or a mononucleosis-like illness 2-4 weeks post exposure while others have no significant symptoms.
Symptoms occur in 40-90% of the cases and most commonly include fever, large tender lymph nodes, throat inflammation, a rash, headache, and/or sores of the mouth and genitals. The rash, which occurs in 20-50% of cases, presents itself on the trunk and is classically maculopapular.
Some people also develop opportunistic infections at this stage. Gastrointestinal symptoms such as nausea, vomiting or diarrhoea may occur, as may neurological symptoms of peripheral neuropathy or Guillain-Barre syndrome. The duration of the symptoms varies, but is usually one or two weeks.

HIV is transmitted by three main routes: sexual contact, exposure to infected body fluids or tissues and from mother to child during pregnancy, delivery, or breastfeeding (known as vertical transmission). There is no risk of acquiring HIV if exposed to feces, nasal secretions, saliva, sputum, sweat, tears, urine, or vomit unless these are contaminated with blood. It is possible to be co-infected by more than one strain of HIV, a condition known as HIV super infection.

Prevention from AIDS :
Sexual contact:.
Consistent protection use reduces the risk of HIV transmission by approximately 80% over the long term. When one partner of a couple is infected, consistent protection use results in rates of HIV infection for the uninfected person of below 1% per year. There is some evidence to suggest that female protection may provide an equivalent level of protection.
Application of a vaginal gel containing tenofovir (a reverse transcriptase inhibitor) immediately before sex seems to reduce infection rates by approximately 40% among African women. By contrast, use of the spermicide nonoxynol-9 may increase the risk of transmission due to its tendency to cause vaginal and rectal irritation. Circumcision in Sub-Saharan Africa “reduces the acquisition of HIV by heterosexual men by between 38% and 66% over 24 months”.
Based on these studies, the World Health Organization and UNAIDS both recommended male circumcision as a method of preventing female-to-male HIV transmission in 2007. Whether it protects against male- to-female transmission is disputed and whether it is of benefit in developed countries and among men who have sex with men is undetermined.
Some experts fear that a lower perception of vulnerability among circumcised men may result in more sexual risk-taking behavior, thus negating its preventive effects. Women who have undergone female genital cutting have an increased risk of HIV.
Programs encouraging sexual abstinence do not appear to affect subsequent HIV risk. Evidence for a benefit from peer education is equally poor. Comprehensive sexual education provided at school may decrease high risk behavior.
A substantial minority of young people continues to engage in high-risk practices despite knowing about HIV/AIDS, underestimating their own risk of becoming infected with HIV. It is not known if treating other sexually transmitted infections is effective in preventing HIV.
Mother-to-child:
Programs to prevent the transmission of HIV from mothers to children can reduce rates of transmission by 92-99%. This primarily involves the use of a combination of antivirals during pregnancy and after birth in the infant but also potentially includes bottle feeding rather than breastfeeding.
If replacement feeding is acceptable, feasible, affordable, sustainable and safe, mothers should avoid breast-feeding their infants, however exclusive breast-feeding is recommended during the first months of life if this is not the case. If exclusive breast feeding is carried out, the provision of extended antiretroviral prophylaxis to the infant decreases the risk of transmission.
Vaccination:
As of 2012 there is no effective vaccine for HIV or AIDS. A single trial of the vaccine RV 144 published in 2009 found a partial reduction in the risk of transmission of roughly 30%, stimulating some hope in the research community of developing a truly effective vaccine. Further trials of the RV 144 vaccine are on-going.
Related Articles:
- AIDS: Paragraphs on AIDS Diseases (384 Words)
- Frequently Asked Questions on AIDS
No comments yet.
Leave a reply click here to cancel reply..
You must be logged in to post a comment.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee to Study HIV Transmission Through Blood and Blood Products; Leveton LB, Sox HC Jr., Stoto MA, editors. HIV And The Blood Supply: An Analysis Of Crisis Decisionmaking. Washington (DC): National Academies Press (US); 1995.

HIV And The Blood Supply: An Analysis Of Crisis Decisionmaking.
- Hardcopy Version at National Academies Press
8 Conclusions and Recommendations
The HIV epidemic has taught scientists, clinicians, public health officials, and the public that new infectious agents can still emerge. The nation must be prepared to deal with a fatal illness whose cause is initially unknown but whose epidemiology suggests it is an infectious disease. The AIDS epidemic has also taught us another powerful and tragic lesson: that the nation's blood supply—because it is derived from humans—is highly vulnerable to contamination with an infectious agent. A nation's blood supply is a unique, essential, life-giving resource. Whole blood and many blood products are lifesaving for many people. As a whole, our nation's system works effectively to supply the nation with necessary blood and blood products and its quality control mechanisms check most human safety threats. The events of the early 1980s, however, revealed an important weakness in the system—in its ability to deal with a new threat that was characterized by substantial uncertainty. The potential for recurring threats to the blood supply led this Committee to reappraise the processes, policies, and resources through which our society attempts to preserve its supply of safe blood and blood products.
- General Conclusions
The events and decisions that the Committee has analyzed underscore the difficulty of decisionmaking when the stakes are high, when decisionmakers may have personal or institutional biases, and when knowledge is imprecise and incomplete. The Committee attempted to understand the complexities of the decisionmaking process during the period analyzed in this report and develop lessons to protect the blood supply in the future. In retrospect, the system was not dealing well with contemporaneous blood safety issues such as hepatitis, and was not prepared to deal with the far greater challenge of AIDS .
By January 1983, the Centers for Disease Control (CDC) had accumulated enough epidemiological evidence to conclude that the agent causing AIDS was almost certainly transmitted through blood and blood products and could be sexually transmitted to sexual partners. The conclusion that the AIDS agent was blood-borne rested on two findings. First, AIDS was occurring in transfusion recipients and individuals with hemophilia who had received AHF concentrate; these AIDS patients did not belong to any other known high-risk group for contracting AIDS. Second, the epidemiologic pattern of AIDS was similar to hepatitis B, another blood-borne disease. However, the magnitude and consequences of the risk for transfusion and blood product recipients was not known at this time. Furthermore, the epidemiological pattern of the new disease was difficult to interpret because, unlike most infectious diseases, there seemed to be several years between exposure leading to infection and the development of symptoms. As a result, physicians and public health officials underestimated the large number of infectious people who had no symptoms of AIDS but could transmit the disease to others and therefore substantially understated the risk of infection.
Compared to the pace of many regulatory and public health decision processes, the federal government responded relatively swiftly to the early warnings that AIDS might be transmitted through blood and blood products. Public and private sector officials considered a range of clinical and public health interventions for reducing the risk of AIDS transmission through blood and blood products. This period, however, was characterized by a great deal of scientific uncertainty about the risks of HIV infection through blood and blood products and about the costs and benefits of the available options. The result, the Committee found, was a pattern of responses which, while not in conflict with the available scientific information, was very cautious and exposed the decisionmakers and their organizations to a minimum of criticism. This limited response can be seen in the refusal of blood banks in 1983 and 1984 to screen for and defer homosexuals or use surrogate tests ( Chapter 5 ), in the Food and Drug Administration's (FDA) cautious and inadequate regulatory approach to the recall of potentially contaminated AHF concentrate ( Chapter 6 ), and in the failure of physicians and the National Hemophilia Foundation to disclose completely the risks of using AHF concentrate and the alternatives to its use ( Chapter 7 ).
Blood safety is a shared responsibility of many diverse organizations. They include U.S. Public Health Service agencies such as the CDC, the FDA, and the National Institutes of Health (NIH), and private-sector organizations such as community blood banks and the American Red Cross, blood and plasma collection agencies, blood product manufacturers, groups such as the National Hemophilia Foundation (NHF), and others. The problems the Committee found were inadequate leadership and inadequate institutional decisionmaking processes in 1983 and 1984. No person or agency was able to coordinate all of the organizations sharing the public health responsibility for achieving a safe blood supply.
Decisionmaking Under Uncertainty
The management of a public health risk requires an evolving process of decisionmaking under uncertainty. It includes interpretive judgment in the presence of scientific uncertainty and disagreement about values. Public health officials must characterize and estimate the magnitude of the risk, which involves considering both the likelihood that infection might occur in various circumstances, and the costs and benefits associated with each of the possible uncertain outcomes. They must also develop and test public health and clinical care strategies, and communicate with the public about the risk and strategies for reducing it. When confronted with a poorly understood and anomalous public health threat, inertia often influences decisions. It is often easier to maintain the status quo than to make a change. In fact, regulatory policymakers, health scientists, and medical experts often require substantial scientific evidence before informing the public and adopting remedial action. Lack of scientific consensus becomes a kind of amplifier for the usual discord and conflict that can be expected whenever an important science-based public policy decision—one profoundly affecting lives and economic interests—must be made. First, uncertainty creates opportunities for advocates of self-interested and ideological viewpoints to advance plausible arguments that favor their desired outcome. Second, uncertainty intensifies bureaucrat cautiousness.
In the course of its investigations, the Committee learned several lessons about decisionmaking under uncertainty. These are set out here both as general lessons and to provide a framework for the recommendations that follow.
Risk Perception
Risk perception is shaped by social tensions, and cultural, political, and economic biases (Douglas 1985). It is important to understand the different contexts in which risk is perceived and the complex system of beliefs, values, and ideals that shape risk perception (Nelkin 1989). There are several other factors that influence risk perception, including locus of control, the type of risk posed by the threat, and the time interval involved in evaluating the risk. For example, people tend to underestimate risks that they perceive to be under their control, risks associated with a familiar situation, and low probability events (Douglas 1985). People have difficulty accepting estimates of a risk that is involuntary, uncertain, unfamiliar, and potentially catastrophic (Fischoff 1987). The epidemic caused by HIV in the blood supply illustrates these patterns of perception and behavior with respect to risk.
Risk Assessment Versus Risk Management
A central precept of risk management is to separate the assessment of risk from the management of its consequences (NRC 1983). Otherwise, risk managers tend to bias their estimates of the magnitude of the risk in favor of their preconceived notions about appropriate or desirable policy choices. The events that the Committee studied provide examples of what can happen when this precept is not followed. When there is uncertainty, it may be necessary to assess risk by making subjective estimates rather than by obtaining objective measures. Such was the case in 1983 when, as part of implicit risk-benefit calculations about donor screening and deferral, blood banks and blood product manufacturers had to make judgments about the risk that their products could transmit AIDS (see Chapter 5 ). Anticipating the consequences of taking action, which is in the domain of risk management, may bias risk estimates toward values that support risk-averse action. When blood bank officials estimated the risk of transmitting AIDS as ''one per million" transfusions, they chose a rate that was low enough to justify their reluctance to take further action. Despite mounting evidence that the risk was much higher, they maintained their original estimate throughout 1983. If the CDC had made numeric estimates of the risk, and the blood banks, blood product manufacturers, or the FDA had used these estimates in a formal analysis of the decision problem, they might have reached different conclusions about, for example, surrogate testing for AIDS.
Consider the Full Range of Possibilities
When there is uncertainty about the facts that will determine the consequences of a decision, a systematic approach is usually best (NRC 1994). One important principle is to consider the full range of assumptions and alternative actions, not only worst-case scenarios. In the events studied by the Committee, systematic denial of worst-case scenarios was a recurring theme, as can be seen in the way that the NHF and the FDA discussed the CDC's warnings in 1982 and early 1983. The plasma fractionators introduced a worst case scenario of their own at the July 1983 Blood Products Advisory Committee (BPAC) meeting, when they estimated that three or four suspect donors and an automatic recall policy could lead to recall of all of the nation's supply of AHF concentrate ( Chapter 6 ). A closely related principle is to scrutinize the evidence to ascertain what is based on fact, what is a "best-guess" estimate, and what is simply untested conventional wisdom.
One approach to such an analysis would be to use a formal group process to systematically sample expert opinion on relevant factors such as the probability of infection and the economic and noneconomic costs and benefits of each of the possible outcomes. Often these officials should use decision analysis, which takes into account the likelihood of events and the magnitude of their outcomes, as a tool to compare the expected value of the outcome of the policy alternatives under consideration. Two somewhat analogous models to consider include those used in Institute of Medicine studies to establish priorities for the development of new vaccines (IOM 1985) and to evaluate the artificial heart program of the National Heart, Lung, and Blood Institute (IOM 1991). The book Acceptable Risk (Fischoff, et al. 1981) also offers sensible approaches to dealing with this kind of situation.
Risk Reduction Versus Zero Risk
Decisionmakers tend to seek zero-risk solutions even when they are unattainable or unrealistically costly (NRC 1994). In doing so, they may run the risk of failing to implement solutions that are less effective but are certain to reduce illness. The failure to adopt risk-reduction strategies can be seen in the resistance of blood banks to screening for homosexual activity or using surrogate tests for AIDS ( Chapter 5 ) and in FDA's limited approach to product recall decisions ( Chapter 6 ). Chapter 7 also points out that many risk-reduction strategies for individuals with hemophilia were available but not fully disclosed or recommended. The perfect should not be the enemy of the good.
Risk Communication
Risk communication is a sensitive area because of its influence on the perceptions and behaviors of health professionals and consumers, regulatory policies, and public decisionmaking (Nelkin 1989). Many public health officials and physicians wish to appear in command and infallible. When uncertain, they remain silent rather than disclose their ambivalence (NRC 1989). In the Committee's view, however, the greater the uncertainty, the greater the need for communication. The Committee's analysis of physician–patient communications at the beginning of the AIDS era illustrates the tragedies that can accompany silence about risks ( Chapter 7 ). Risk-communication skills are equally important when presenting information to the general public. The blood banks' reluctance to acknowledge the risk of transfusion-associated AIDS ( Chapter 5 ) seems to have been due in part to the difficulties that they foresaw in presenting this information to potential donors and recipients.
Other important principles of risk communication are that the source of the information must be credible, the process should be open and two-way, and the message should be balanced and accurate (NRC 1989). When there was no other sources of information for physicians treating people with hemophilia and for their patients, the NHF and its Medical and Scientific Advisory Council (MASAC) took on an important risk-communication role—providing what would now be called "clinical practice guidelines." The NHF's credibility in this area was eventually seriously compromised by its financial connections to the plasma fractionation industry.
Bureaucratic Management of Potential Crises
Federal agencies had the primary responsibility for dealing with the national emergency posed by the AIDS epidemic. The Committee scrutinized bureaucratic function closely, and came to the following conclusions about the management of potential crises.
Coordination and Leadership
A crisis calls for extraordinary leadership. Legal and competitive concerns may inhibit effective action by agencies of the federal government. Similarly, when policymaking occurs against a backdrop of a great deal of scientific uncertainty, bureaucratic standard operating procedures designed for routine circumstances seem to take over unless there is a clear-cut decisionmaking hierarchy. An effective leader will insist upon coordinated planning and execution. Focusing efforts and responsibilities, setting timetables and agendas, and assuming accountability for expeditious action cannot be left to ordinary standard operating procedures. These actions are the responsibilities of the highest levels of the public health establishment.
The Public Health Service failed to bring these leadership functions to bear when CDC scientists raised concerns about the blood supply at the January 4, 1983 meeting but received no public support from the director of the CDC or the office of the Assistant Secretary for Health. Similarly, the record does not indicate that the highest levels of the FDA or the PHS were involved in responding to advice from the BPAC regarding donor deferral or product recall. Part of this leadership problem may stem from major changes in the PHS leadership that took place during this period: the leadership of the FDA, the CDC, and the NIH, and the person serving as the Assistant Secretary for Health all changed between 1982 and 1984.
Advisory Mechanisms
In the early 1980s, the FDA and other agencies did not have a systematic approach to conducting advisory committee proceedings. Such an approach requires that agencies tell their advisory committees what is expected of them, keep attention focused on high-priority topics, and independently evaluate the advice offered. No regulatory process should have its information base effectively controlled by an advisory panel. Public agencies must be able to generate and analyze the information that they need to assure that decisions serve the needs of the public. The FDA failed to observe this principle when it allowed statements and recommendations of the BPAC to go unchallenged, apparently because it could not independently analyze the information ( Chapter 6 ).
Because mistakes will always be made and opportunities sometimes missed, regulatory structures must be organized and managed to assure both the reality and the continuous appearance of propriety. The prominence of representatives from blood banks and blood product manufacturers on the BPAC, with no balancing influence from consumers and no process within the FDA to evaluate its recommendations ( Chapter 6 ), is a failure of advisory committee management. Perhaps advisory committees should contain fewer topical experts and more members with expertise in principles of good decisionmaking and the evaluation of evidence. A committee so constituted might run a reduced risk of standing accused of having conflicts of interest.
Analytic Capability and Long-Range Vision
Leadership passes to the organization that has access to information and the ability to analyze it. Federal agencies should avoid exclusive reliance upon the entities which they regulate for analysis of data and modeling of decision problems. The FDA should have had some independent capacity to analyze the information presented at the July 1983 BPAC meeting that suggested that with only three or four suspect donors, an automatic recall policy would completely deplete the nation's supply of AHF concentrate ( Chapter 6 ). In addition, there did not seem to be any focus within the Public Health Service prepared to, or charged to, analyze the options, costs, and benefits of the options for protecting the blood supply that were discussed at the January 4, 1983, meeting convened by CDC.
In addition, agencies need to monitor more systematically the long-term outcomes of blood transfusion and blood product infusion and to think far ahead to anticipate both new technologies and new threats to the safety of the blood supply. Because new pathogens can enter the blood supply and be propagated very rapidly through it, a low level of suspicion about a threat should trigger high-level consideration of how to manage and monitor the problem.
Through its fact-finding interviews and through written documents, the Committee found little evidence that the PHS agency heads and the Assistant Secretary for Health were involved in making decisions about protecting the blood supply in 1983 and 1984 when HIV was becoming increasing apparent as a threat. Most decisions and interagency communication seems to have occurred several levels below the top.
Presumptive Regulatory and Public Health Triggers
The Committee believes that the Public Health Service should prepare for future threats to the blood supply by specifying in advance the types of actions that should occur once the level of concern passes a threshold. In the face of scientific uncertainty, the PHS needs a series of criteria or triggers for taking regulatory or other public health actions to protect the safety of blood and blood products. The Committee favors a series of triggers in which the response is proportional to the magnitude of the risk and the quality of the information on which the risk estimate is based. Not all triggers should lead to drastic or irrevocable actions; some merely require careful consideration of the options or developing new information. This general principle is detailed by examples in each of the Committee's four areas of inquiry. Table 8.1 summarizes these triggers and corresponding actions.
Triggers for Taking Actions in Response to Uncertain.
Product Treatment
Whenever they propose new methods of protecting the safety of the blood supply, blood regulatory agencies must perform cost-utility or cost-benefit analyses to evaluate whether the intervention will advance the public health at reasonable costs. If manufacturers do not have market incentives, resources, or access to data to test promising methods, public agencies should create incentives or provide resources or access to data. In this case, the trigger is a new proposal to increase safety, and the action is for the public sector to assume responsibility for thorough analysis and development, or to create incentives for industry to do so.
When performing a cost-effectiveness analysis of new treatments for blood products, the potential to protect against other threats should always be a part of the analysis. Here, the trigger is the initiation of a cost-effectiveness analysis, and the action is to ensure that the analysis takes into account secondary benefits.
Donor Screening
Whenever epidemiologists identify a high-risk donor group, the FDA should immediately tell blood banks to create a way to defer that group and tell collection agencies to segregate and separately treat supplies obtained from those populations. Concerns about stigmatizing subpopulations and maintaining the supply of blood products should influence the means of taking actions, not whether to take action. In this case, the trigger to action is the identification of a high-risk population, and the action is deferral and segregation of lots.
Whenever any segment of the industry institutes a donor screening program, the FDA should require all segments of the industry to follow suit with actions that they believe will be at least as effective in promoting safety. Public regulators have a responsibility to monitor these efforts and to forge consensus or to impose the most effective methods as information concerning efficacy becomes available. Here, the trigger is one company's action to take an additional safety measure, and the response is for all companies to follow suit, or to be held accountable when they do not.
Blood banks should use a partially effective intervention that has little or no risk unless they can show that a better method will rapidly supersede it. In this case the trigger is the availability of an inherently risk-free, partially effective intervention, and the response to use that test/intervention unless it is certain to become redundant prior to realizing its full benefits.
When a test or treatment makes a product safer, manufacturers should withdraw all stocks of untested or untreated product as quickly as possible. Where immediate complete withdrawal might injure the public health, withdrawals should be partial or staged. Here, the trigger is the implementation of a new test or treatment process, and the action is to recall untested or untreated products as expeditiously as possible, given other considerations of public health.
A limited, staged, or selective recall places responsibility on public regulatory agencies to establish criteria for selecting lots for recall, to provide processes to permit effective implementation of the recall by industry, and to monitor the recall to assure that removal of the products occurs in the prescribed manner. In this case the trigger is the initiation of a recall action, and the response is to provide clear guidance and monitoring.
Communication to Patients and Providers
Whenever new information triggers inquiry into a possible threat to the blood supply, both patients and their physicians should have access to the information. Public officials should presume that candid statements and rigorous actions will enhance rather than erode public confidence and that persons using blood or blood products have the right to understand fully the risks and benefits of using these products. In this case, the trigger is new information relevant to the public health, and the action is to tell affected individuals what they need to make an informed choice: the facts, the gaps in knowledge, and the implications thereof.
- Recommendations
The Committee's charge was to learn from the events of the early 1980s the lessons that would help the nation prepare for future threats to the blood supply. The Committee identified potential problems with the system in place at that time (as summarized earlier in this chapter) and proposes changes that, if implemented in the early 1980s, might have moderated some of the effects of the AIDS epidemic on recipients of blood and blood products. This analysis has led the Committee to the following recommendations for Public Health Service agencies, for the blood and plasma fractionation industry, and for health care providers and the public. These recommendations address both public health options and individual clinical options.
The Committee is mindful of several caveats. First, the Committee is acutely aware of the difficulties of retrospective analysis, as described in Chapter 1 . Second, the Committee has not considered its recommendations from perspectives other than blood safety. Finally, the Committee tried to identify opportunities for institutional change that would respond to the problems that the Committee diagnosed. The Committee based its recommendations on the institutions as they functioned in the early 1980s, not as they exist now. The organizations responsible for blood safety and public health will have to evaluate their current policies and procedures to see if they fully address the issues raised by our recommendations.
The Public Health Service
Several federal agencies necessarily play important, often different roles in managing a public health crisis such as the contamination of blood and blood products by the AIDS virus. The National Blood Policy of 1973 charged the Public Health Service (including the CDC, the FDA, and the NIH) with responsibility for protecting the nation's blood supply.
The Committee has come to believe that a failure of leadership contributed to delay in taking effective action, at least during the period from 1982 to 1984. This failure led to incomplete donor screening policies, weak regulatory actions, and insufficient communication to patients about the risks of AIDS .
In the event of a threat to the blood supply, the PHS must, as in any public health crisis, insist upon coordinated action. The Secretary of Health and Human Services is responsible for all the agencies of the Public Health Service, 1 and therefore the Committee makes
Recommendation 1: The Secretary of Health and Human Services should designate a Blood Safety Director, at the level of a deputy assistant secretary or higher, to be responsible for the federal government's efforts to maintain the safety of the nation's blood supply.
Choosing a "lead person" is important because it is in the nature of federal agencies and their leaders to be at once competitive and protective. This condition is healthy in reasonable measure and in normal times. However, a serious threat to public health requires that agencies communicate, cooperate, and learn to view the world through each other's lenses. Once there is an action plan, the Secretary of Health and Human Services must hold the agency leaders accountable for enforcing cooperation in implementing the plan.
To be effective in coordinating the various agencies of the PHS, the Blood Safety Director should be at the level of a deputy assistant secretary or higher, and should not be a representative of any single PHS agency. When a threat does arise, the Blood Safety Director should create a crisis management team.
One such action was to establish, in July 1982, the Committee on Opportunistic Infections in Hemophiliacs (see Chapter 3 ). This group seems to have been organized by the CDC, but there is no record of its operations after August of that year.
Blood Safety Council
The AIDS crisis revealed that the institutions in place to ensure blood safety, both public and private, were unable to work cooperatively toward a common goal of a safe blood supply. The institutions were not accountable to anyone but themselves, and they failed to cooperate, to coordinate their activities, and to communicate effectively with physicians and the public. The Committee has become convinced that the nation needs a far more responsive and integrated process to detect, evaluate, and respond to emerging threats to the blood supply. To this end the Committee makes
Recommendation 2: The PHS should establish a Blood Safety Council to assess current and potential future threats to the blood supply, to propose strategies for overcoming these threats, to evaluate the response of the PHS to these proposals, and to monitor the implementation of these strategies. The Council should report to the Blood Safety Director (see Recommendation 1). The Council should also serve to alert scientists about the needs and opportunities for research to maximize the safety of blood and blood products. The Blood Safety Council should take the lead to ensure the education of public health officials, clinicians, and the public about the nature of threats to our nation's blood supply and the public health strategies for dealing with these threats.
Supplying safe blood and blood products to the nation—a public good—requires the cooperation of public and private institutions. The Blood Safety Council would give voice to the public's interest in having these institutions cooperate and would provide opportunities for them to do so.
The lessons of HIV transmission through blood and blood products show the need for an advisory council with a significantly greater level of diversity, responsibility, and authority than the current Blood Products Advisory Committee of the FDA. The BPAC is limited by the regulatory mission of the FDA which it advises, and there is no other body primarily concerned with blood safety as a whole. Representatives from governmental agencies, academia, the blood bank community, industry, and the public all have relevant expertise and perspectives and should be involved in the Blood Safety Council. A broad-based range of expertise in areas of hematology, infectious diseases, epidemiology, blood product manufacturing, blood collection and delivery, risk assessment, consumer advocacy, and cost-benefit analysis is essential.
The proposed Blood Safety Council would facilitate the timely transmission of information, assessment of risk, and initiation of appropriate action both during times of stability and during a crisis. The Council should report to the Blood Safety Director (see Recommendation 1). The Council would not replace the PHS agencies responsible for blood safety but would complement them by providing a forum for them to work together and with private organizations. The PHS agencies would be represented on the Council (see below and Figure 8.1 ). The Council would not have its own surveillance capability, but would work with CDC and FDA to interpret the information that those organizations can provide. It would not carry out or fund research itself, but would work with those at NIH and in the private sector to identify priorities for blood safety research. The Council would not have regulatory power, but would inform FDA actions from a blood safety rather than a product-specific perspective.
Figure 8.1.
Blood Safety Council relationships.
The organizations and groups that should be included in the Blood Safety Council, and the reasons for including them, are as follows:
- The FDA can provide a direct link between itself, the essential regulatory agency responsible for the safety of blood and blood products, and important sources of information, scientific support, and disease surveillance findings.
- The CDC can provide expertise in epidemiology, infectious diseases, and immunology as well as communicate the results of ongoing disease surveillance studies. The CDC's newly established emerging infectious disease program would also provide valuable information.
- The NIH can provide scientific expertise and the means to communicate information about essential research needs to the appropriate institutes for support of research.
- Representatives from academia can bring independent scientific and medical expertise, especially in hematology, infectious diseases, epidemiology, risk assessment, and cost-effectiveness analysis.
- Representatives from the volunteer blood collection community can bring experience with blood safety concerns and the knowledge of blood bank operations that is necessary to evaluate proposed change.
- Representatives from the private-sector blood product manufacturers and biotechnology companies can bring both experience with blood safety concerns and knowledge of plasma fractionation operations.
- Representatives of the general public (who may in the future require blood transfusions) and individuals who currently require frequent use of blood products, such as hemophilia patients, bring important perspectives on the trade-offs that must be considered in evaluating response options.
The Blood Safety Council should consider the following activities and issues:
Surveillance. Although the FDA and the CDC keep track of events in blood and blood product recipients, their surveillance systems are passive and incomplete. The Blood Safety Council should work with the CDC to design a system of active surveillance for adverse reactions in blood recipients, as described in Recommendation 5 below. If such a system is established, the Council would benefit from its results and should participate in its governance.
Expert Panel on Best Practices . Drawing on its members' knowledge about blood and blood product safety concerns, and about clinical alternatives, the Blood Safety Council could establish a panel of experts to provide the public and providers of care with information about risks and benefits, alternatives to using blood products, and recommended best practices, as described in more detail in Recommendation 13 below.
Investigate Methods to Make Blood Products Safer. The Council should evaluate new methods to make blood and blood products safer. One promising approach is double inactivation in the preparation of blood products, which minimizes the risk of transmission of infectious pathogens in the blood of the donor pool. At present, the FDA requires only a single inactivation process (usually solvent detergent or heat treatment) for most blood products manufactured in the United States. With the goal of maximizing the safety of the blood supply at minimal added cost, the Blood Safety Council should encourage the FDA to evaluate double inactivation methods and expeditiously relicense products manufactured by the improved technologies, if appropriate. The Blood Safety Council should also consider, at least yearly, in a public forum, opportunities to maximize the safety of the blood supply.
Another promising approach is to reconsider minimum pool size requirements in plasma product manufacturing. The FDA currently requires a large number of donors to be included in plasma pools used in the manufacture of plasma products in order to ensure a wide range of antibodies in preparations of intravenous gamma globulin. Pooling of plasma obtained from numerous donors, although permitting some economy of scale, also increases the risk that a large fraction of manufactured blood products will be contaminated by a single infected donor. The Blood Safety Council should consider this issue and address the safety and efficiency trade-offs in changing the minimum pool size.
The Blood Safety Council would provide information relevant to the decisions that individuals as well as public and private decisionmakers need to make. The forum would not have direct regulatory or other authority, but would function as a forum for holding the organizations with authority responsible for blood safety. In short, the Blood Safety Council could advocate the public's need for a responsible process for decisionmaking about public health policy. The following examples illustrate how regular public discussions of blood safety issues, in the presence of representatives from the relevant organizations' perspectives, could provide an opportunity to hold the organizations with authority accountable for blood safety.
If it had existed in the 1970s, for instance, the Blood Safety Council might have called for the development of heat-treated AHF concentrate to reduce the risk of hepatitis, which would have also reduced the risk of HIV transmission. It would have been able to do so if the NIH and blood products industry representatives on the Council had been called upon to make periodic reports to the Council during the 1970s about their efforts to deal with the hepatitis problem. These representatives would have fed the discussions of the Council back into their own organizations' decisionmaking.
In 1983, the Council could have provided a forum for CDC to present its concerns about HIV in the blood supply and held the FDA, the NHF, and the blood banks and fractionators accountable for responding constructively. CDC created a forum on its own by convening the January 4, 1983, meeting in Atlanta, but as the Committee's analysis indicates, the follow-up on this meeting was insufficient. If a standing Blood Safety Council had existed, the CDC scientists who had concerns about the safety of blood and blood products would have had an opportunity to hold blood collection organizations accountable for their decisions regarding donor deferral and surrogate testing. It would also provide an opportunity to hold plasma fractionators and the FDA accountable for its decisions with regard to heat-treated AHF.
Later that year, the Council could have provided a mechanism to evaluate the claims that automatic recall of AHF would have virtually eliminated the supply of AHF. As the analysis in Chapter 6 indicates, neither the BPAC nor the FDA staff had the capacity to analyze claims that a automatic recall would have such an effect. The Blood Safety Council could have insisted that the FDA commission a formal decision analysis of the options for surrogate testing, or the Council might have performed such an analysis itself. The FDA would retain its regulatory authority, and continue to get advice from the BPAC, but the Council would have provided critical information relevant to the agency's decision.
Finally, if the Council had established an expert panel on best practices as described above and in Recommendation 13, hemophilia patients and their physicians would have had a more credible source of information about the risks of HIV infection and their clinical options than the NHF was able to provide. The operations of such a panel are described below under Recommendation 13.
Compensation Policy
When a product or service provided for the public good has inherent risks, the common law tort system fails to protect the rightful interests of patients who suffer harm resulting from the use of those products or services. Each claim requires extended, costly, and complex adjudicative procedures to establish liability. The results are erratic and unpredictable, and therefore inequitable (IOM 1985).
The doctrine of strict liability holds manufacturers accountable for injuries that are incurred from products that are inherently dangerous because diligence cannot fully eliminate their risks. The public health imperative of assuring enough vaccine for widespread use argues for limits on the strict liability doctrine for vaccine-related injuries. The chief concern is that fear of liability will discourage manufacturers from producing a vital public good. To vitate this concern, a federal compensation system has removed vaccine-related injuries from the scope of strict liability laws (Mariner 1992). The federal government established a mechanism for compensating individuals suffering harm from vaccine-related complications. Its rationale is that consent to undergo vaccination confers benefits to the entire community.
Blood -product-related injuries have also been removed from the scope of strict liability law by blood shield laws, which are in force in most states, and which protect society's interests in having an adequate blood supply. The blood shield laws serve to protect providers and manufacturers of blood and blood products from liability claims in instances where they take all due care to ensure the safety of the product. These laws, however, are unique in the manner in which they limit liability. The shield laws have made it difficult, and often impossible, to obtain compensation for HIV infection acquired from blood or blood products. To address this asymmetry between the protection that blood shield laws offer for manufacturers and adequate protection of individual rights, the Committee makes
Recommendation 3: The federal government should consider establishing a no-fault compensation system for individuals who suffer adverse consequences from the use of blood or blood products. 2
An effective no-fault system requires prospective standards and procedures to guide its operations. In a no-fault system, individual plaintiffs would not have to prove that their adverse outcome was a result of negligence related to manufacture of a blood product. Therefore, there needs to be an objective, science-based process to establish which categories of adverse outcomes are caused by blood-borne pathogens and which individual cases deserve compensation. As with vaccines, a tax or fee paid by all manufacturers or by the recipients of blood products could finance a compensation system. Rather than attempt to allocate blame for HIV infections through blood and blood products, some countries have established such no-fault compensation programs for individuals infected with HIV as a result of their use of blood and blood products. Countries fund these programs in a variety of ways, including direct government support and joint public/private resources.
Making recommendations about compensating affected individuals for damages incurred in the past is outside the Committee's mandate. However, had there been a no-fault compensation system in the early 1980s, it could have relieved much financial hardship suffered by many who became infected with HIV through blood and blood products in the United States. The no-fault principles outlined in this recommendation might serve to guide policymakers as they consider whether to implement a compensation system for those infected in the 1980s.
The Centers for Disease Control and Prevention
The CDC has an indispensable role to play in protecting our nation's health: to detect potential public health risks and sound the alert. Because of its expertise in detecting and evaluating possible infectious disease outbreaks, the Committee believes that the CDC should take responsibility for a surveillance system to detect adverse outcomes from blood and blood products. The following two recommendations embody an important principle: separating the assessment of risk from the management of the consequences of risk. The FDA, in its role as guarantor of the safety of the blood supply, has the responsibility for managing threats to the blood supply. The CDC should detect potential threats and assess the magnitude of the danger.
Early Warning Systems
A nation needs individuals and organizations that identify problems and raise concerns that may be difficult to confront. The CDC plays this role in the Public Health Service. The CDC appears to have been prescient in raising the possibility that the blood supply was contaminated early in the AIDS epidemic, but it was relatively ineffective in convincing other agencies of the potential gravity of the situation. In order to improve CDC's efficacy in this critical role, the Committee makes
Recommendation 4: Other federal agencies must understand, support, and respond to the CDC's responsibility to serve as the nation's early warning system for threats to the health of the public.
Officials in the government, scientists, and physicians in the private sector seem to have discounted the CDC warnings about the transmissibility of AIDS through blood and blood products because the swine flu episode in the 1970s had cost the agency considerable credibility. If, in 1983, the involved public and private organizations had the attitude called for in this recommendation, CDC's recommendations regarding donor screening and surrogate testing might have led to earlier, more effective screening and donor deferral policies.
Consistent with the precept of separating risk assessment and risk management as described above, CDC's role is to characterize and assess risks, and communicate this to others. The FDA and other organizations have the responsibility to manage the risks through regulation, clinical practice guidelines, and other means. The Committee believes that CDC should be able to play its designated role without fearing loss of credibility if it sometimes proves to be wrong. Implementing this recommendation may be difficult. As a start, the Secretary of Health and Human Services should insist that an agency that wishes to disregard a CDC alert should support its position with evidence that meets the same standard as that used by the CDC in raising the alert.
Surveillance
In order to carry out its early warning responsibility effectively, the CDC needs good surveillance systems. Because blood products are derived from human beings and may contain harmful biologic agents that were present in the blood of a donor, blood products are inherently risky, a principle long recognized by blood shield laws. The Committee, believing that the degree of surveillance should be proportional to the level of risk, makes
Recommendation 5: The PHS should establish a surveillance system, lodged in the CDC, that will detect, monitor, and warn of adverse effects in the recipients of blood and blood products.
If such a system had existed in 1982, data about the risks of HIV transmission through blood and blood products might have been available sooner and might have been more definitive. In dealing with newly approved pharmaceuticals, the FDA increasingly demands careful post-approval study of potential adverse effects (the so-called ''Phase IV Trial"). Two existing systems for vaccine adverse events—the CDC/FDA Vaccine Adverse Event Reporting System (VAERS) and the CDC's Large-Linked Database (LLDB)—might be useful models (Institute of Medicine 1994).
The Food and Drug Administration
The FDA has legal authority to protect the safety of the nation's blood supply. Accordingly, it is the lead federal agency in regulating blood-banking practice, the handling of source plasma, and the manufacture of blood products from plasma. The Committee found cause for concern when it evaluated the FDA's actions in protecting the public from HIV in the nation's blood supply during the 1980s. The record reveals many opportunities to improve the agency's capacity to deal with crises involving the blood supply, most notably with respect to the safety of AHF concentrate. In responding to these opportunities, the Committee's recommendations focus on decisionmaking and the role of advisory committees in formulating the FDA's response to crises.
Risk Reduction
In a crisis, decisionmakers may become so preoccupied with seeking solutions that will dramatically reduce danger that they will fail to implement solutions that are less effective but are likely to improve public safety to some degree. Partially effective risk-reducing improvements, as described herein, can save lives, pending the development of more efficacious safety measures. In order that the perfect not be the enemy of the good, the Committee makes
Recommendation 6: Where uncertainties or countervailing public health concerns preclude completely eliminating potential risks, the FDA should encourage, and where necessary require, the blood industry to implement partial solutions that have little risk of causing harm.
In the event of a future threat to the blood supply, the FDA should encourage small, low-risk solutions to large, difficult problems. The FDA's actions during the early 1980s are evidence that the agency should change its attitude toward regulation in order to adopt this proactive approach. Some examples from Chapter 6 illustrate how the FDA might have encouraged practices that would have reduced the risk faced by recipients of blood or a blood product.
Example: Destroy Unscreened Blood When Possible . When hospital blood banks first started to screen donors by questioning them for risk factors, there was a period of transition during which its stocks contained two classes of blood or plasma: blood from screened donors, which was relatively safe; and blood from unscreened donors, which had a higher probability of containing HIV. Within a few weeks of starting to screen donors, blood from unscreened donors would have been either used or discarded. In the instructions contained in its letter of March 24, 1983, the FDA could have recommended that blood banks adopt a policy of using blood from screened donors whenever possible during the transition period, a policy that some blood banks may have adopted on their own. Requiring all blood banks to adopt this policy would not have compromised the nation's blood supply, and it would have prevented at least a few instances in which a patient received an infected unit of blood.
Example: Destruction of Potentially Contaminated Cryoprecipitate . Blood banks store cryoprecipitate from a single unit of donated blood in the frozen state for up to one year. The FDA could have issued a directive that required the blood banks to check their inventory of frozen cryoprecipitate and destroy possibly contaminated units whenever they learned of a previous donor who had AIDS or was strongly suspected of having AIDS.
Example: Phased Recall. In July 1983, there was considerable reluctance to recall untreated Factor VIII concentrate at a time when much of the supply was almost certainly contaminated with HIV. The FDA apparently feared that the ensuing shortage of Factor VIII would have caused more harm than the HIV virus. A phased withdrawal would have been a compromise between no withdrawal and immediate total withdrawal. This middle path might have avoided a factor concentrate shortage and still reduced the number of hemophiliacs who became infected.
Example: Lookback. The FDA formally instituted a "lookback" policy in 1991, years after it was clear that AIDS had a long incubation period during which a patient could transmit HIV through sexual contact or contact with blood. Lookback required blood banks to contact recipients of blood from infected donors and notify them that they might be a HIV carrier and should be tested for HIV antibodies. Earlier action on lookback might have reduced secondary transmission of HIV.
Decision Processes
In all fields, decisionmaking under uncertainty requires an iterative process. As the knowledge base for a decision changes, the responsible agency should reexamine the facts and be prepared to change its decision. The agency should also assign specific responsibility for monitoring conditions and identifying opportunities for change. In order to implement these principles at the FDA, the Committee makes
Recommendation 7: The FDA should periodically review important decisions that it made when it was uncertain about the value of key decision variables.
An example illustrates the principle of iterative decisionmaking. During 1983, most blood bank officials opposed asking prospective male donors if they had ever had sex with a man. They were worried that regular donors might take offense and stop donating blood. They were also concerned about some gays would lie about their homosexuality and donate blood in reprisal for being singled out as the target of the questioning. Eventually, some blood collection centers began to ask questions about sexual preference. If the FDA had carefully monitored these experiments, it would have soon learned that the blood bank officials' fears were groundless. The FDA might then have revised its requirements for donor screening to include direct questions about high-risk sexual practices.
Regulatory Efforts
Although the FDA has a great deal of regulatory power over the blood products industry, the agency appears to regulate by expressing its will in subtle, understated directives. This informal approach to regulation is often necessary to permit a timely response and to preserve needed flexibility. The FDA used this approach, for example, in July 1983 when it issued recommendations to withdraw lots of AHF concentrate that plasma fractionators had identified as containing material from a donor that had AIDS . The language in the July 1983 communication failed to specify, however, whether the agency considered the recommendations to be binding on industry. While most regulated industries might have interpreted these letters as mandatory, that question should not have been left to the judgment of individual entities. Taking this into account, the Committee makes
Recommendation 8: Because regulators must rely heavily on the performance of the industry to accomplish blood safety goals, the FDA must articulate its requests or requirements in forms that are understandable and implementable by regulated entities. In particular, when issuing instructions to regulated entities, the FDA should specify clearly whether it is demanding specific compliance with legal requirements or is merely providing advice for careful consideration.
In 1983, the FDA chose a middle ground when faced with the decision to withdraw all AHF concentrate. The agency recommended that plasma fractionators withdraw individual lots of AHF concentrate when a donor was suspected of having AIDS . This decision was certainly defensible. However, the process for this "case-by-case" withdrawal was seriously compromised by the vagueness of the criteria specified for a recall. The agency failed to specify a process for deciding whether a donor may have had AIDS. The agency should have specified a process for reviewing donors who did not fully satisfy the diagnostic criteria for AIDS but who were suspected of having the disease. When deciding whether to withdraw a lot of AHF concentrate, the FDA asked plasma fractionators to take into account the time of the donation in relation to the diagnosis of AIDS and the effect of the recall on product availability. However, the FDA did not specify parameters for assessing either of these decision criteria. With greater forethought, the FDA could have avoided the potential for a seriously flawed implementation of a policy that otherwise appeared to balance benefits, risks, and harms.
Advisory Committees
The FDA made several decisions in 1983 that appear to have been influenced by the blood-industry-based (profit and nonprofit) members of the BPAC. The BPAC membership did not include individuals with expertise in the social, ethical, political, and economic aspects of the issues that BPAC was deliberating at the time. The FDA apparently did not seek independent analysis of the recommendations made by the members of the BPAC, some of whom were employed by the blood industry. In the early 1980s, the FDA appeared too reliant upon analyses provided by industry-based members of the BPAC and the BPAC. For example, see the discussion in Chapter 6 of the July 19, 1983, BPAC meeting which resulted in the decision for case-by-case rather than automatic recall of lots of AHF when one donor was suspected of having AIDs. Chapter 6 also contains a discussion of the December 15, 1983, BPAC meeting, which effectively curtailed actions on surrogate testing of blood for months. The Committee's analysis of the FDA's management of its advisory committee leads to the following three recommendations:
Recommendation 9: The FDA should ensure that the composition of the Blood Products Advisory Committee reflects a proper balance between members who are connected with the blood and blood products industry and members who are independent of industry.
The FDA should select some BPAC members because they can provide independent judgment, question the analyses provided by blood-industry-based BPAC members, and hold the FDA accountable for a high standard of public responsiveness. The BPAC should have at least one voting member who is a representative of consumer interests. BPAC members who vote to establish policy should have neither the appearance of a conflict of interest nor a true conflict of interest.
An agency that is practiced in orderly decisionmaking procedures will be able to respond to the much greater requirements of a crisis. The BPAC meetings cited before Recommendation 9 above provide examples to support this recommendation. Applying this principle to the use of advisory committees, the Committee makes
Recommendation 10: The FDA should tell its advisory committees what it expects from them and should independently evaluate their agendas and their performance.
The FDA staff and its advisory committees should structure their relationship so that they invigorate each other. The agency should hold an advisory committee accountable for its performance through periodic independent evaluation. By placing unresolved issues on future agendas, the committee can hold the FDA accountable for taking follow-up action between committee meetings. The IOM Committee to Study the Use of Advisory Committees by the Food and Drug Administration makes further recommendations to strengthen the FDA advisory committee system (IOM 1992).
Advisory committees provide scientific advice to the FDA; they do not make regulatory decisions for the agency (IOM 1992). As Chapter 6 indicates, the FDA in 1983 did not independently verify the estimates of the risk of blood-product-related HIV infection. The FDA did not analyze the public health implications of the BPAC's recommendation against automatic recall of AHF concentrate that contained plasma from donors suspected of having AIDS . The FDA's lack of independent information and its own analytic capacity meant that it had little choice but to incorporate the advice of the BPAC into its policy recommendations. To ensure the proper degree of independence between the FDA and the blood products industry, the Committee makes
Recommendation 11: The FDA should develop reliable sources of the information that it needs to make decisions about the blood supply. The FDA should have its own capacity to analyze this information and to predict the effects of regulatory decisions.
Communication to Physicians and Patients
One of the crucial elements of the system for collecting blood and distributing blood products to patients is the means by which to convey concern about the risks inherent in blood products. In today's practice of medicine, in contrast to that of the early 1980s, patients and physicians each accept a share of responsibility for making decisions. Patients' informed consent is required for risky procedures. From early 1983, it was clear that AHF concentrate was a risky product. The failure to tell hemophilia recipients of Factor VIII concentrate about the risks of this treatment and about alternative treatments seems especially serious in the light of present-day emphasis on the autonomy of patients in decisions involving their health.
Clinical Practice
One powerful lesson of the AIDS crisis is the importance of telling patients about the potential harms of the treatments that they are about to receive. The NHF dedicated itself to providing information to individuals with hemophilia and their physicians. Their strategy, however, was seriously flawed. As discussed in Chapter 7 , the NHF provided treatment advice, not the information on risks and alternatives that would enable physicians and patients to decide for themselves on a course of treatment. Hemophilia patients did not have the basis for informed choice about a difficult treatment decision.
Considerable scientific and medical uncertainties characterized the early years of the AIDS epidemic. For individuals medically dependent on the use of blood and blood products, these uncertainties created complex dilemmas about clinical options for their continued care. In instances of great uncertainty, it is crucial for patients to be fully apprised of the full range of options available to them and to become active participants in the evaluation of the relative risks and benefits of alternative treatments. As the case studies in Chapter 7 indicate, the failure to communicate adequately about these options prevented many hemophiliacs from making choices in which they accepted responsibility for balancing the risk of AIDS and the risks of bleeding. Ultimately the failure to communicate led to a powerful sense of betrayal that exacerbated the tragedy of the epidemic for many patients and their families. To encourage better communication, the Committee makes
Recommendation 12: When faced with a decision in which the options all carry risk, especially if the amount of risk is uncertain, physicians and patients should take extra care to discuss a wide range of options.
Medicine has many "gray areas" in which the correct course of action is not clear. Guidelines should identify these areas and spotlight the importance of full disclosure of risks, discussion of the broadest range of clinical options, and incorporation of the patient's preferences into an individualized recommendation. Given the inherent risks and uncertainties in all blood products, the public and the providers of care need expert, unbiased information about the blood supply. This information includes risks and benefits, alternatives to using blood products, and recommended best practices. As Chapter 7 indicates, the NHF (the only organization that stepped in to provide information to hemophiliacs and the physicians who were treating them) focused on practice recommendations rather than complete information on risks and options. In order to provide the public and providers of care with the information they need, the Committee makes
Recommendation 13: An expert panel should be created to inform the providers of care and the public about the risks associated with blood and blood products, about alternatives to using them, and about treatments that have the support of the scientific record.
One lesson of the AIDS crisis is that a well-established, orderly decisionmaking process is important for successfully managing a crisis. This applies as much to clinical decisionmaking as to the public health decision process addressed by the earlier recommendations. As the narrative indicates, there are both public health and clinical approaches to reducing the risk of blood-borne diseases. The Blood Safety Council called for in Recommendation 2 would deal primarily with risk assessment and in the public health domain, actions that would reduce the chance that blood products could be vectors of infectious agents. The primary responsibility of the expert panel on best practices called for in Recommendation 13 would be to provide the clinical information that physicians and their patients need to guide their individual health care choices. To be most effective, this panel should be lodged in the Blood Safety Council (see Recommendation 2) so that both bodies can interact and coordinate their activities in order to share information about emerging risks and clinical options.
Any organization that supplies this information must adhere to accepted norms for documenting evidence. The Committee believes that the public's interest would be best served by creating one publicly accountable source of this information. This function would build on the experience of the Agency for Health Care Policy and Research, which has an established guideline development process and issues guidelines on topics such as the management of chronic pain, screening for AIDS , and management of urinary incontinence (El-Sadr, et al. 1994; Jacox, et al. 1994).
Experience in developing practice guidelines for hemophilia treatment and blood transfusion is an important element of preparedness for future threats to the blood supply. There are now well-established processes such as those recommended by the IOM Committee to Advise the Public Health Service on Practice Guidelines (IOM 1990, 1992) and used by the Agency for Health Care Policy and Research. The U.S. Preventive Services Task Force (1989) uses another system process. Guideline developers should perform a thorough literature search, identify well-designed studies, describe fully the evidence on harms and benefits, and explain the connection between the evidence and the recommendations. They should seek critical evaluation from a wide spectrum of individuals and organizations and should periodically reexamine the recommendations in the light of changing knowledge.
Credibility
During the early 1980s, in its role as the guardian of the interests of the hemophilia patient community, the NHF was the principal source of information about using blood products. The outcome of the NHF efforts was that individuals with hemophilia and their families lost faith in the NHF as the rightful steward of their interests. The reasons discussed in Chapter 7 include the NHF's unwavering recommendation to use AHF concentrate, its dependence on funds contributed by the plasma fractionation industry, and the composition of the NHF expert panel (MASAC) that formulated treatment recommendations (e.g., the panel's lack of infectious disease experts and decision analysts).
Toward the end of providing the highest-quality, most credible information to patients and providers, the Committee makes
Recommendation 14: Voluntary organizations that make recommendations about using commercial products must avoid conflicts of interest, maintain independent judgment, and otherwise act so as to earn the confidence of the public and patients.
One of the difficulties with using experts to give advice is the interconnections that experts accumulate during their careers. Organizations that regulate an industry may get advice from the same experts who advise the industries. Organizations that give treatment advice may rely on experts whose employer relies upon support from industry. As a result, an expert may have a history of relationships that raise concerns about whether he or she can be truly impartial when advising a course of action in a complex situation. The Committee believes that the best way to avoid these risks is to choose some panelists who are not expert in the subject of the panel's assignment but have a reputation for expertise in evaluating evidence, sound clinical judgment, and impartiality.
Financial conflicts of interest influence organizations as well as individuals. As indicated in Chapter 7 and above, the financial relationships between the NHF and the blood products industry seriously compromised the NHF's credibility. The standards for acknowledging conflicts of interest are higher than they were 12 years ago. Public health officials and the medical professions must uphold this new standard. Failure to do so will threaten the fabric of trust that holds our society together.
- Douglas, M. Risk Acceptability According to the Social Sciences . Russell Sage Foundation, New York; 1985.
- El-Sadr, W., et al. Evaluation and Management of Early HIV Infection, Clinical Practice Guideline No. 7 . AHCPR Publication No. 94-0572. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; January 1994.
- Fischoff, B. Treating the Public with Risk Communications: A Public Health Perspective . Science, Technology and Human Values , vol. 12 ; 1987. [ PubMed : 11649904 ]
- Fischoff, B., et al. Acceptable Risk . 1981.
- Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program . M.J. Field, editor; and K.N. Lohr, editor. , eds. National Academy Press, 1990. [ PubMed : 25144032 ]
- Institute of Medicine. Guidelines for Clinical Practice: From Development to Use . M.J. Field, editor; and K.N. Lohr, editor. , eds. National Academy Press, 1992. [ PubMed : 25121254 ]
- Institute of Medicine . Research Strategies for Assessing Adverse Events Associated with Vaccines , National Academy Press, 1994.
- Institute of Medicine. Vaccine Supply and Innovation , National Academy Press, 1985. [ PubMed : 25032425 ]
- Institute of Medicine. The Artificial Heart: Prototypes, Policies and Patents . J.R. Hogness, editor; and M. VanAntwerp, editor. , eds., National Academy Press, 1991. [ PubMed : 25121317 ]
- Jacox, A., et al. Management of Cancer Pain, Clinical Practice Guideline No. 9 . AHCPR Publication No. 94-0592. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; March 1994.
- Mariner, Wendy K., "Legislative Report: The National Vaccine Injury Compensation Program," Health Affairs , Spring 1992 vol. II. [ PubMed : 1577380 ]
- National Research Council. Improving Risk Communication . National Academy Press, Washington, D.C.; 1989. [ PubMed : 25032320 ]
- National Research Council. Risk Assessment in the Federal Government: Managing the Process . National Academy Press, Washington, D.C.; 1983. [ PubMed : 25032414 ]
- National Research Council . Science and Judgment in Risk Assessment . National Academy Press, Washington, D.C.; 1994. [ PubMed : 25032408 ]
- Nelkin, D. Communicating Technological Risk: The Social Construction of Risk Perception . Annu. Rev. Public Health , 1989. [ PubMed : 2655644 ]
- U.S. Preventive Services Task Force. Guide to Clinical Preventive Services . Williams and Wilkins, Baltimore, Maryland; 1989.
- Cite this Page Institute of Medicine (US) Committee to Study HIV Transmission Through Blood and Blood Products; Leveton LB, Sox HC Jr., Stoto MA, editors. HIV And The Blood Supply: An Analysis Of Crisis Decisionmaking. Washington (DC): National Academies Press (US); 1995. 8, Conclusions and Recommendations.
- PDF version of this title (6.4M)
- Disable Glossary Links
In this Page
Related information.
- PubMed Links to PubMed
Recent Activity
- Conclusions and Recommendations - HIV And The Blood Supply Conclusions and Recommendations - HIV And The Blood Supply
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

Expository Essay On HIV/Aids 450 Words
Our body has a protection system called the immune system. The immune system helps protect us from diseases by fighting off germs. But HIV weakens the immune system making a person prone to other diseases.
Table of Contents
Essay On HIV AND AIDS
What is hiv.
HIV stands for Human Immunodeficiency Virus. HIV destroys CD4 called white blood cells which helps the immune system fight diseases. When HIV destroys too many of these cells, the body can’t fight off diseases and infections. This leads to AIDS.
HIV spreads from one person to another through certain body fluids like blood, semen, and vaginal fluids. When these fluids from a person with HIV enter the body of another person, then the HIV can infect that person. This can happen in three main ways.
1. Unprotected sex. This includes anal and vaginal sex without condoms.
2. Sharing of needles and syringes used for injecting drugs or other substances.
3. From an HIV-positive mother to her baby during pregnancy, birth or through breastfeeding.
You cannot get HIV from everyday activities like hugging, holding hands, sharing food or drink, mosquito bites, or toilet seats. Some people think you can get HIV from these things but this is not true.
Symptoms of HIV:
Some people have a flu-like illness within a month or two after getting HIV. But many people don’t notice any symptoms for 10 years or more.
As the virus destroys CD4 cells over time, the following symptoms may appear:
- Weight loss
- Night sweats
- Swelling of lymph nodes
- Red or purple blotches on or under the skin.
Diagnosis of HIV
It can be can determined by a blood test, if a person is infected with HIV. The earlier HIV is detected, the sooner treatment can begin to manage the virus. HIV tests are very accurate and most can detect HIV within 3 months of exposure.
How To Prevent HIV
The best ways to prevent getting HIV are:
- Abstinence from sex. Avoiding sexual contact with anyone will prevent HIV transmission.
- Use condoms correctly every time you have sex. Condoms provide a barrier that prevents fluids from passing between people.
- Limit your number of sexual partners. The more people you have sex with, the greater your risk of HIV exposure.
- Don’t share needles or syringes, even for illegal drugs. If you do inject drugs, always use a clean needle. If you are at high risk of HIV exposure, medicines like PrEP can help 99% protect from HIV by having sex.
Treatment for HIV
With proper treatment and care People with HIV can live healthy lives. Antiretroviral therapy or ART uses a combination of HIV medicines to suppress the virus and stop it from multiplying. While there is no cure for HIV yet, ART can dramatically slow disease progression, helping people with HIV live close to normal lifespans.
Stigma and Support
Having HIV should not be stigmatized. The more people talk openly and honestly about HIV, the more awareness and understanding will grow. It is important to support those living with HIV through active listening, empathy, and compassion. With care and treatment, as well as love and support from family and friends, people with HIV/AIDS can lead fulfilling lives.
In conclusion, education and testing are powerful tools for preventing the spread of HIV/AIDS. While much progress has been made in HIV treatment and research, there is still no cure. Therefore, prevention through safer practices and awareness remains essential. With care, empathy, and commitment to fighting the stigma, we can all play a part in supporting those living with HIV/AIDS and ultimately ending this epidemic.

Hello! Welcome to my Blog StudyParagraphs.co. My name is Angelina. I am a college professor. I love reading writing for kids students. This blog is full with valuable knowledge for all class students. Thank you for reading my articles.
Related Posts:

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Three essays on HIV/AIDS related issues in Southern Africa
Add to collection, downloadable content.
- March 21, 2019
- Affiliation: Gillings School of Global Public Health, Department of Health Policy and Management
- For many years, the number of HIV/AIDS-related deaths in developing countries has been increasing at such an alarming rate that it is no longer whether it will be an epidemic, but rather how severe the epidemic will be. This study addresses three important aspects of the epidemic, including effects as well as causes. The first paper identifies the potential effects of HIV on labor market participation, which affects economic outcomes. Using Heckman selection models and Demographic and Health Survey data from Lesotho, Malawi, Swaziland, and Zimbabwe, results show a significant negative association between being HIV positive and currently working, as well as having worked in the past 12 months, for men and women. The second paper measures the spillover effects of fostering to help inform welfare policies. Linear probability models with fixed effects are estimated using data from the Cape Area Panel Study to quantify the effects of orphan fostering on the school enrollment, employment, and health status of young adults living in households which foster orphans. Results indicate that young adults from higher wealth quintile households which foster orphans have a higher probability of being enrolled in school. The third paper highlights the role played by parental investment in influencing concurrent sexual partners, a risk factor affecting the rate of HIV transmission, which can help make HIV prevention campaigns more effective. Results from multinomial logistic regressions on data from the Cape Area Panel Study show that financial support from fathers significantly decreased the probability of sexual concurrency among Black and Colored males, 11% of whom reported having been in sexually concurrent relationships. The findings have important implications for the macroeconomic stability and future growth of the countries under investigation. The first paper suggests a need for employment protection for HIV positive individuals and their households. The second paper indicates that further research into subsidies for families taking on orphans is warranted. The third paper recommends health education programs on the risks of sexual concurrency for young adults. By providing empirical evidence, HIV policies can be made more effective, thereby mitigating any negative impacts on vulnerable individuals and families.
- December 2010
- https://doi.org/10.17615/2fah-3f09
- Dissertation
- In Copyright
- "... in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Health Policy and Management of the Gillings School of Global Public Health."
- Stearns, Sally
- University of North Carolina at Chapel Hill
- Chapel Hill, NC
- Open access
- March 18, 2013
This work has no parents.
Select type of work
Master's papers.
Deposit your masters paper, project or other capstone work. Theses will be sent to the CDR automatically via ProQuest and do not need to be deposited.
Scholarly Articles and Book Chapters
Deposit a peer-reviewed article or book chapter. If you would like to deposit a poster, presentation, conference paper or white paper, use the “Scholarly Works” deposit form.
Undergraduate Honors Theses
Deposit your senior honors thesis.
Scholarly Journal, Newsletter or Book
Deposit a complete issue of a scholarly journal, newsletter or book. If you would like to deposit an article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
Deposit your dataset. Datasets may be associated with an article or deposited separately.
Deposit your 3D objects, audio, images or video.
Poster, Presentation, Protocol or Paper
Deposit scholarly works such as posters, presentations, research protocols, conference papers or white papers. If you would like to deposit a peer-reviewed article or book chapter, use the “Scholarly Articles and Book Chapters” deposit option.
The HIV/AIDS epidemic in Nigeria: progress, problems and prospects
Affiliation.
- 1 Vanderbilt University Institute for Global Health, Nashville, Tennessee, USA.
- PMID: 21416794
Nigeria is Africa's most populous country, and is home to the third largest number of persons living with HIV/ AIDS in the world. Poverty, stigma, discrimination, and a poorly coordinated health system constitute major barriers to HIV treatment and prevention efforts. The purpose of this paper is to review the current status of the HIV/AIDS epidemic in Nigeria, analyze the challenges facing provision of HIV/AIDS services, examine the prospects of attaining universal access to HIV prevention, treatment, care and support, and advance recommendations for developing quality, sustainable and efficient HIV/AIDS services in Nigeria. HIV programs in Nigeria must emphasize sustainability of current foreign-donor driven treatment and prevention initiatives by engaging all segments of the society and enhancing community leadership and ownership of the programs.
Publication types
- Acquired Immunodeficiency Syndrome / epidemiology*
- Acquired Immunodeficiency Syndrome / prevention & control
- Capacity Building
- Delivery of Health Care / organization & administration
- Delivery of Health Care / trends
- Financing, Organized
- HIV Infections / epidemiology*
- HIV Infections / prevention & control
- Health Knowledge, Attitudes, Practice*
- Health Promotion / organization & administration*
- Health Services Accessibility / organization & administration*
- Health Services Accessibility / trends
- Nigeria / epidemiology
- Nigeria / ethnology
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

CONTROLLING HIV/AIDS IN NIGERIA

HIV is the short form for ..........AIDS is the abbreviation for ............ it can be contacted and controlled.....
Related Papers
Ndakaitei Chikonzo
By submitting this assignment electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification.
Chiedozie I Atuonwu
Maxwell Musingafi
HIV-infection - Impact, Awareness and Social Implications of living with HIV/AIDS
PROFESSOR OSARO ERHABOR
Love Ogundipe
International Journal of Asian Social Science
Hafisa Abubakar
Special journal of the Medical Academy and other Life Sciences
Nikolaos Tzenios
Nigeria has experienced a tremendous impact from HIV/AIDS on both a personal and societal level. The report presents a comprehensive problem overview referencing existing research, relevant literature, and expert insights. Due to HIV/AIDS, the Nigerian healthcare industry has experienced significant difficulties. With rising demand for testing, counseling, anti-retroviral medication (ART), and supportive care, the disease has strained healthcare resources. The disease has decreased production and resulted in a loss of human capital. The workforce has been impacted, which has reduced output across several industries, including agriculture. Concerns about the disease's prevalence have hurt foreign investment, preventing economic expansion and employment development. HIV/AIDS has impacted communities' social dynamics. Discrimination and stigma still exist, putting obstacles in support, treatment, and testing. The disease has put a strain on social welfare institutions, needing ...
Research on Humanities and Social Sciences
Saheed Amusa
Oluwatoyin Obinyan
RELATED PAPERS
Biomedical Journal of Scientific & Technical Research
Ariel Rokach
Estudios Irlandeses
María Gaviña Costero
Rachid Tiberkak
Sovremennye issledovaniya sotsialnykh problem
Vladimir Kravchenko
Gamal Hashem
Antimicrobial Agents and Chemotherapy
Jiun-Ling Wang
Tilla Zilmardah
Dermatol. rev. …
Roberto Arenas
The European Physical Journal C
Andy Buckley
Indian Journal of Science and Technology
sandra zuñiga
Journal of Building Engineering
Mahir Korkmaz
febby febby
Journal of Agricultural Extension
Sekender Ali
Journal of the Korea Institute of Military Science and Technology
Seongil Hong
Luis Castells Arteche
MOHAMED AMINE ENNOUHI
Journal of Biological Chemistry
Kum Kum Rajiv Khanna
Damian Ricci
Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology
Linda Peterson
Investigación
Journal of Natural Remedies
Dr. M. Loganathan
Research Square (Research Square)
Muaaz alajlani
Jurnal Ilmiah Mahasiswa Ekonomi Pembangunan
fakhruddin rudi
MeTra1. Epica e tragedia greca: una mappatura a cura di Andrea Rodighiero, Giacomo Scavello, Anna Maganuco
Andrea Taddei
See More Documents Like This
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Snapsolve any problem by taking a picture. Try it in the Numerade app?

COMMENTS
500+ Words Essay on AIDS. Acquired Immune Deficiency Syndrome or better known as AIDS is a life-threatening disease. It is one of the most dreaded diseases of the 20 th century. AIDS is caused by HIV or Human Immunodeficiency Virus, which attacks the immune system of the human body. It has, so far, ended more than twenty-nine million lives all ...
Get custom essay. In addition, the cost of the treatment has fallen from $ 10, 000 to approximately $100. The initiative has partnered with many countries around the world in formulating programs aimed at fighting HIV/AIDS ( HIV/AIDS, 2014). The best example of such partnerships includes the partnership with Ukraine.
Here you have Expository essay on HIV AIDS, Learn how to write an Essay by this example. Introduction. One of the most feared diseases of the twentieth century is HIV. The Human Immunodeficiency Virus (HIV) causes AIDS by destroying the human immune system. It has claimed the lives of more than 29% of the world's population.
AIDS Essay. HIV (human immunodeficiency virus) is an infection that causes cells in the body that help it fight infections, making a person more susceptible to other infections and diseases. Interaction with certain bodily secretions of an HIV-positive individual, most commonly during unprotected intercourse (sex without the use of a condom or ...
HIV is a virus that attacks the body's white blood cells. White blood cells circulate around the body to detect infection and faults in other cells. HIV targets and infiltrates CD4 cells, a type ...
The. HIV/AIDS catastrophe has been one of the defining features of the past quarter of a century. Although it is short-lived in the scheme of public-health crises, the pandemic ranks among the ...
Students are often asked to write an essay on HIV AIDs Awareness in their schools and colleges. And if you're also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic. ... 500 Words Essay on HIV AIDs Awareness Understanding HIV/AIDS.
An essay on AIDS and HIV needs a clear conceptual understanding of the subject. At first, you need to do a little research on what AIDS and HIV are. Then you need to know the causes and methods of how the disease is spread. People have a limited understanding of this disease globally. For that reason, there is a need to add explanatory concepts ...
The structure of your expository essay will vary according to the scope of your assignment and the demands of your topic. It's worthwhile to plan out your structure before you start, using an essay outline. A common structure for a short expository essay consists of five paragraphs: An introduction, three body paragraphs, and a conclusion.
HIV is the short form of human immunodeficiency virus. When the person is infected with HIV, it is more likely that the acquired immunodeficiency syndrome (AIDS) will develop. HIV is a terrible condition because it cannot be cured. Once one has HIV, he or she will have to live with it for the rest of life. The virus infects T cells or CD4 cells.
According to WHO (2020), the global prevalence of HIV is estimated to be over 37.7 million people, including 1.7 million children. The percentage prevalence in adults is 0.7%. Additionally, the incidence of HIV infection was 1.5 million (WHO, 2020). Most people living with HIV live in low and middle-income countries, with East and Southern ...
AIDS as a term stands for Acquired Immune Deficiency Syndrome as symptoms caused by the HIV (human immunodeficiency virus) (Faria et al., 2014). Therefore, when a person has AIDS his or her immune system has become weakened and as such, cannot fight off diseases and infections. In return, they will develop particular defining illnesses and ...
ADVERTISEMENTS: Essay on HIV/AIDS: Signs, Symptoms and Prevention! Human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS) is a disease of the human immune system caused by the human immunodeficiency virus (HIV). During the initial infection a person may experience a brief period of influenza-like illness. ADVERTISEMENTS: This is typically followed by a prolonged ...
The HIV epidemic has taught scientists, clinicians, public health officials, and the public that new infectious agents can still emerge. The nation must be prepared to deal with a fatal illness whose cause is initially unknown but whose epidemiology suggests it is an infectious disease. The AIDS epidemic has also taught us another powerful and tragic lesson: that the nation's blood supply ...
Check HIV essay examples from EduBirdie Database of HIV essay papers about prevention and awareness. Easy way to get good results! ... Get essay writing help. Essay Service Examples Health Illnesses. Find HIV Essay. 56 samples in this category. ... HIV/AIDS pandemic, we examine the association between declining natural capital and engaging in ...
Global HIV/AIDS Epidemic. According to UNAIDS, since the beginning of the epidemic, more than 70 million people have been infected with HIV and about 35 million people have died of HIV. Globally, 36.7 million [30.8-42.9 million] people were living with HIV at the end of 2016.
Expository Essay On HIV/Aids 450 Words. By Angelina August 8, 2023 October 6, 2023. ... Essay On HIV AND AIDS What is HIV? HIV stands for Human Immunodeficiency Virus. HIV destroys CD4 called white blood cells which helps the immune system fight diseases. ... Writing A Compare And Contrast Essay About Presentation Of Ideas; Argumentative Essay ...
HIV and AIDS Essay Persuasive Essay About Aliens Expository Writing Rough Draft On School An Exposition Of Ephesians 2 : 1-10 The Importance Of Expository. Skip to document. University; High School. Books; ... HIV and AIDS Essay. HIV is the human immunodeficiency virus that causes AIDS. A member of a group of viruses called retroviruses, HIV ...
For many years, the number of HIV/AIDS-related deaths in developing countries has been increasing at such an alarming rate that it is no longer whether it will be an epidemic, but rather how severe the epidemic will be.
Poverty, stigma, discrimination, and a poorly coordinated health system constitute major barriers to HIV treatment and prevention efforts. The purpose of this paper is to review the current status of the HIV/AIDS epidemic in Nigeria, analyze the challenges facing provision of HIV/AIDS services, examine the prospects of attaining universal ...
This expository essay will delve into the challenges faced by Nigeria in controlling HIV/AIDS and the strategies employed to mitigate its impact. **Challenges:**. 1. **Stigma and Discrimination:** Deep-rooted stigma associated with HIV/AIDS leads to discrimination against affected individuals, hindering efforts to encourage testing, treatment ...
CONTROLLING HIV/AIDS IN NIGERIA. HIV/AIDS is an abbreviation or acronym for ''Human Immunodeficiency Virus '' and this virus causes AIDS which is an acronym for ''Acquired Immune Deficiency Syndrome'' .It is a Sexually Transmitted Disease (STD) and AIDS is presently the most deadly Sexually Transmitted Disease.It was originated from a chimpazee while being hunted by humans for meat and its ...
Write an expository essay on the topic of controlling HIV/AIDS in Nigeria. 00:33 Write an essay on "ROLE OF YOUTH IN CONTROLLING HIV/AIDS IN SOCIETY" (Essay should have an Introduction, some key points and describe them and lastly a conclusion) (Length of the essay should be between 2500 to 3000 words).