An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Sputum analysis.
Fan Shen ; Consolato Sergi .
Affiliations
Last Update: February 20, 2023 .
- Introduction
Mucus is the fluid secreted by the airways (also known as bronchial and windpipes) and lungs. In the setting of an infection or a longstanding health condition, the term phlegm is also used. The mixture of saliva and mucus specifically coughed up from the respiratory tract, often either following an infection or an irritation of the mucosa, is precisely labeled "sputum." The term phlegm arises from the Greek word "ϕλ?γμ?" which in the ancient books of medicine was used to label humor caused by heat.
The sputum is examined grossly and microscopically to aid medical diagnosis. The sputum contains various cells and molecular compounds such as soluble lipids and proteins. Its analysis is crucial in medicine. The sputum analysis involves an analytical approach to investigate the cellular and acellular components expelled from the patient's upper respiratory tract. This procedure is essential in the evaluation and management of lower respiratory infections or other longstanding health conditions. [1] Clinically, sputum molecular biomarkers or gene sequencing of the microorganisms have increased medicine accuracy and represent a milestone in the current evaluations of the algorithms running for precision medicine.
- Specimen Requirements and Procedure
The procedure of sputum specimen collection is usually non-invasive. In medicine, it is comparatively simple. However, in some clinical settings, the approach may be more vigorous due to the inability of the patient to expel such fluid from the upper respiratory tract. Thus, some maneuvers of physiotherapy may be considered adjuvant for getting some material for the analysis. Commonly, the "deep cough" sample of the early morning is collected before eating or drinking anything to avoid bias in interpreting the results. At first, the patients need to rinse out the mouth with clear water for 10-15 seconds to eliminate any contaminants in the oral cavity. After expelling saliva, the patients then breathe in deeply three times to cough at 2-minutes intervals until bringing up some sputum. The sputum is then released in a sterile well-closed container provided by the medical professionals to the patient.
The medical professionals will check the amount and gross qualities of the sputum, which should be thick to allow a proper investigation by the laboratory medical staff. In several institutions, clear and runny samples are not acceptable for further microscopic or microbiological studies. In some settings, the procedure can be repeated until 10 to 20 mL of sputum sample has been collected. It is paramount that the lid of the container where the first fluid is collected is changed to avoid contaminations. If the patient has difficulties coughing up enough sputum, the medical professionals may apply some physiotherapeutic maneuvers, which allow the progressive release of the sputum. Routine sputum culture requires that one sample is collected and sent to the lab on the same day of collection. If the patient leaves the specimen in the refrigerator after collection, there is often a tolerance range, which may run well over 24 hours. In tuberculosis (TB), three sputum samples must be collected on three consecutive days and be returned to the clinical lab each day.
Sputum induction is a procedure used to collect adequate lower respiratory secretions from patients who have trouble producing sputum to aid the diagnosis of TB. In particular, patients with suspicion of miliary tuberculosis and/or tuberculous pleural effusion are often targeted using this adjuvant procedure. In such settings, the patient inhales nebulized hypertonic saline solution to liquefy airway secretions. This solution stimulates the patient's coughing and promotes the expectoration of airway secretions. The medical professionals prepare a 20 ml 3% hypertonic saline solution and inject it into the nebulizer cup filled with water. Similar to the non-adjuvant procedure, the patients are always required to wash their mouths thoroughly.
Moreover, the patients wear the nebulizer cup to cover the face and nose after sitting in an upright position. The patients inhale and exhale through the mouthpiece. An expectorate saliva into an emesis bowl and expectorate sputum coughed up are collected into a sterile well-closed container. The medical staff turns on the nebulizer device to allow the patient to inhale the hypertonic mist for approximately five minutes. Then the patients take several deep breaths before attempting to cough. If there are difficulties for the patients to cough up the sputum, the medical staff may use gentle chest physiotherapy to aid the patients to produce sputum. During the procedure, the patients should be observed closely by the medical staff to identify any potential rupture of pleural bullae, triggering a life-threatening pneumothorax. The patients should stop when 1 to 2 mL sputum specimen is collected for each sample or reach 15 minutes of nebulization, or the patient complains of chest tightness, dyspnoea, or wheeze. Imaging is advised if there is the persistence of these symptoms at the end of the sputum collection.
Bronchoscopy is a procedure used to investigate the throat and airway through a thin viewing camera. It is also used to collect the sputum samples in some special situations such as a persistent infection, cough, or something unusual seen on clinical laboratory tests or chest X-rays. The sputum specimen will be examined under a microscope to detect whether abnormal cells are present. Flexible bronchoscopy is used more often than rigid bronchoscopy to collect the sputum samples. Before having a flexible bronchoscopy, the doctor may give the patient anesthetic to relax the throat muscles and numb the mouth, nasal passages, or throat. The procedure is performed using a thin and lighted bronchoscope inserted through the mouth or nose, down to the throat into the windpipe (trachea), and then to the major bronchi leading to the lungs. Sputum samples may be taken using the devices passed through the bronchoscope by the doctor.
- Diagnostic Tests
Clinical diagnostic sputum tests aim to detect the causes of lower respiratory tract infections and some other diseases. It also provides an efficacious tool for monitoring the effectiveness of clinical treatment. Sputum culture is the most common test needed to be performed when the patient has pneumonia. It is used to identify the bacteria or fungi causing the airways or lung infection.
Sputum smear microscopy is the initial step taken in laboratory sputum analysis. It is a fast and inexpensive technique, precisely, in resource-limited settings. Gram stain is used to differentiate bacteria into two broad groups (gram-positive and gram-negative microorganisms). The Gram stain is the first staining technique performed in preliminary bacterial identification, which helps determine if there is an adequate amount of pathogens in the culture and make a definitive diagnosis. It is also crucial because it can address antibiotic therapy more specifically. With the Gram stain, the bacterial species are distinguished into gram-positive and gram-negative groups by the differences in cell walls' physical and chemical properties. Some bacteria have a thick peptidoglycan layer cell wall stained with crystal violet (gram-positive).
In contrast, some other bacteria have a thinner peptidoglycan layer stained red or pink by counterstain (gram-negative). [2] When the physician suspects that the patient may have TB, acid-fast bacilli (AFB), stain testing must be performed. TB is a lung infection disease caused by Mycobacterium tuberculosis . Mycobacteria are a group of rod-shaped acid-fast bacilli. They can be distinguished under the microscope after an AFB staining procedure where the bacilli retain the stain color after an acid-fast wash. The Grocott-Gomori's methenamine silver stain (GMS) is a standard staining method used to detect fungal microorganisms. GMS staining is critical in identifying Pneumocystis jirovecii . This microorganism first appeared in patients with human immunodeficiency virus (HIV) infection in the 1980s, and it was used to be classified as a protozoan. This microorganism, which is now classified as a fungus, was initially called Pneumocystis carinii . Colony morphology is a method that describes the characteristics of an individual colony of bacteria growing on agar in a Petri dish. It can help the lab technologist to identify some specific bacteria.
However, only relying on microscopic observation and colony morphology maybe not be enough to get the relevant information of the species and genus of etiologic microorganisms. Biochemical tests of bacterial growth are the next step to perform to recognize the bacteria. The common biochemical tests used to identify bacterial growth include motility, McFarland standard, fluid thioglycollate medium (FTM), catalase, and oxidase tests.
Respiratory viruses have been tested in sputum specimens from patients with cystic fibrosis, asthma, and chronic obstructive pulmonary disease (COPD). [3] [4] [5] Typically, viral testing is also performed on upper airway samples such as nasopharyngeal swabs or nasal washes. However, some viral pathogens such as severe acute respiratory syndrome (SARS) coronavirus, H1N1 influenza, Middle Eastern respiratory syndrome coronavirus (MERS-CoV), and SARS coronavirus 2 (SARS-CoV-2), the causative agent of Coronavirus Virus disease 2019 (COVID-19), may be absent in upper airway secretions. [6] [7] [8] [9] So the sputum samples are also frequently used for viral diagnosis testing by using the clinical real-time polymerase chain reaction (RT-PCR) method or the newly developed next-generation sequencing (NGS) method. Potentially, face masks, which reduce the aerosol-related risk of transmission in the current era of the COVID-19 pandemic, may also represent a useful source for NGS investigations. [10]
Sputum cytology examination is using a microscope to determine whether abnormal cells are present in sputum samples. The thin layer of sputum placed on a slide before specific staining and diagnosed directly under the microscope helps find out some abnormal cells. Sputum cytology helps detect both lung cancer cells and non-cancer cellular and acellular material useful for the diagnosis of conditions such as pneumonia, tuberculosis, interstitial lung diseases, or pneumoconiosis (e.g., asbestosis). Hematoxylin and eosin stain is the worldwide most performed tissue stain in medical laboratory diagnosis. It is often considered the gold standard. [11] [12] . It is mostly used for suspected lung cancer samples. Periodic acid–Schiff (PAS) stain is used to detect polysaccharides and mucosubstances in tissue specimens. It is mainly helpful for the detection of living fungi in sputum specimens.
Further, Wright stain, Giemsa stain, and Wright-Giemsa mixture stain are used for staining the sputum smears. These stain methods facilitate the differentiation of blood cell types by using specific solutions. These staining methods help detect abnormal white blood cells of sputum, which are vital signs of lung infection.
Sputum molecular analysis is a new insight and advanced technique used to detect lung cancer-related biomarkers to assist in the early stage of a lung cancer diagnosis. Many DNA mutations, such as p53 , KRAS , EML4-ALK, and GFR mutations, have been investigated on sputum specimens. [13] [14] [15] DNA hypermethylation has also been reported in lung cancer sputum samples. Loss of heterozygosity (LOH) and microsatellite instability (MSI) have been found in lung cancer patients' sputum specimens by using DNA markers. [16] MicroRNAs (miRNAs) such as miR-21 and miR-155, proteins such as proliferation-inducing ligand (APRIL) and complement factor H were significantly overexpressed, and some messenger RNAs (mRNAs) such as APRIL , MAGE , Telomerase , CEA, et al., have been found rapidly degraded in sputum specimens of lung cancer patients by RT-PCR and immunocytochemistry. [17] Still, other molecular biomarkers such as free DNA and mitochondrial DNA (mtDNA) variants seem to exhibit some promise. [17]
Finally, sputum antimicrobial susceptibility testing is performed on the bacteria or fungi, leading to lung infection after being identified in a sputum culture sample. The most common approaches consist of the disk diffusion and minimum inhibitory concentration (MIC) methods. These tests are used to detect the effectiveness of the specific antibiotics on the bacteria or to detect whether the bacteria have already developed resistance to certain antibiotics or not. The results of antimicrobial susceptibility testing help to select the most likely effective antibiotics in treating lung infection.
- Testing Procedures
Sputum Culture Procedure
The sputum sample is added to a culture plate with a specific substance that promotes the growth of bacteria or fungi. Then cover the lid of the dish and place it in a 37 degree C incubator for bacteria and 30 degrees C for fungus. The lab specialist should check the bacteria or fungi growth in the sputum plate every day. Once the sputum culture is positive, microscopy, colony morphology, or biochemical tests of bacterial growth will be performed to identify the specific type of bacterium or fungus.
Sputum Staining Tests Procedure
The sputum specimen is a smear on a microscope slide. Different staining dyes are added to the cells, bacteria, or fungi of the sample on the slides and then washed with water, alcohol, or acid solutions. The slides are then diagnosed under a microscope. If the bacteria, fungi, or specific cells are identified in the specimens, the results are positive.
Sputum Biochemical Tests Procedure
To identify a suspected organism, at first, the bacteria will be inoculated in a series of differential media. Then use different indicators to observe the specific end products of metabolism inside of the medium.
Sputum Cytology Examination Procedure
The smear sputum slide is stained with different dyes according to the instructions. Then the pathology specialist examines the stained slide under the microscope to find the abnormal cells from the sputum specimen.
Sputum Nucleic Acid Amplification Test Procedure
The RNA or DNA is extracted from the sputum specimen according to the instruction of different commercial kits. The DNA or RNA is added to a PCR reaction tube with designed primers, Taq polymerase, deoxynucleoside triphosphates [dNTPs], and a fluorescent-labeled probe. Then the tube with RT-PCR reaction mixture is placed in a real-time PCR device for amplifying the molecules at specific temperatures.
Sputum Antimicrobial Susceptibility Tests Procedure
For the MIC method, the bacteria or fungi isolated from sputum specimens were diluted in saline and swabbed onto the MIC panels. For the dish diffusion method, selected different concentrations of antibiotics are placed directly onto the bacteria swabbed agar plates. Panels or plates are incubated at 35 degrees C for about 16 to 18 hours or longer. The minimal concentration of the antibiotic that inhibits the growth of organisms or MIC panel is read according to the guidelines of different manufacturers. Then the result is reported.
- Interfering Factors
Many interfering factors affect the results of every step of the sputum diagnosis. Any deviation from the standard procedure of sample collection, culture, staining, biochemical, molecular, and antimicrobial susceptibility tests can significantly impact the diagnostic result, directly affecting the patient's clinical management. Therefore, strict laboratory workflow procedures and well-trained laboratory technologists are required to perform the sputum analysis.
Collecting a good quality sputum sample is the first step for getting an , the pathogens identified from sputum culture do not always originate from lower respiratory tract infections because they may be part of contaminant sites or preexisting in the oral flora. Thus, standard microbiological procedures for organisms' isolation and identification are critical for the sputum quality assessment (QA).
QA remains an essential tool in the lab for distinguishing the real respiratory pathogens from the possible colonizing flora. Finally, it is vital to check the quality of commercial products that need to be approved by the United States Food and Drug Administration, the Public Health Agency of Canada, and European and Australian similar agencies. Inferior quality culture plates, expired staining kits, or ineffective molecular biology kits are directly related to poor performance. Several agencies determine the quality performed in a laboratory, and the College of the American Pathologists plays a major in dictating laboratory standards and quality control procedures, which are essential to avoid different interfering factors.
- Results, Reporting, and Critical Findings
Sputum Culture
If the pathogenicity organisms grow after 24 hours of incubation in the culture dish, the result is positive. Some sample dishes will keep incubating longer, depending on microbial flora present and the need to identify and semiquantitative isolates and perform antimicrobial susceptibility tests. Conversely, if no bacteria or fungi grow in 6 to 8 weeks for solid culture media or six weeks for liquid culture media, the result is negative.
Sputum Staining Tests
- Common Gram-positive bacteria include Staphylococcus , Streptococcus , Bacillus , Listeria , Enterococcus, and Clostridium .
- Common Gram-negative bacteria include E. coli , Klebsiella species , Proteus species, and Pseudomonas aeruginosa .
- AFB stain test positive result: the acid-fast bacilli, such as Mycobacterium tuberculosis , retain the red or pink color.
- AFB stain test negative result: no red or pink bacteria are found in the stained slide.
- GMS test positive result: black or brown wall from fungal organisms or worms such as Pneumocystis jirovecii are found .
- GMS test negative result: no black or brown stained fungal organisms or worms are found.
Sputum biochemical tests: The motility, McFarland standard, catalase, and oxidase tests are positive or negative.
- Motile (positive): organisms will spread out from the stab line and produce cloudiness or turbidity throughout the medium.
- Nonmotile (negative): organisms will remain along the stab line of inoculation.
- McFarland standard test: The test is used to standardize the number of bacteria in liquid suspensions by the turbidity of bacteria in the McFarland standard vial or tube. The test result arises by comparing the turbidity of a bacterial suspension to different concentrations of McFarland standard solutions.
- Obligate aerobes, such as Pseudomonas spp. , requiring oxygen for growth, will only grow toward the oxygen-rich surface layer.
- Obligate anaerobes, which cannot grow with oxygen, will only grow on the bottom of the tube.
- Microaerophiles frequently grow below the oxygen-rich layer.
- Gram-negative, facultative or aerotolerant anaerobes generally can grow throughout the broth but will mostly grow between the oxygen-rich and oxygen-free area.
- Catalase-positive: the organisms can produce catalase, which will generate oxygen bubbles after adding 3% hydrogen peroxide.
- Catalase-negative: the organisms cannot produce catalase, and there is no reaction after adding 3% hydrogen peroxide.
- Oxidase-positive: there is a deep purple-blue or blue color change within 10 to 30 seconds.
- Oxidase-negative: no purple-blue color or no color change.
Sputum Nucleic Acid Amplification Test
If the RT-PCR amplification is successful, the result is positive. However, if it is not successful, the result is negative.
Sputum Cytology Examination
If a few white blood cells and no abnormal cells have been found in the sputum sample, that means the sputum cytology examination is regular, and other reasons may cause the patient's symptoms.
Sputum Antimicrobial Susceptibility Test
If antibiotics inhibit the growth of an organism, that means the antibiotics are working to treat the patient infected by the organism, and the antibiotics are susceptible. Conversely, if the antibiotic does not inhibit the organism's growth, it means the antibiotics are not adequate for the patient's treatment, and the antibiotics are resistant.
- Clinical Significance
The analysis of sputum is essential for discovering the reasons leading to different airway and lung diseases. The accurate diagnosis is directly related to the strategy that the physician uses to treat the patients. For infectious diseases, a lab technician first tries to find out the pathogens, and then they test different drugs that can be used for the treatment. For the suspected lung cancers and certain non-cancerous lung conditions, the lab will inspect whether abnormal cells exist in the sputum specimens or not.
- Quality Control and Lab Safety
Sputum analysis quality control is essential and directly affects the diagnostic results. From sputum specimens collection, samples transport, samples storage, diagnostic test procedures environment, to tests results reporting, every step should be strictly performed under the standard guidelines. In 1988, the Clinical Laboratory Improvement Amendments (CLIA) was passed by the Congress of the United States of America. The Clinical and Laboratory Standards Institute (CLSI) was established to minimize the analysis errors and maximize the control of test variables. Similar bills are present for other countries. During the diagnostic tests, a standard positive, sensitivity, and negative control should always be performed when the laboratory technician carries out the sputum samples tests. No diagnostic results should be reported when measurements of the quality control are not authorized.
Strict lab safety rules and measures should be conducted by the staff working in the lab. Depending on specific diagnostic tests, the results of the procedural risk assessment may be different. It is always good to consider the sputum samples as potentially infectious, and essential biosafety measures should be set to limit or reduce the risks of laboratory infection when specific procedures are performed. The CLSI has provided new practice guidelines for applying risk management to both QC plans and statistical QC. [18] [19]
- Enhancing Healthcare Team Outcomes
Sputum analysis's diagnostic results involve many different diseases, such as respiratory infections, lung cancers, and non-cancerous lung conditions. Pneumonia is the most common lung infection disease and may relate to potential spreading. Untreated pneumonia may lead to severe complications. Lung cancer is ranked the fourth most commonly diagnosed malignancy in developed countries, and the first in cancer-caused death worldwide. [20] Despite the high incidence and mortality of pneumonia and lung cancer, the treatments are few. [21] [22] [23] . Thus, requiring an interprofessional approach is essential for the patient to receive the most appropriate evidence-based laboratory diagnosis decisions, clinical treatment, and healthcare support. This interdisciplinary team approach includes a laboratory diagnostic specialist, a radiologist, a pulmonologist, an infectious disease expert, a pharmacist, and a public health nurse.
According to the symptoms of the patient, the pulmonologist or infectious disease expert orders the laboratory tests. A nurse or a healthcare supporter will then collect the sputum sample and send it to the clinical lab. The laboratory specialist will conduct the diagnostic tests for the sputum specimen and report the results. The radiologist may also need to carry out a chest X-ray for the patient. When the physician receives the sputum diagnostic reportings, they may prescript the drugs for the patient's treatment. Then the nurse will perform the clinical therapy.
Once the patient is discharged from the hospital, the nurse should educate the patient and the family members on how to take care of good health. After that, a pharmacist will observe the outpatient therapy and make sure the patient follows the treatment. When the patient has an allergy to some drugs or has difficulties in obeying the treatment, the pharmacist should report the situation to the interprofessional team. Besides, a social worker is needed to support the outpatient spiritually and financially. To ensure the treatment at home is working for the patient, regular following up serial monitoring in the clinical lab and imaging studies by the radiologist are essential.
The multidisciplinary team approach facilitates the delivery of high-quality healthcare services to the patient. Previous evidence suggested that patients are more satisfied with the traditional care model and have improved life quality if they receive multidisciplinary care. [24] [25]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Fan Shen declares no relevant financial relationships with ineligible companies.
Disclosure: Consolato Sergi declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Shen F, Sergi C. Sputum Analysis. [Updated 2023 Feb 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Sputum collection and analysis: what the nurse needs to know. [Nurs Stand. 2017] Sputum collection and analysis: what the nurse needs to know. Myatt R. Nurs Stand. 2017 Mar 1; 31(27):40-43.
- Analyzing the Treatment of Patients with Acute Exacerbation of COPD with the Aid of Intelligent Diagnosis Method. [J Healthc Eng. 2022] Analyzing the Treatment of Patients with Acute Exacerbation of COPD with the Aid of Intelligent Diagnosis Method. Jiang Q, Zhao G, Song S, Chen Y. J Healthc Eng. 2022; 2022:3962074. Epub 2022 Mar 12.
- [Biomarkers and inflammatory characteristics for microcosmic syndrome differentiation of cold-phlegm syndrome and heat-phlegm syndrome in patients with bronchial asthma]. [Zhongguo Zhong Xi Yi Jie He Za...] [Biomarkers and inflammatory characteristics for microcosmic syndrome differentiation of cold-phlegm syndrome and heat-phlegm syndrome in patients with bronchial asthma]. Cao YX, Dong JC, Du YJ. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010 Aug; 30(8):828-32.
- Review Mucus, phlegm, and sputum in cystic fibrosis. [Respir Care. 2009] Review Mucus, phlegm, and sputum in cystic fibrosis. Rubin BK. Respir Care. 2009 Jun; 54(6):726-32; discussion 732.
- Review Physiology of airway mucus clearance. [Respir Care. 2002] Review Physiology of airway mucus clearance. Rubin BK. Respir Care. 2002 Jul; 47(7):761-8.
Recent Activity
- Sputum Analysis - StatPearls Sputum Analysis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Hosted content
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 57, Issue 10
- Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- R H Green ,
- C E Brightling ,
- G Woltmann ,
- A J Wardlaw ,
- Institute for Lung Health, Department of Respiratory Medicine and Thoracic Surgery, Glenfield Hospital, Groby Road, Leicester LE3 9PQ, UK
- Correspondence to: Dr I D Pavord, Institute for Lung Health, Department of Respiratory Medicine and Thoracic Surgery, Glenfield Hospital, Groby Road, Leicester LE3 9PQ, UK; trina.raftery{at}uhl-tr.nhs.uk
Background: The debate as to whether asthma is a single or heterogeneous disease remains unresolved although pathological studies, mostly using fibreoptic bronchoscopy on small numbers of subjects, have emphasised the similarities between different clinical phenotypes.
Methods: Lower airway inflammation was assessed non-invasively using induced sputum in 34 normal controls and 259 adults with symptomatic asthma receiving treatment at steps 1–3 of the British Thoracic Society (BTS) guidelines. A subgroup of 49 patients treated with as required β 2 agonists only who met BTS criteria for a step up in treatment were studied before and 2 months after treatment with inhaled budesonide 400 μg twice daily.
Results: There was considerable heterogeneity in induced sputum cell counts, particularly in non-atopic patients. A subgroup of 60 patients had a distinctive sputum cell profile with a neutrophil count higher than our normal range (>65.3%) and a normal sputum eosinophil count (<1.9%). These patients were older, predominantly female, and were more likely to be non-atopic but otherwise had similar clinical and physiological features to the group as a whole. Among the 49 subjects studied before and after inhaled budesonide, 11 patients had an isolated sputum neutrophilia. Following treatment, these patients showed significantly less improvement in visual analogue symptom scores (–5.5 v –19.4 mm; mean difference 13.9; 95% CI 0.7 to 27.0), forced expiratory volume in 1 second (FEV 1 ) (–0.08 v 0.13 l; mean difference 0.21; 95% CI 0.03 to 0.39), and concentration of methacholine provoking a fall in FEV 1 of 20% or more (PC 20 ) (0.15 v 1.29 doubling doses; mean difference 1.11; 95% CI 0.13 to 2.15) than the remaining 38 patients.
Conclusions: These results suggest the presence of a distinct subgroup of patients with mild to moderate asthma who have predominantly neutrophilic airway inflammation and who respond less well to treatment with inhaled corticosteroids.
- induced sputum
- neutrophilic inflammation
https://doi.org/10.1136/thorax.57.10.875
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Clinicians have long regarded asthma as a heterogeneous disease, 1, 2 although detailed clinicopathological studies have tended to emphasise the similarities in the underlying airway pathology and disordered function between patients. 3– 9 Airway inflammation in asthma has usually been assessed invasively using bronchoscopic techniques, so studies are largely confined to young adults with mild atopic asthma. Whether the findings can be generalised to a wider more heterogeneous population analogous to that seen in clinical practice is unclear.
More recent studies where airway inflammation has been assessed non-invasively using induced sputum in a more diverse range of patients have shown predominant neutrophilic airway inflammation in some patients with severe asthma 10, 11 and in others studied during acute exacerbations. 12 Whether these changes reflect the severity of the disease or the effect of treatment is unclear. We have measured airway inflammation in 34 normal and 259 subjects with symptomatic asthma receiving treatment at British Thoracic Society (BTS) steps 1–3 13 and have related sputum cell counts to the response to inhaled corticosteroids in 49 subjects. We have used these data to test the hypothesis that a predominant neutrophilic airway inflammation is present in a subset of patients with milder asthma and that this phenotype is associated with a poor response to inhaled corticosteroids.
Patients and controls were recruited from patients, staff, and volunteers attending the Department of Respiratory Medicine at the Glenfield Hospital. Normal controls had no symptoms suggestive of asthma, were non-smokers or ex-smokers who had not smoked within 12 months of study entry and had a past history of less than 10 pack years, had normal spirometric values (forced expiratory volume in 1 second (FEV 1 ) >80% predicted and ratio of FEV 1 to forced vital capacity (FVC) >80%), and normal methacholine airway responsiveness (PC 20 >16 mg/ml). Subjects with asthma had consistent symptoms and one or more of the following: a methacholine PC 20 of <8 mg/ml; a >15% increase in FEV 1 10 minutes after 200 μg salbutamol or a >20% maximum within day variability in peak expiratory flow (PEF) measured twice daily over 14 days. Patients had no clinical or radiological evidence of bronchiectasis and no symptoms suggesting acute lower respiratory tract infection within a month of entering the study. All patients had an FEV 1 % predicted of >65% and a smoking history of less than 10 pack years. Clinical records were used to corroborate patients’ smoking histories and exhaled carbon monoxide was measured where there was any doubt.
All patients with asthma treated at BTS steps 1–3 attending our respiratory outpatient clinic who fulfilled the entry criteria and who agreed to participate were included. Assessments were carried out following informed consent as part of a project examining the validity, repeatability, and responsiveness of induced sputum differential inflammatory cell counts which was approved by the Leicestershire Hospitals research ethics committee.
Study design and protocol
Patients and controls attended on two occasions. On the first occasion allergen sensitivity was measured by radioallergosorbent tests for specific IgE or skin prick testing to Dermatophagoides pteronyssinus , cat fur, grass pollen, and Aspergillus fumigatus and atopy was defined as one or more positive skin tests (weal >2 mm larger than negative control) or raised specific IgE (>0.34 kU/l) to one or more antigen. Spirometric tests before and after inhaled salbutamol and chest radiography were performed. Subjects recorded PEF twice daily as the best of three blows over a 14 day period.
On the second visit methacholine airway responsiveness was measured using the tidal breathing method 14 followed, after recovery, by sputum induction and processing as previously described. 15 The duration of inhalation of hypertonic saline was standard. A subgroup of patients taking as required β 2 agonists only who met the BTS criteria for a step up in treatment (using rescue β 2 agonists more than once per day, having nocturnal wakening or limitations in activities, peak flow variability ≥20%, or PEF ≤80% of predicted or best) 13 were given inhaled budesonide 400 μg twice daily for 2 months. These patients identified their predominant symptom (breathlessness, wheeze, or cough) and the severity of this was assessed using a 100 mm visual analogue scales (VAS) from no symptom (0 mm) to the worst ever symptom (100 mm). This scale was the most responsive outcome measure in our earlier study 16 and has been validated. 17
The patients then attended for a third visit when the spirometric tests, methacholine inhalation test, and VAS symptom scores were repeated 12 hours after the last dose of treatment.
Data from some of these patients have been presented previously. 16
Analysis of data
Normal ranges were derived from the eosinophil and neutrophil counts of the control subjects as the mean + 2SD and the mean + 1.7SD using one tailed and two tailed tests, respectively. One tailed tests were used for eosinophil counts since there is no lower reference limit. Spirometric values, induced sputum macrophage, neutrophil, lymphocyte and epithelial differential cell counts and maximum PEF amplitude % mean were described as mean (SE) values. Methacholine PC 20 results were log normally distributed and were log transformed and described as geometric mean (log SE) values. Sputum eosinophil counts were expressed as median and interquartile range (IQR). Differences between groups were analysed for normally distributed variables using the independent t test and for variables not observing a normal distribution using the Mann-Whitney U test. The correlation between sputum eosinophils and methacholine PC 20 , PEF amplitude % mean (A%M), and FEV 1 were assessed using the Spearman rank test. Differences in methacholine PC 20 were expressed as doubling doses. The χ 2 test was used to compare the percentage of patients using inhaled steroids and the percentage of atopic patients between groups.
All subjects
Normal ranges derived from normal subjects were <65.3% for sputum neutrophil counts and <1.9% for sputum eosinophil counts. 143 patients had intermittent asthma treated with as required β 2 agonists only (step 1 of the BTS guidelines) 13 ; 116 had more persistent symptoms requiring regular inhaled corticosteroids (steps 2 and 3) (table 1). 13 Twenty patients (11 steroid naïve, nine atopic) were current smokers and 78 (42 steroid naïve, 26 atopic) were ex-smokers, but all had a history of <10 pack years. Patient details categorised according to atopic status and use of inhaled corticosteroids are shown in table 1. The mean (SE) daily dose of inhaled steroid (in beclomethasone equivalent doses) for atopic and non-atopic subjects was 424 (56) μg and 416 (50) μg, respectively. Non-atopic asthma was associated with less methacholine airway responsiveness (methacholine PC 20 1.34 mg/ml v 0.68 mg/ml; geometric mean difference 1.0 doubling doses; 95% CI of difference 0.4 to 1.6; p=0.002) and higher mean neutrophil count (54.1% v 45.0%; mean difference 9.1%; 95% CI of difference 2.3 to 15.8; p=0.008).
- View inline
Patient details and sputum cell counts in normal controls, atopic and non-atopic subjects
Sputum evidence of eosinophilic airway inflammation was the most common abnormality in the group as a whole with 135 patients (52%) having an induced sputum eosinophil count outside our normal range (fig 1). The median sputum eosinophil count was significantly lower in atopic subjects receiving inhaled corticosteroids (1.1%) than in similarly treated non-atopic subjects. (3.3%, p<0.05; table 1). Among the whole study population there was no correlation between the sputum eosinophil count and the methacholine PC 20 ( r =–0.03; p>0.05), the maximum PEF amplitude % mean ( r =–0.02, p>0.05), or the % predicted FEV 1 ( r =–0.03, p>0.05). Among the 114 atopic patients a weakly negative correlation was observed between the sputum eosinophil count and the methacholine PC 20 ( r =–0.30, p<0.01), while the 145 non-atopic patients demonstrated a weakly positive correlation between these two measurements ( r =0.22, p<0.05).
- Download figure
- Open in new tab
- Download powerpoint
Change in (A) concentration of methacholine provoking a fall in forced expiratory volume in 1 second (FEV 1 ) of 20% or more (PC 20 ), (B) FEV 1 , and (C) VAS symptom score following 2 months of treatment with budesonide 400 μg twice daily in neutrophilic patients (open bars) and all other patients (closed bars).
Subgroup with isolated neutrophilic inflammation
There was considerable heterogeneity in induced sputum eosinophil and neutrophil cell counts, even among those patients treated with as required β 2 agonists alone. A subgroup of 60 patients, including 35 who were steroid naive, had a distinctive sputum cell profile with a sputum neutrophil count outside the normal range and a normal sputum eosinophil count. Five of these were current smokers and 20 were ex-smokers. These patients were older, tended to develop asthma later, and were more likely to be female and non-atopic than the group as a whole. Clinical and physiological features were otherwise similar (table 2).
Characteristics of patients with isolated sputum neutrophilia and all other patients studied
Patients studied before and after treatment with inhaled corticosteroids
Ninety two of the patients treated with as required β 2 agonists only met the BTS criteria for a step up in treatment. Forty nine such patients were randomly selected and agreed to attend again 2 months after treatment with inhaled budesonide 400 μg twice daily. Of these subjects, 11 were included in the subgroup described above, having an isolated sputum neutrophilia with a normal sputum eosinophil count (table 3). Compared with the other 38 patients studied before and after treatment, these subjects had significantly less improvement in VAS scores (–5.5 v –19.4 mm; mean difference 13.9; 95% CI 0.7 to 27.0; p=0.04), FEV 1 (–0.08 v 0.13 l; mean difference 0.21; 95% CI 0.03 to 0.39; p=0.026), and PC 20 (0.15 v 1.29 doubling doses; mean difference 1.11; 95% CI 0.13 to 2.15; p=0.029; fig 1).
Baseline characteristics of patients studied before and after treatment with budesonide 400 μg twice daily for 2 months
We have analysed the extent and nature of airway inflammation in induced sputum in normal controls and in a large population of well characterised patients with asthma. Our estimates of normal ranges, although derived from small numbers, are very similar to findings in larger populations. 18, 19 In the adults with asthma receiving treatment at BTS stages 1–3 and with relatively normal spirometric parameters, we found considerable heterogeneity in induced sputum inflammatory cell counts. Importantly, a number of predominantly female, non-atopic patients with adult onset asthma had a distinctive sputum inflammatory cell profile consisting of sputum neutrophilia and a normal sputum eosinophil count. Furthermore, a subgroup of steroid naïve subjects with this isolated neutrophilic inflammation had an impaired response to treatment with inhaled corticosteroids.
Previous studies have noted sputum evidence of isolated neutrophilic airway inflammation in some patients with severe asthma 10, 11 and in a minority of adults studied during asthma exacerbations. 12 Gibson et al used induced sputum to assess 56 patients with persistent asthma taking high doses of inhaled corticosteroids and found that 59% of patients had suppressed sputum eosinophil counts but evidence of neutrophilic inflammation. 20 Wenzel et al used bronchoscopic techniques to characterise the underlying airway immunopathology of a group of patients with severe refractory asthma who had severely impaired lung function and were treated with high dose inhaled steroids and oral prednisolone and have suggested the presence of a subgroup who have a predominant neutrophilic airway inflammation, absence of eosinophils, and normal basement membrane thickness. 21 It is not clear whether the findings are peculiar to severe asthma or reflect the effects of treatment with high doses of corticosteroids. Our results provide support for the presence of such a distinct asthma phenotype and, for the first time, show that it is a relatively common finding in patients with milder asthma and, in some subjects at least, that it is not an artefact due to corticosteroid treatment. The incidence of neutrophilic inflammation was higher in the population studied by Gibson et al 20 and in the patients with severe asthma studied by Wenzel et al , 21 and it remains possible that this phenotype is particularly associated with more severe disease. We have further extended these earlier findings by showing a significantly impaired response to inhaled corticosteroids in a subgroup of the subjects with an isolated neutrophilia. The poor response to inhaled corticosteroid is not only of obvious clinical significance, but it also provides a possible mechanism by which subjects might be particularly likely to evolve into more severe refractory cases.
We do not have a clear explanation for the development of neutrophilic airway inflammation in these patients. All patients had a smoking history of less than 10 pack years, only a few were current smokers, and the patients with isolated sputum neutrophilia were no more or less likely to have ever smoked than the remaining group. We therefore doubt that current smoking and early chronic obstructive pulmonary disease were important explanations for the unusual inflammatory cell profile. All the patients presented with symptoms consistent with asthma, had normal chest radiographs and no clinical evidence of acute infection, although we cannot exclude the possibility of subtle subclinical bronchiectasis or lower respiratory tract infections. Idiopathic chronic cough has a similar female predominance and age at onset of symptoms and is associated with a sputum neutrophilia. 22 These similarities suggest there might be parallels between these conditions. Further work is required to define the lower airway immunopathology in more detail and to investigate its aetiology.
This large observational study of adults with asthma provided us with the opportunity to compare sputum markers of airway inflammation in subjects categorised according to atopic status and use of inhaled corticosteroids, variables that have been traditionally used to phenotype asthma. We identified several differences between atopic and non-atopic subjects that have not been reported before. The higher sputum neutrophil count in non-atopic subjects could reflect the higher incidence of neutrophilic asthma in this group. Non-atopic subjects also had less airway hyperresponsiveness and were more likely to have sputum evidence of persistent eosinophilic airway inflammation despite treatment with inhaled corticosteroids.. These differences support suggestions that non-atopic and atopic asthma represent distinct disease phenotypes. 1 Further work is required to determine whether they are clinically significant. The sputum eosinophil count was significantly lower in atopic subjects treated with inhaled corticosteroids than in non-atopic subjects, so one possibility is that atopic patients might not respond as well to a higher dose of inhaled corticosteroids.
There was no correlation between airway hyperresponsiveness and eosinophilic airway inflammation in the population as a whole, although there was a weak negative correlation when atopic subjects were considered alone. These findings challenge the widely held view, reflected by recent definitions of asthma, 23, 24 that there is a simple causal association between eosinophilic airway inflammation and disordered airway function and suggest a more complex relationship. Other studies examining the relationship between the sputum eosinophil count and airway responsiveness have produced mixed results, 25– 30 although it is notable that those studies showing a significant correlation have been largely confined to atopic subjects. 28– 30
We describe a single observation, and in a disease characterised by variability we cannot be sure that the distinctive phenotype seen in our population of adults with asthma is stable. Our estimates of incidence might also be incorrect since we have studied subjects referred for secondary care who might be particularly likely to display unusual features. Longer term studies of a more typical population of asthmatic subjects are required to estimate the true prevalence of this asthma phenotype and to determine whether it is stable. Placebo controlled longer term intervention studies with inhaled corticosteroids and other treatments are also required to assess the efficacy of these interventions fully. Our findings raise the possibility of a distinct phenotype of asthma, with active neutrophilic and suppressed eosinophilic airway inflammation, across the range of severity of asthma that differs in response to treatment and could have important implications for our understanding and treatment of the disease.
Acknowledgments
We thank William Monteiro and Richard Ward for help with sputum processing and members of the department of respiratory physiology for performing the sputum inductions. This work was supported by grants from Astra Zeneca, Trent Region and Glenfield Hospital Research fund. Ruth Green is supported by a National Asthma Campaign grant.
- ↵ Rackemann F .M. A clinical classification of asthma. Am J Med Sci 1921 ; clxii : 802 . OpenUrl
- ↵ Aas K . Heterogeneity of bronchial asthma. Sub-populations or different stages of the disease. Allergy 1981 ; 36 : 3 –14. OpenUrl PubMed Web of Science
- ↵ Humbert M , Menz G, Ying S, et al . The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today 1999 ; 20 : 528 –33. OpenUrl CrossRef PubMed Web of Science
- Humbert M , Durham SR, Ying S, et al . IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med 1996 ; 154 : 1497 –504. OpenUrl CrossRef PubMed Web of Science
- Humbert M , Ying S, Corrigan C, et al . Bronchial mucosal expression of the genes encoding chemokines RANTES and MCP-3 in symptomatic atopic and nonatopic asthmatics: relationship to the eosinophil-active cytokines interleukin (IL)-5, granulocyte macrophage-colony-stimulating factor, and IL-3. Am J Respir Cell Mol Biol 1997 ; 16 : 1 –8. OpenUrl CrossRef PubMed Web of Science
- Tang C , Rolland JM, Ward C, et al . IL-5 production by bronchoalveolar lavage and peripheral blood mononuclear cells in asthma and atopy. Eur Respir J 1997 ; 10 : 624 –32. OpenUrl Abstract
- Bentley AM , Menz G, Storz C, et al . Identification of T lymphocytes, macrophages, and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial responsiveness. Am Rev Respir Dis 1992 ; 146 : 500 –6. OpenUrl CrossRef PubMed Web of Science
- Folkard SG , Westwick J, Millar AB. Production of interleukin-8, RANTES and MCP-1 in intrinsic and extrinsic asthmatics. Eur Respir J 1997 ; 10 : 2097 –104. OpenUrl Abstract
- ↵ Bentley AM , Durham SR, Kay AB. Comparison of the immunopathology of extrinsic, intrinsic and occupational asthma. J Invest Allergol Clin Immunol 1994 ; 4 : 222 –32. OpenUrl PubMed
- ↵ Jatakanon A , Uasuf C, Maziak W, et al . Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 1999 ; 160 : 1532 –9. OpenUrl CrossRef PubMed Web of Science
- ↵ Hoskins G , McCowan C, Neville RG, et al . Risk factors and costs associated with an asthma attack. Thorax 2000 ; 55 : 19 –24. OpenUrl Abstract / FREE Full Text
- ↵ Fahy JV , Kim KW, Liu J, et al . Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 1995 ; 95 : 843 –52. OpenUrl CrossRef PubMed Web of Science
- ↵ British Thoracic Society . The British guidelines on asthma management. Thorax 1997 ; 52 (Suppl 1): S1 –20. OpenUrl PubMed
- ↵ Juniper EF, Cockcroft DW, Hargreave FE . Histamine and methacholine inhalation tests: a laboratory tidal breathing protocol . 2nd ed. Lund, Sweden: Astra Draco AB, 1994.
- ↵ Pavord ID , Pizzichini MM, Pizzichini E, et al . The use of induced sputum to investigate airway inflammation. Thorax 1997 ; 52 : 498 –501. OpenUrl PubMed Web of Science
- ↵ Pavord ID , Brightling CE, Woltmann G, et al . Non-eosinophilic corticosteroid unresponsive asthma. Lancet 1999 ; 353 : 2213 –4. OpenUrl CrossRef PubMed Web of Science
- ↵ Brightling CE , Monterio W, Green RH, et al . Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med 2001 ; 95 : 999 –1002. OpenUrl CrossRef PubMed Web of Science
- ↵ Belda J , Leigh R, Parameswaran K, et al . Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med 2000 ; 161 : 475 –8. OpenUrl CrossRef PubMed Web of Science
- ↵ Spanevello A , Confalonieri M, Sulotto F, et al . Induced sputum cellularity. Reference values and distribution in normal volunteers. Am J Respir Crit Care Med 2000 ; 162 : 1172 –4. OpenUrl PubMed Web of Science
- ↵ Gibson PG , Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001 ; 119 : 1329 –36. OpenUrl CrossRef PubMed Web of Science
- ↵ Wenzel SE , Schwartz LB, Langmack EL, et al . Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999 ; 160 : 1001 –8. OpenUrl CrossRef PubMed Web of Science
- ↵ Hunter CJ , Ward R, Woltmann G, et al . The safety and success rate of sputum induction using a low output ultrasonic nebuliser. Respir Med 1999 ; 93 : 345 –8. OpenUrl CrossRef PubMed Web of Science
- ↵ Barnes PJ , Grunstein M, Leff A, et al . Asthma . Philadelphia: Lippincott Raven, 1997.
- ↵ Global Initiative for Asthma . Global strategy for asthma management and prevention. Publication no 95-3659. Bethesday, MD: National Heart, Lung and Blood Institute, 1995.
- ↵ Crimi E , Spanevello A, Neri M, et al . Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med 1998 ; 157 : 4 –9. OpenUrl CrossRef PubMed Web of Science
- Rosi E , Ronchi MC, Grazzini M, et al . Sputum analysis, bronchial hyperresponsiveness, and airway function in asthma: results of a factor analysis. J Allergy Clin Immunol 1999 ; 103 : 232 –7. OpenUrl CrossRef PubMed Web of Science
- Adelroth E , Rosenhall L, Johansson SA, et al . Inflammatory cells and eosinophilic activity in asthmatics investigated by bronchoalveolar lavage. The effects of antiasthmatic treatment with budesonide or terbutaline. Am Rev Respir Dis 1990 ; 142 : 91 –9. OpenUrl PubMed Web of Science
- ↵ Lim S , Jatakanon A, John M, et al . Effect of inhaled budesonide on lung function and airway inflammation. Assessment by various inflammatory markers in mild asthma. Am J Respir Crit Care Med 1999 ; 159 : 22 –30. OpenUrl PubMed Web of Science
- Pizzichini E , Pizzichini MM, Efthimiadis A, et al . Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 1996 ; 154 : 308 –17. OpenUrl CrossRef PubMed Web of Science
- ↵ Claman DM , Boushey HA, Liu J, et al . Analysis of induced sputum to examine the effects of prednisone on airway inflammation in asthmatic subjects. J Allergy Clin Immunol 1994 ; 94 : 861 –9. OpenUrl CrossRef PubMed Web of Science
This work was supported by grants from Astra Zeneca, Trent Region and Glenfield Hospital Research fund. Ruth Green is supported by a National Asthma Campaign grant.
Read the full text or download the PDF:
Proteomic analysis of sputum reveals novel biomarkers for various presentations of asthma
Affiliations.
- 1 Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
- 2 State Key Lab. for Respiratory Diseases, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China.
- 3 National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.
- 4 Shanghai Science Research Center, Chinese Academy of Sciences, Shanghai, China.
- 5 National Heart & Lung Institute, Imperial College, London, UK.
- 6 State Key Lab. for Respiratory Diseases, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China. [email protected].
- 7 Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China. [email protected].
- 8 Department of Pharmacology, Zhejiang University School of Medicine, Hangzhou, China. [email protected].
- 9 Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China. [email protected].
- 10 State Key Lab. for Respiratory Diseases, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China. [email protected].
- PMID: 28778200
- PMCID: PMC5544989
- DOI: 10.1186/s12967-017-1264-y
Background: It is now recognized that asthma can present in different forms. Typically, asthma present with symptoms of wheeze, breathlessness and cough. Atypical forms of asthma such as cough variant asthma (CVA) or chest tightness variant asthma (CTVA) do not wheeze. We hypothesize that these different forms of asthma may have distinctive cellular and molecular features.
Methods: 30 patients with typical or classical asthma (CA), 27 patients with CVA, 30 patients with CTVA, and 30 healthy control adults were enrolled in this prospective study. We measured serum IgE, lung function, sputum eosinophils, nitric oxide in exhaled breath (FeNO). We performed proteomic analysis of induced-sputum supernatants by mass spectrometry.
Results: There were no significant differences in atopy and FEV 1 among patients with CA, CVA, and CTVA. Serum IgE, sputum eosinophil percentages, FeNO, anxiety and depression scores were significantly increased in the three presentations of asthmatic patients as compared with healthy controls but there was no difference between the asthmatic groups. Comprehensive mass spectrometric analysis revealed more than a thousand proteins in the sputum from patients with CA, CVA, and CTVA, among which 23 secreted proteins were higher in patients than that in controls.
Conclusions: Patients with CA, CVA, or CTVA share common clinical characteristics of eosinophilic airway inflammation. And more importantly, their sputum samples were composed with common factors with minor distinctions. These findings support the concept that these three different presentations of asthma have similar pathogenetic mechanism in terms of an enhanced Th2 associated with eosinophilia. In addition, this study identified a pool of novel biomarkers for diagnosis of asthma and to label its subtypes. Trial registration http://www.chictr.org.cn (ChiCTR-OOC-15006221).
Keywords: Airway inflammation; Chest tightness variant asthma; Classic asthma; Clinical characteristics; Cough variant asthma; Proteomic characteristics.
Publication types
- Research Support, Non-U.S. Gov't
- Asthma / complications
- Asthma / metabolism*
- Asthma / pathology
- Biomarkers / metabolism*
- Case-Control Studies
- Cough / complications
- Eosinophils / metabolism
- Immunoglobulin E / blood
- Nitric Oxide / metabolism
- Proteomics / methods*
- Sputum / metabolism*
- Nitric Oxide
- Immunoglobulin E
Associated data
- ChiCTR/ChiCTR-OOC-15006221
Grants and funding
- G1000758/MRC_/Medical Research Council/United Kingdom
- Research article
- Open access
- Published: 05 April 2016
Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory?
- Sophie Demarche 1 , 2 , 3 ,
- Florence Schleich 1 , 2 ,
- Monique Henket 1 , 2 ,
- Virginie Paulus 1 , 2 ,
- Thierry Van Hees 3 &
- Renaud Louis 1 , 2
BMC Pulmonary Medicine volume 16 , Article number: 46 ( 2016 ) Cite this article
4189 Accesses
77 Citations
1 Altmetric
Metrics details
The technique of induced sputum has allowed to subdivide asthma patients into inflammatory phenotypes according to their level of granulocyte airway infiltration. There are very few studies which looked at detailed sputum and blood cell counts in a large cohort of asthmatics divided into inflammatory phenotypes. The purpose of this study was to analyze sputum cell counts, blood leukocytes and systemic inflammatory markers in these phenotypes, and investigate how those groups compared with healthy subjects.
We conducted a retrospective cross-sectional study on 833 asthmatics recruited from the University Asthma Clinic of Liege and compared them with 194 healthy subjects. Asthmatics were classified into inflammatory phenotypes.
The total non-squamous cell count per gram of sputum was greater in mixed granulocytic and neutrophilic phenotypes as compared to eosinophilic, paucigranulocytic asthma and healthy subjects ( p < 0.005). Sputum eosinophils (in absolute values and percentages) were increased in all asthma phenotypes including paucigranulocytic asthma, compared to healthy subjects ( p < 0.005). Eosinophilic asthma showed higher absolute sputum neutrophil and lymphocyte counts than healthy subjects ( p < 0.005), while neutrophilic asthmatics had a particularly low number of sputum macrophages and epithelial cells. All asthma phenotypes showed an increased blood leukocyte count compared to healthy subjects ( p < 0.005), with paucigranulocytic asthmatics having also increased absolute blood eosinophils compared to healthy subjects ( p < 0.005). Neutrophilic asthma had raised CRP and fibrinogen while eosinophilic asthma only showed raised fibrinogen compared to healthy subjects ( p < 0.005).
Conclusions
This study demonstrates that a significant eosinophilic inflammation is present across all categories of asthma, and that paucigranulocytic asthma may be seen as a low grade inflammatory disease.
Peer Review reports
The technique of induced sputum has been pivotal in the emergence of the concept of inflammatory asthma phenotypes. Although it is technically demanding and time-consuming, several centers have applied the technique of induced sputum to characterize asthma inflammatory phenotypes in routine [ 1 – 7 ]. It has been suggested that airway inflammation may be subdivided into four phenotypes according to the level of granulocyte airway infiltration: eosinophilic, neutrophilic, mixed granulocytic and paucigranulocytic [ 2 , 3 ]. This latter has been considered as a non-inflammatory type of asthma as the sputum analysis of these patients was suggested not to differ from healthy subjects [ 8 ].
These inflammatory phenotypes have been analyzed with respect to their demographic, functional and clinical characteristics in several studies [ 2 , 3 , 9 , 10 ]. However, there has been fewer studies looking in detail at sputum cells [ 2 ], blood leukocytes [ 11 ], systemic inflammatory markers or at these variables in combination in a large cohort of asthmatics classified according to the extent of airway granulocytic inflammation, and investigating how those groups compared with healthy subjects.
Here, in a retrospective analysis, we report on sputum cell counts, blood leukocytes, C-reactive protein (CRP) and fibrinogen in a large series of asthmatics seen in daily practice of a University Clinic and compare the results with those in healthy subjects.
Our results show that paucigranulocytic asthmatics may display a low grade airway and systemic inflammation.
Study design, setting and participants
We conducted a retrospective cross-sectional study on asthmatic patients and healthy subjects recruited from the University Asthma Clinic of Liege. Asthmatic patients were eligible for the study if they had a first visit with a successful sputum analysis between 1 October 2003 and 5 February 2015. The diagnosis of asthma was based on the presence of typical symptoms (wheezing, breathlessness, chest tightness, cough) and at least one of the following: forced expiratory volume in one second (FEV 1 ) increase of ≥12 % and 200 ml after inhalation of 400 μg salbutamol or a provocative concentration of methacholine causing a 20 % fall in FEV 1 (PC20M) less than 16 mg/ml. Atopy was defined by the presence of at least one positive specific IgE (>0.35 kU/L; Phadia; Groot-Bijgaarden, Belgium) to one or more common aeroallergen (cat, dog, grass pollen, tree pollen, house dust mite and a mixture of moulds). Fractional exhaled nitric oxide (FENO) measurements were performed at 50 ml/s of flow rate (NIOX, Aerocrine, Sweden). Healthy subjects were recruited by advertisement and in the hospital staff, during this 12-year period of study. They had no diagnosis of respiratory disease, a FEV 1 ≥ 80 % and a Tiffeneau index ≥0.7. Only healthy subjects with a successful sputum analysis were selected. This retrospective study was conducted with the approval from the ethics committee of the University Hospital of Liege (Reference 2015/193). Informed consents were obtained from healthy subjects. As for asthmatic patients, all procedures were performed in the context of clinical practice and the retrospective data collection was conducted with the approval from the above-mentioned ethics committee.
Sputum induction and analysis
Sputum was induced and processed as previously described [ 12 ]. Briefly, sputum was induced by hypertonic (5 %) or isotonic saline with salbutamol when post-bronchodilator FEV 1 was >65 % or ≤65 % predicted, respectively. Sputum was then processed using the whole expectorate technique. Dithiothreitol (DTT) was used as the mucolytic agent. Sputum sample was considered as adequate for cell count when squamous cell count was <80 % [ 13 ]. Differential cell count was performed on cytospins after a Diff-Quick staining.
Asthma phenotypes
Threshold values used to define the eosinophilic and neutrophilic phenotypes were a sputum eosinophil count ≥3 % and a sputum neutrophil count ≥76 %, respectively [ 14 ]. The mixed granulocytic phenotype was defined as both raised sputum eosinophil and neutrophil counts and the paucigranulocytic one as sputum eosinophil and neutrophil counts lower than the thresholds.
Statistical analysis
Results were expressed as frequencies and percentages for categorical variables and as median (interquartile range) or mean ± standard deviation for continuous variables. Comparisons were performed using a Pearson’s chi-squared test for categorical variables, an ANOVA or a Student’s t -test for parametric variables, and a Kruskal-Wallis test for non-parametric variables. The Pearson correlation coefficient was used to measure the association between the percentage of sputum neutrophils and eosinophils, and the number of pack-years in asthmatics. For this parametric test, sputum eosinophils were logtransformed and the 0 values replaced by 0.1. A p value <0.05 was considered statistically significant. When multiple tests were performed, the statistically significant level was corrected according to the Bonferroni principle. Statistical analysis was done using STATA version 13.0 (Statistical Software, College Station, TX: StataCorp LP).
Subject characteristics
The demographic, functional and treatment characteristics of asthmatics and healthy subjects are given in Table 1 . In our cohort of asthmatics with a successful sputum analysis ( n = 833), inflammatory phenotypes were distributed as follows: 42 % of patients were eosinophilic, 16 % neutrophilic, 4 % mixed granulocytic and 38 % paucigranulocytic. The group of neutrophilic asthma presented a higher proportion of women while eosinophilic asthma had a lower proportion of women. Paucigranulocytic asthmatics were the youngest and had the best FEV 1 (% predicted) and FEV 1 /forced vital capacity (FVC) ratio. All asthma subgroups had a higher prevalence of atopy as compared to healthy subjects ( p < 0.005: level of statistical significance after Bonferroni correction).
Sputum cell counts and viability
The sputum cell counts and viability of asthma phenotypes and healthy subjects are shown in Table 2 . The total non-squamous cell count per gram of sputum and the cell viability were greater in mixed granulocytic and neutrophilic phenotypes compared to eosinophilic, paucigranulocytic asthma and healthy subjects. Eosinophilic asthmatics also had significantly greater total sputum cell counts than paucigranulocytic asthmatics and healthy subjects. The fraction of squamous cells was less than 20 % in a majority of the patients, and was particularly low in eosinophilic, neutrophilic and mixed granulocytic asthma. While levels were obviously the highest in eosinophilic and mixed granulocytic phenotypes compared to other asthmatics, sputum eosinophils (in absolute values and percentages) were increased in all asthma phenotypes, compared to healthy subjects (Table 2 and Fig. 1a ). Absolute neutrophil counts were increased not only in neutrophilic and mixed granulocytic asthma, but also in eosinophilic asthma subjects, compared with healthy subjects. The group of neutrophilic asthma was the only one to have a lower absolute sputum macrophage count than eosinophilic, paucigranulocytic asthmatics and healthy subjects. Absolute sputum lymphocyte count was greater in eosinophilic asthma ( p < 0.005, versus paucigranulocytic asthma and healthy subjects), while absolute epithelial cell count was remarkably lower in case of intense neutrophilic inflammation ( p < 0.005, versus paucigranulocytic and eosinophilic asthma, Table 2 and Fig. 1b ).
Absolute sputum eosinophils ( a ) and absolute sputum epithelial cells ( b ) in asthma phenotypes and healthy subjects. * p < 0.005. Values of 0 were assigned to 0.1 because of the use of a logarithmic scale
Blood leukocyte counts and systemic inflammatory markers
The blood leukocyte counts and systemic inflammatory markers of asthma phenotypes and healthy subjects are shown in Table 3 . All subgroups of asthmatics showed an increased level of blood leukocytes compared with healthy subjects (Table 3 and Fig. 2a ). As expected, eosinophilic and mixed granulocytic asthmatics had higher blood eosinophils (in absolute values and percentages) than neutrophilic, paucigranulocytic asthma and healthy subjects, but paucigranulocytic asthmatics also showed higher levels of absolute blood eosinophils than healthy subjects. Neutrophilic asthmatics had the highest level of blood neutrophils, compared to eosinophilic, paucigranulocytic asthma and healthy subjects, but both eosinophilic and paucigranulocytic asthmatics also had greater absolute blood neutrophils than healthy subjects. Absolute lymphocyte counts were mainly increased in eosinophilic and paucigranulocytic asthma. Only the group of eosinophilic asthma had increased absolute blood basophil counts compared to healthy subjects. Neutrophilic asthma was characterized by raised CRP and fibrinogen levels, while eosinophilic asthma had raised fibrinogen only, compared with healthy subjects (Table 3 and Fig. 2b ).
Blood leukocytes ( a ) and blood fibrinogen ( b ) in asthma phenotypes and healthy subjects. * p < 0.005
Analysis of asthma phenotypes according to treatment with inhaled corticosteroids (ICS) and comparison with healthy subjects
For each phenotype, we compared sputum and blood cell counts between patients treated and not treated with ICS and we also compared these subgroups with healthy subjects (Table 4 and Additional file 1 : Table S1). In steroid-naïve paucigranulocytic patients, as in the whole group of paucigranulocytic asthma, there was an increase in sputum eosinophil counts, compared to healthy subjects. With respect to systemic inflammation, blood leukocyte, eosinophil and lymphocyte counts were increased in steroid-naïve paucigranulocytic asthmatics, compared to healthy subjects ( p < 0.0042: level of statistical significance after Bonferroni correction, Table 4 ). Paucigranulocytic asthmatics receiving inhaled corticosteroids showed an increase in sputum eosinophils and epithelial cells, and an increase in blood leukocyte, neutrophil and lymphocyte numbers ( p < 0.0042, Table 4 ). We did not find any statistically significant difference between patients treated and not treated with ICS in the subgroups of paucigranulocytic, neutrophilic and mixed granulocytic asthma ( p > 0.0042, Table 4 and Additional file 1 : Table S1). In eosinophilic asthmatics, patients treated with ICS presented higher levels of sputum epithelial cells, blood leukocytes and blood neutrophils, as compared with eosinophilic patients not treated with ICS ( p < 0.0042, Additional file 1 : Table S1).
Analysis of asthma phenotypes classified with a threshold of sputum eosinophils of 1.01 %
We also used 1.01 % as the threshold of sputum eosinophils to define asthma phenotypes. When using this cutoff value, there was no statistically significant difference in sputum and blood eosinophils (in absolute values and percentages) between neutrophilic or paucigranulocytic asthma, and healthy subjects (the level of statistical significance after Bonferroni correction being a p value <0.005, Table 5 ). However, there was a strong trend for greater absolute sputum eosinophil count in paucigranulocytic asthmatics versus healthy subjects ( p = 0.005). In the paucigranulocytic asthma subgroup defined with this cutoff value, absolute sputum epithelial cells (50 (24-115) × 10 3 /g), blood leukocytes (7.21 (5.82–8.66) × 10 3 /μL), absolute blood neutrophils (3900 (3083–5299)/μL) and absolute blood lymphocytes (2334 (1891–2904)/μL) were still greater than in healthy subjects ( p < 0.005).
Analysis according to atopy
The blood and sputum eosinophil counts of asthmatics and healthy subjects according to their atopic status are presented in Table 6 . We did not observe any statistically significant difference in these cells between atopic and non-atopic healthy subjects or asthmatics (the level of statistical significance after Bonferroni correction being a p value <0.0083).
Analysis according to smoking habits
The proportion of asthma inflammatory phenotypes was analyzed according to the smoking status of patients (Fig. 3 ), and was not different between non-smokers, ex-smokers and current smokers ( p = 0.6). There was however a correlation between the number of pack-years and the percentage of sputum neutrophils but this correlation was absent with sputum eosinophils (Fig. 4 ).
Proportion of asthma inflammatory phenotypes according to the smoking status of asthmatics
Relationship between sputum neutrophils ( a ) and eosinophils ( b ) and the number of pack-years in ex- and current smoker asthmatics. For sputum eosinophils, values of 0 were assigned to 0.1 because of the use of a logarithmic scale
Our study shows that the four inflammatory asthma phenotypes display raised sputum eosinophilia compared to healthy subjects. Eosinophilic asthma showed higher levels of absolute sputum neutrophils and lymphocytes than healthy subjects, while neutrophilic asthmatics had a reduced number of sputum macrophages and epithelial cells. In addition, all phenotypes had increased systemic inflammation reflected by raised total circulating leukocyte counts, mainly accounted for by raised granulocytic blood cell counts compared to healthy subjects.
As previously shown [ 2 ], our data indicate that neutrophilic and mixed granulocytic inflammation is more intense than pure eosinophilic and paucigranulocytic ones as the total number of cells per gram of sputum was clearly increased in these two first forms of groups. This might be related to the larger pool of circulating neutrophils and the variety of stimuli able to recruit neutrophils into the airways [ 15 ]. Our results showing greater viability in neutrophilic and mixed granulocytic asthma are also in keeping with those reported by Simpson et al. [ 2 ]. It may seem paradoxical as neutrophils are recognized to be more fragile than eosinophils in cell culture [ 16 ], but this may suggest greater cell turnover and short time of residency of neutrophils in the airways [ 17 ].
It has been suggested that non-eosinophilic asthmatics are poorly responsive to inhaled corticosteroids [ 8 , 18 ]. One interesting finding of our study is the fact that the so called “non-eosinophilic phenotypes” (neutrophilic and paucigranulocytic phenotypes) actually had more sputum eosinophils than healthy subjects, pointing to a low grade eosinophilic inflammation in these phenotypes. This could explain the fact that some patients with non-eosinophilic asthma responded to inhaled corticosteroids in the study of Cowan et al. [ 19 ], even though a limitation of this study was the absence of placebo-controlled design. Also of interest is the fact that eosinophilic asthmatics had more absolute sputum neutrophils compared to healthy subjects. It is likely to reflect that pathways leading to the recruitment of eosinophils and neutrophils in the airways may co-exist or act through a same effector in asthma [ 20 ].
It is noteworthy that absolute sputum lymphocyte counts were increased when there was significant eosinophilic infiltration. Although little attention has been paid to lymphocytes in sputum so far because of their small number (representing approximately 1–2 %), our finding is in keeping with the role of lymphocytes in organizing eosinophilic inflammation, a concept which has been established by bronchial biopsy and bronchoalveolar lavage analyses [ 21 , 22 ].
A particularly low absolute number of macrophages was observed in neutrophilic asthma. Further to this decreased amount of macrophages in the airways, Simpson et al. [ 23 ] described an impaired capacity of sputum macrophages to phagocytose apoptotic material in non-eosinophilic asthma, which may explain chronic inflammation and accumulation of airway neutrophils.
In keeping with epithelial alteration in asthma [ 24 ], we found an increased epithelial cell number in sputum from eosinophilic and paucigranulocytic asthma. By contrast, epithelial cell count was particularly low in the groups displaying intense neutrophilic inflammation. The presence of epithelial cells in sputum samples may reflect epithelial desquamation. Therefore, it is somewhat surprising to find lower epithelial cell counts in neutrophilic asthma. Our clinical feeling is that the cough effort during sputum induction may favor epithelial shedding and our experience is that effort to produce sputum is less when patients have intense neutrophilic inflammation, which may prevent epithelial cell desquamation.
In contrast to COPD [ 25 ], little attention has been paid to systemic inflammation in asthma, so far. Our data show striking elevation of total blood leukocyte counts in all asthmatic phenotypes compared to healthy subjects. There was however no difference between asthma phenotypes in this respect, which is in keeping with the recent study of Zhang et al. [ 11 ]. Although raised circulating leukocytes might have been expected in patients with eosinophilic, neutrophilic and mixed granulocytic asthma, this finding was somewhat more surprising in paucigranulocytic asthma.
As previously shown [ 3 , 11 ], patients with a raised sputum eosinophilic inflammation also had the highest levels of blood eosinophils (absolute values or percentages). Moreover, we found that the highest levels of absolute blood neutrophils were observed in neutrophilic and mixed granulocytic asthma, although the results did not reach statistical significance in this last phenotype, probably due to the small number of patients. Interestingly, paucigranulocytic asthmatics also had raised absolute circulating eosinophils and neutrophils compared to healthy subjects. Overall it points to a granulocytic inflammation in all asthma phenotypes.
Like in sputum, absolute lymphocyte counts were increased in blood of patients with eosinophilic asthma. Interestingly, levels of absolute blood basophils were also higher in these patients, which is consistent with a previous report showing a concomitant increase in circulating eosinophils and basophils after allergen inhalation in atopic asthmatics [ 26 ].
Supporting the concept of low grade systemic inflammation in asthma, we found a mild but significant increase in fibrinogen levels in eosinophilic and neutrophilic asthma, while increase in CRP was essentially observed in asthmatics with intense airway neutrophilic infiltration, as previously shown [ 27 ].
The group of paucigranulocytic asthmatics may be considered heterogeneous as almost two third of the patients were receiving ICS, which may have attenuated an airway inflammation initially present before the start of the treatment. Therefore, it is interesting to notice that mild airway and systemic eosinophilic inflammation, together with raised circulating lymphocyte number, were still present in those patients not treated with ICS. One limitation of our study is the retrospective design that precludes any further phenotypic characterization of circulating lymphocytes.
Several interventional studies have shown that ICS therapy decreases sputum eosinophils [ 28 , 29 ] and increases sputum neutrophils [ 19 ]. In our study, we did not find any statistically significant difference in these sputum cells between patients treated and not treated with ICS. However, because of its cross-sectional design, our study does not question the impact of ICS on eosinophil and neutrophil counts. We believe that the persistence of significant sputum eosinophilia in ICS treated asthmatics reflects the severity of the inflammatory process in these patients.
Paucigranulocytic asthma is defined as a sputum eosinophil count <3 % in our study. Others have proposed a cutoff at 1.01 % [ 2 ]. When choosing this cutoff in our patients, we increase the proportion of eosinophilic asthma from 42 to 51 % and we correspondingly decrease the proportion of paucigranulocytic asthma from 38 to 29 %. With this cutoff at 1.01 %, the group of paucigranulocytic asthmatics still displayed signs of airway and systemic inflammation, although they did not show increased circulating blood eosinophils anymore. It is interesting to postulate that the paucigranulocytic phenotype may be composed of some asthmatics presenting low grade inflammation and others without any sign of airway and systemic inflammation.
Some studies have shown that the eosinophilic inflammation is somewhat related to atopy [ 3 , 30 ]. Our data suggest that the eosinophilic trait observed in all asthma phenotypes is independent of the atopic status with non-atopic asthmatics not displaying statistically different sputum and blood eosinophil counts from their atopic counterparts.
There was no significant change in the proportion of inflammatory phenotypes according to the smoking status. However, in contrast to sputum eosinophils, sputum neutrophil count appears to be partly associated with the cumulative smoking history expressed as pack-years. Previous studies failed to find this relationship [ 31 , 32 ] but they were performed on a limited number of patients. This association remains however rather limited based on our results, where pack-years can only account for 5 % of the neutrophil variability.
It is known that asthma inflammatory phenotypes may somewhat vary over time [ 2 , 33 ]. One limitation of our study is its cross-sectional design with sputum analyzed at only one time point.
Overall, this study demonstrates that a significant eosinophilic inflammation is present across all categories of asthma, and that paucigranulocytic asthma may be seen as a low grade inflammatory disease. Whether steroid-naïve or a part of steroid-treated paucigranulocytic asthmatics may do without ICS should be investigated in long term trials, but our data incite to be cautious in clinical practice for now.
Ethics approval and consent to participate
This retrospective study was conducted with the approval from the ethics committee of the University Hospital of Liege (Reference 2015/193). Informed consents were obtained from healthy subjects. As for asthmatic patients, all procedures were performed in the context of clinical practice and the retrospective data collection was conducted with the approval from the above-mentioned ethics committee.
Consent for publication
Not applicable.

Availability of data and material
The data supporting our results are presented within the article (and its Additional file 1 : Table S1).
Abbreviations
body mass index
C-reactive protein
fractional exhaled nitric oxide
forced expiratory volume in 1 second
forced vital capacity
inhaled corticosteroid
long-acting β2-agonist
leukotriene receptor antagonist
oral corticosteroid
provocative concentration of methacholine causing a 20 % fall in FEV 1
Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498–501.
Article CAS PubMed PubMed Central Google Scholar
Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirol Carlton Vic. 2006;11:54–61.
Article Google Scholar
Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13:11.
in’t Veen JC, de Gouw HW, Smits HH, Sont JK, Hiemstra PS, Sterk PJ, et al. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J. 1996;9:2441–7.
Bacci E, Cianchetti S, Carnevali S, Bartoli ML, Dente FL, Di Franco A, et al. Induced sputum is a reproducible method to assess airway inflammation in asthma. Mediators Inflamm. 2002;11:293–8.
Vlachos-Mayer H, Leigh R, Sharon RF, Hussack P, Hargreave FE. Success and safety of sputum induction in the clinical setting. Eur Respir J. 2000;16:997–1000.
Article CAS PubMed Google Scholar
Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–9.
Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007;119:1043–52. quiz 1053‑4.
Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–36. e13.
Porsbjerg C, Lund TK, Pedersen L, Backer V. Inflammatory subtypes in asthma are related to airway hyperresponsiveness to mannitol and exhaled NO. J Asthma Off J Assoc Care Asthma. 2009;46:606–12.
Article CAS Google Scholar
Zhang X-Y, Simpson JL, Powell H, Yang IA, Upham JW, Reynolds PN, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2014;44:1137–45.
Delvaux M, Henket M, Lau L, Kange P, Bartsch P, Djukanovic R, et al. Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax. 2004;59:111–5.
Gershman NH, Wong HH, Liu JT, Mahlmeister MJ, Fahy JV. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996;9:2448–53.
Louis R, Godinas L, Schleich F. Induced Sputum - Towards Normal Values. Non Invasive Assessment of airways inflammation in asthma and COPD. Athens: Paschalidis Medical Publications; 2011. p. 113–23.
Google Scholar
Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–8.
Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol Baltim Md 1950. 1996;156:4422–8.
CAS Google Scholar
Kamath AV, Pavord ID, Ruparelia PR, Chilvers ER. Is the neutrophil the key effector cell in severe asthma? Thorax. 2005;60:529–30.
Louis R, Schleich F, Barnes PJ. Corticosteroids: still at the frontline in asthma treatment? Clin Chest Med. 2012;33:531–41.
Article PubMed Google Scholar
Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65:384–90.
Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–98.
Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani AM, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88:661–74.
Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–42.
Simpson JL, Gibson PG, Yang IA, Upham J, James A, Reynolds PN, et al. Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2013;43:29–35.
Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–62. quiz 463‑4.
Dickens JA, Miller BE, Edwards LD, Silverman EK, Lomas DA, Tal-Singer R, et al. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res. 2011;12:146.
Gibson PG, Manning PJ, O’Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, et al. Allergen-induced asthmatic responses. Relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils, and their progenitors. Am Rev Respir Dis. 1991;143:331–5.
Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142:86–93.
Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–9.
Article PubMed PubMed Central Google Scholar
Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54:108–14.
Lewis SA, Pavord ID, Stringer JR, Knox AJ, Weiss ST, Britton JR. The relation between peripheral blood leukocyte counts and respiratory symptoms, atopy, lung function, and airway responsiveness in adults. Chest. 2001;119:105–14.
Boulet L-P, Lemière C, Archambault F, Carrier G, Descary MC, Deschesnes F. Smoking and asthma: clinical and radiologic features, lung function, and airway inflammation. Chest. 2006;129:661–8.
Telenga ED, Kerstjens HAM, Ten Hacken NHT, Postma DS, van den Berge M. Inflammation and corticosteroid responsiveness in ex-, current- and never-smoking asthmatics. BMC Pulm Med. 2013;13:58.
McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–9.
Download references
Acknowledgements
We acknowledge the Fonds Léon Fredericq for funding training in statistics.
This work was supported by a federal grant IAP P7/30.
Author information
Authors and affiliations.
Department of Respiratory Medicine, CHU Liege, Sart-Tilman B35, 4000, Liege, Belgium
Sophie Demarche, Florence Schleich, Monique Henket, Virginie Paulus & Renaud Louis
GIGA I3 Research Group, University of Liege, Liege, Belgium
Department of Clinical and Hospital Pharmacy, University of Liege, Liege, Belgium
Sophie Demarche & Thierry Van Hees
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sophie Demarche .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
SD designed the study, helped to collect the data, analyzed and interpreted the data and drafted the manuscript. FS collected the data, helped to draft the manuscript and revised it critically for important intellectual content. MH and VP collected the data and revised the manuscript critically for important intellectual content. TVH helped to analyze the data and revised the manuscript critically for important intellectual content. RL designed the study, collected the data, interpreted the data and drafted the manuscript. All authors read and approved the final manuscript.
Additional file
Additional file 1: table s1..
Sputum and blood cell counts of eosinophilic phenotypes classified by ICS treatment and healthy subjects. (DOCX 29.3 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Demarche, S., Schleich, F., Henket, M. et al. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory?. BMC Pulm Med 16 , 46 (2016). https://doi.org/10.1186/s12890-016-0208-2
Download citation
Received : 10 November 2015
Accepted : 18 March 2016
Published : 05 April 2016
DOI : https://doi.org/10.1186/s12890-016-0208-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Healthy subjects
- Sputum cytology
- Blood leukocyte count
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 December 2023
Asthma control and sputum eosinophils in adult patients: a cross-sectional study in southern Brazil
- Vanessa Albano Barcellos ORCID: orcid.org/0000-0002-2250-5259 1 , 2 ,
- Vanessa Cristina Hartmann dos Santos ORCID: orcid.org/0000-0003-0327-2354 1 ,
- Maria Ângela Fontoura Moreira ORCID: orcid.org/0000-0002-9258-1054 2 &
- Paulo de Tarso Roth Dalcin ORCID: orcid.org/0000-0002-9774-9135 1 , 2
Scientific Reports volume 13 , Article number: 21464 ( 2023 ) Cite this article
494 Accesses
Metrics details
- Cytological techniques
Asthma control and health related quality of life are an important goal of asthma management, but their association with sputum eosinophilic inflammation has been less firmly established. To investigate the relationship of asthma control and quality of life with sputum eosinophils in clinical practice. Cross-sectional study with a convenience sample, including patients with asthma, aged between 18 and 65 years, attending to outpatient clinic. Patients underwent sputum induction, pulmonary function tests, Juniper’s Asthma Quality of Life Questionnaire (AQLQ), Asthma Control Test (ACT), Global Initiative for Asthma (GINA) criteria for evaluation of asthma control and severity of the disease, blood count analysis, serum IgE and cutaneous prick test. Sputum sample was considered as eosinophilic if the percentage of eosinophils was ≥ 3%. A total of 45 individuals were enrolled, 15 with eosinophilic sputum (≥ 3% eosinophil cells) and 30 with non-eosinophilic sputum (< 3% eosinophil cells). There were no association of ACT an AQLQ scores with sputum eosinophilia (p > 0.05). This study suggested that the finding of sputum eosinophilia was not related to asthma control neither with health-related quality of life in patients with severe asthma.
Similar content being viewed by others

Deep phenotyping of post-infectious myalgic encephalomyelitis/chronic fatigue syndrome
Brian Walitt, Komudi Singh, … Avindra Nath

Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update
Mark L. Levy, Leonard B. Bacharier, … Helen K. Reddel

Effect of breathwork on stress and mental health: A meta-analysis of randomised-controlled trials
Guy William Fincham, Clara Strauss, … Kate Cavanagh
Introduction
Asthma is a common, chronic respiratory disease affecting 1–18% of the population in different countries, characterized by variable symptoms of wheeze, shortness of breath, chest tightness, cough, and by variable expiratory airflow limitation. It is a heterogeneous disease which consists in multiple phenotypes that are distinguished by clinical, function and inflammatory characteristics. The eosinophil is one of the most important cells related to airway inflammation in asthma 1 .
The type 2 asthma phenotype is based on an eosinophilic inflammation that can occur in the absence of an allergic reaction. This involves biomarkers such as Interleukin (IL) 4, IL-5 and IL-13, Immunoglobulin E (IgE), fractional exhaled nitric oxide (FeNO), peripheral blood eosinophils and eosinophils in induced sputum 2 . These biomarkers can be employed in several ways to aid treatment decisions 3 .
Treatment of asthma can reduce or remove symptoms. The assessment of asthma control (control of symptoms and risk of adverse outcomes) is determined by the interaction between the patient’s genetic background, environment exposure, psychological factors, treatments, and the underlying disease process 1 .
The sputum induction method has been validated and standardized, providing a safe and relatively noninvasive way to collect material from the lower airways 4 , 5 . On the other hand, the method of analyzing the samples is laborious and requires well-trained technicians using highly specialized laboratory equipment 6 .
The importance of examination of sputum cellularity in the management of moderate-to-severe persistent asthma has grown as recent studies have demonstrated that the number of severe exacerbations is lower when induced sputum findings are used to design the anti-inflammatory treatment than when the treatment is based on the current guidelines 7 .
Considering that asthma control is an important goal of asthma management and that its association with sputum eosinophilic inflammation has been less firmly established, in this exploratory study we sought to analyze the implementation in our institution of conventional method for sputum processing in adult patients with severe asthma, investigating the relationship of asthma control and quality of life with sputum eosinophils in clinical practice.
Study design
This was a cross-sectional study approved by the ethics and research committee of the Hospital de Clínicas de Porto Alegre (HCPA), protocol number 5.860.232, and Plataforma Brazil, protocol number 5.860.232. All patients signed an informed consent form. Part of the results has been published previously as an original article 8 . The population studied in the present study was a convenience sample, with no sample size calculation.
Secondarily, this was an exploratory study in which we sought to analyze the preliminary implementation in our institution of conventional method for sputum processing in adult patients with severe asthma. Exploratory research design is conducted for a research problem when the researcher has no past data or only a few studies for reference 9 .
Patients were recruited from the Asthma Outpatient Clinic of HCPA, Porto Alegre, Rio Grande do Sul, Brazil. This Clinic is responsible of the care of patients with severe asthma.
The study included patients aged between 18 and 65 years. The diagnosis of asthma was confirmed by compatible history and evidence of reversible airflow obstruction in spirometry: forced expiratory volume in the first second (FEV 1 ) less than 80% of the predicted value and FEV 1 /forced vital capacity (FVC) ratio less than 75% plus substantial improvement in airflow after inhalation of short-acting beta 2 -agonist bronchodilator (BD) (increase in FEV 1 greater than 12% in relation to the pre-BD value and greater than 200 mL in absolute value or increase in FEV 1 greater than 20% and exceeding 250 mL spontaneously over time or after intervention with medication) 1 .
Asthmatic subjects exposed to smoking were included. Individuals who reported smoking in the last 30 days and had a smoking index greater than 5 pack-years were considered active smokers. Smokers in cessation or ex-smokers were defined as individuals who had quit smoking in the last 30 days or more and had a smoking index greater than 5 pack-years. Nonsmokers were defined as individuals who reported never having smoked and ex-smokers with a smoking index of less than 5 pack-years. The smoking index (pack-years) was calculated as follows: number of cigarettes smoked per day/20 × the number of years the person had smoked.
The exclusion criteria from the study were: pregnant women; patients with other chronic lung diseases such as bronchiectasis, sequelae of pulmonary tuberculosis, diffuse lung fibrosis; lung neoplasm or neoplasm of other sites; human immunodeficiency syndrome, acquired immunodeficiency syndrome, or congenital immunodeficiency syndrome; psychiatric illness or incapacitating chronic neurological disease that could prevent the performance of the study procedures; and patients who did not complete the study evaluation tests.
Induced sputum samples were collected to evaluate cellularity, following institutional protocols. We considered the sputum sample as eosinophilic if the percentage of eosinophils was ≥ 3% 10 .
The level of asthma control was assessed using the Asthma Control Test (ACT) 11 and the GINA table 1 .
Spirometry was performed using a Jaeger v 4.31a spirometer (Jaeger, Wuerzburg, Germany). The carbon monoxide diffusing capacity (DL CO ) was measured by a single sustained breath of a special gaseous mixture, using Master Screen Diffusion equipment (Jaeger, Wuerzburg, Germany). Pulmonary volumes were measured using the Master Screen Body-Plets (Jaeger, Wuerzburg, Germany) 12 , 13 .
All the patients were asked about Health-related Quality of Life using Juniper’s Asthma Quality of Life Questionnaire ( AQLQ) 14 , 15 . The AQLQ comprises four domains: symptoms, activity limitation, emotional function, and environmental exposure. Each domain is scored from 1 to 7; scores of 1 indicate maximal impairment and scores of 7 indicate no impairment.
Blood count analysis was performed, total eosinophils count, and percentage was used. We consider blood count > 300 cell/mm 3 as eosinophilia 16 . Serum IgE dosage was analyzed and considered high if greater than 100 IU/mL, according to the reference value 17 . The skin prick test for allergy was conducted according to published protocols, if the patient had at least one cross positive, it was considered positive test 18 .
Patients were asked about symptoms of allergic rhinitis and were classified about its control.
Statistical analysis
This study was made with a convenience sampling. All data were processed and analyzed using the Statistical Package for the Social Sciences, version 22.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed using mean and standard deviation or median and interquartile range. Categorical variables were expressed using absolute and relative frequencies.
The associations between categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test. To compare means, we used Student’s t-test or one-way analysis of variance, supplemented by Tukey’s test. In case of asymmetry, Mann–Whitney or Kruskal–Wallis tests were used and supplemented by Dunn’s test. A p-value ≤ 0.05 was considered statistically significant and all tests were two-tailed.
Ethics statement
This was an exploratory study using data of a cross-sectional study, approved by the ethics and research committee of the Hospital de Clínicas de Porto Alegre (HCPA) and Plataforma Brasil (protocol number 1.139.117). All patients signed an informed consent form. The authors signed a data use agreement protecting the confidentiality of patient information. All study methods were carried out in accordance with international and national guidelines and regulations for clinical studies of humans (Declaration of Helsinki and Brazilian Governmental regulation—Plataforma Brasil).
During a period of 20 months, a total of 494 adults subjects were assessed in the initial evaluation. Out of them, 87 fulfilled all the criteria, but 15 withdraw consent to sputum induction. Thus 72 subjects completed the study (see Fig. 1 —Flow diagram of study selection and exclusion criteria).
Flow diagram of study selection and exclusion criteria.
Despite performing the protocol correctly, 27 individuals could not achieve the necessary volume of sputum to do the analyses. We classified them as non-adequate sputum sample. Table 1 shows the demographic, clinical data, and comparison of patients in groups with inadequate sputum sample and with adequate sputum sample. The individuals with inadequate sputum sample were younger than those with adequate sputum sample (respectively, 42.1 ± 12.9 vs 49.1 ± 12.5 years, p = 0.027). There was no other statistically difference between the 2 groups.
A total of 45 individuals were enrolled in the study (group with adequate sputum samples). Thirty-five patients (77.8%) were female, the mean age was 49.1 ± 12.5 years, the mean BMI was 31.2 ± 6.8 kg/m 2 and 73.3% were white. The median ACT was 16 (13–21) points. The mean FVC was 79.5 ± 15.8% of predict, the mean FEV 1 was 66.3 ± 18.0% of predict and the mean FEV 1 /FVC was 67.9 ± 11.0%. Ninety-eight percent of subjects were classified as severe asthma (steps 4 and 5 of GINA classification).
Those patients with adequate sputum samples were divided in two groups: 15 individuals with eosinophilic sputum (≥ 3% eosinophil cells) and 30 with non-eosinophilic sputum (< 3% eosinophil cells). The comparison of demographic and clinical data between patients with non-eosinophilic and eosinophilic sputum is showed in Table 2 . There were no statistically significant differences between groups. In both groups female sex was predominant, and the main ethnicity was white. When asthma control was assessed according to GINA, only 13% of all patients had well controlled asthma. The median of ACT score was 15.5 (13–20) in the non-eosinophilic and 16 (12–23) in the eosinophilic sputum group, p = 0.621. The ACT score was ≥ 20 points in only 9 (20%) of patients in non-eosinophilic sputum group and in 6 (13.3%) of patients in eosinophilic sputum group, p = 0.513.
Table 3 presents comparison between lung function, cutaneous prick test, serum eosinophils and serum IgE between patients with non-eosinophilic and eosinophilic sputum. The individuals with eosinophilic sputum had higher serum eosinophils (%) levels than the non-eosinophilic group (respectively, 3.3% vs 2.1%, p = 0.037) and when considering serum eosinophilia as eosinophils count > 300 mm 3 , there was association with sputum eosinophilia too (p = 0.044), Fig. 2 . There were no other significant statistical differences between groups. The proportion on patients with blood eosinophilia was 33.3%.
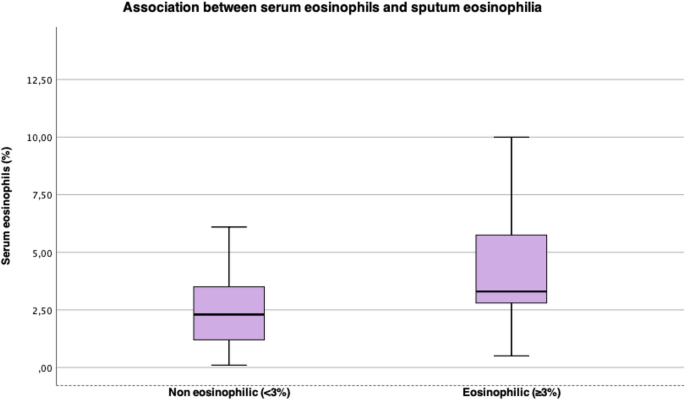
Association between serum eosinophils and sputum eosinophilia.
Table 4 shows comparison between ER visits, hospital admissions and AQLQ domain scores between patients with non-eosinophilic and eosinophilic sputum. Graphics comparing AQLQ scores are on Fig. 3 . There were no significant statistical differences between groups for ER visits, estimated number of days away from job or school, number of hospital admissions and scores of AQLQ. The ER visits and hospital admission rates were low in both groups in the present study. The AQLQ domain scores showed a moderate impairment in quality of life in both groups.
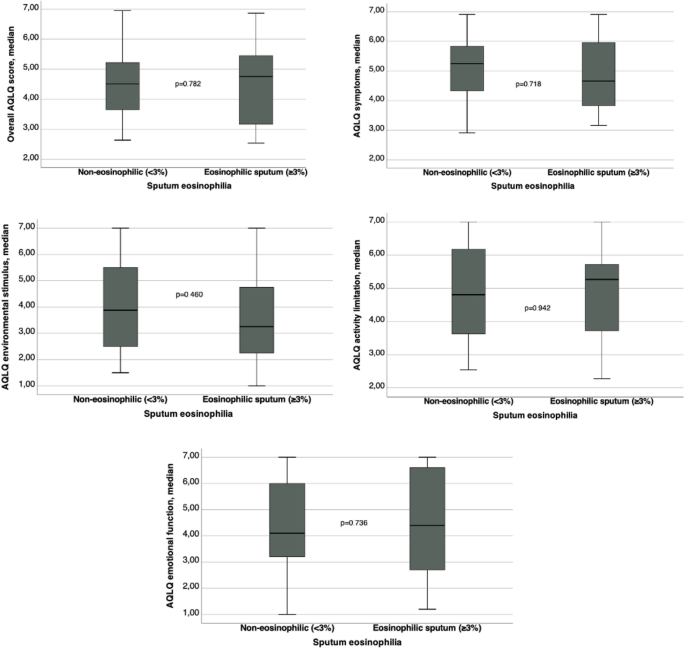
Comparison between AQLQ domain scores.
The knowledge about sputum cellularity in inflammatory respiratory diseases allows more comprehensive approach and care for patients with moderate or severe conditions 7 . This cross-sectional study suggested that the finding of eosinophilic sputum was not related to asthma control neither with health-related quality of life in a population with severe asthma attending to an asthma outpatient clinic in a tertiary and academic hospital in Southern Brazil.
Duncan et al. used the measures of inflammatory cells in induced sputum to search for asthma severity relationship with eosinophils. This was research with chronic stable asthma 19 which included former smokers, comparing forced expiratory volume, symptoms, and sputum eosinophils apoptosis. It was demonstrated that reduced eosinophil apoptosis and sputum eosinophil load are correlated with degree of self-reported symptoms and severity of the disease.
In a retrospective longitudinal study of 187 patients, Demarche et al. demonstrated that asthma control was associated with fluctuations in sputum eosinophilic inflammation 20 . Furthermore, they have calculated a minimal important increase and decrease in sputum eosinophils associated with a change of at least 0.5 in the Asthma Control Questionnaire (ACQ). The age and smoking status were like the present study, but the asthma severity was lower than that identified in our study.
Pizzichini et al. studied a population of 130 patients with asthma that had been receiving treatment for asthma for at least a year and were lifelong non-smokers or ex-smokers, who were assessed to induced sputum and to answer quality of life questionnaires 21 . The sputum was labelled eosinophilic if sputum eosinophils were ≥ 3%, neutrophilic if neutrophils were ≥ 60%, and pauci-granulocytic if neither. It was found that near 70% of subjects with controlled asthma had pauci-granulocytic sputum, suggesting that answering “No” to the four questions of GINA table (1) is a good indicator of control of air inflammation. On the other hand, in patients with partially controlled or uncontrolled asthma, there was no difference between the groups with eosinophilic, neutrophilic, or pauci-granulocytic sputum. The population of this research differs from ours in some features: they did not include active smokers and they had number of individuals with controlled asthma higher than ours. In our population, with GINA criteria, only 6.7% had control of the disease.
Recently, Athanazio et al. evaluated prevalence of eosinophilic phenotype in patients with severe asthma, with blood eosinophils count. The prevalence of patients with severe asthma and eosinophils > 300 cell/mm 3 in Brazil was 40% 16 . Our research found similar data, 33% of our severe asthma population studied had sputum eosinophilia (defined as sputum eosinophils ≥ 3%). When comparing serum and sputum eosinophils, blood eosinophils count > 300 cell/mm 3 was associated with sputum eosinophilia (p = 0.044), secondarily there was association between percentual of serum eosinophils and sputum eosinophilia.
Our study has some limitations. First, this was a cross-sectional study, so it was not possible to establish a temporal link between sputum eosinophilia and asthma control and health related quality of life. Second, the sample size was too small with a convenience sample and would be associated with low statistical power. Third, our study did not encompass all the spectrum of asthma severity, and the study only included steps 4 and 5 of GNA severity classification. Also, the study population was selected from patients referred to a reference center and was probably biased toward the more severe disease. It is important to emphasize that the patients in our sample were on high doses of inhaled steroids, the majority was using more than 1600mcg of budesonide. Fourth, the study population includes only those patients who were able to produce a sputum sample of sufficient quality.
Another important issue we found was to implement the induced sputum in our service. Although we followed straightly a standardized protocol 5 almost 40% of the first study sample could not achieve adequate sputum to do the analyzes. This group’s characteristics had no statistical differences when compared to who expectorated adequate sputum. The proportion of inadequate samples in our research was higher than other studies 5 , 21 , 22 , probably because induced sputum to investigate inflammatory features is laborious as it demands time, trained professionals, and a qualified laboratory, able to process and count inflammatory cells 7 . It is not performed in our daily practice.
In conclusion, this cross-sectional study suggested that the finding of sputum eosinophilia in a population with severe asthma and in use of high doses of inhaled steroids was not related to asthma control, neither with health-related quality of life.
Data availability
The datasets generated and/or analysed during the current study are available in the file “ datasets.xlsx ”, which is attached on “Supplementary Material”.
GINA-Main-Report-2022-FINAL-22-07-01-WMS .
de Carvalho-Pinto, R. M. et al. Recomendações para o manejo da asma da Sociedade Brasileira de Pneumologia e Tisiologia—2021. J. Bras. Pneumol. 47 , 1–24 (2021).
Google Scholar
Robinson, D. et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 47 , 161–175. https://doi.org/10.1111/cea.12880 (2017).
Article CAS PubMed Google Scholar
Pizzichini, M. M. et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 154 (4), 866–869. https://doi.org/10.1164/ajrccm.154.4.8887576 (1996).
Pavord, I. D., Pizzichini, M. M., Pizzichini, E. & Hargreave, F. E. The use of induced sputum to investigate airway inflammation. Thorax 52 , 498 (1997).
Article CAS PubMed PubMed Central Google Scholar
Fireman, E., Toledano, B., Buchner, N., Stark, M. & Schwarz, Y. Simplified detection of eosinophils in induced sputum. Inflamm. Res. 60 , 745–750 (2011).
Moritz, P. et al. Determination of the inflammatory component of airway diseases by induced sputum cell counts: Use in clinical practice* Determinação do componente inflamatório das doenças das vias aéreas através do escarro induzido: Utilização na prática clínica. J. Bras. Pneumol. 34 , 913 (2008).
Article PubMed Google Scholar
dos Santos, V. C. H. et al. Association of quality of life and disease control with cigarette smoking in patients with severe asthma. Braz. J. Med. Biol. Res. 55 , 1 (2022).
Article Google Scholar
Singh, A. An introduction to experimental and exploratory research. SSRN Electron. J. https://doi.org/10.2139/ssrn.3789360 (2021).
Chakir, J. et al. Monitoring sputum eosinophils in mucosal inflammation and remodelling: A pilot study. Eur. Respir. J. 35 , 48–53 (2010).
Petroni Faria Roxo, J. et al. Portuguese-language version of the asthma control test: Validation for use in Brazil* Validação do Teste de Controle da Asma em português para uso no Brasil. J. Bras. Pneumol. 36 , 159 (2010).
Crapo, R. O., Morris, A. H. & Gardner, R. M. Reference Spirometric Values Using Techniques and Equipment that Meet ATS Recommendations 13 .
Prata, T. A. et al. Spirometry reference values for black adults in Brazil. J. Bras. Pneumol. 44 , 449–455 (2018).
Article PubMed PubMed Central Google Scholar
Juniper, E. F. et al. Evaluation of impairment of health related quality of life in asthma: Development of a questionnaire for use in clinical trials. Thorax 47 , 76–83 (1992).
Silva et al . Validação do questionário de qualidade de vida em asma (Juniper) para o português brasileiro (2007).
Athanazio, R. et al. Prevalence of the eosinophilic phenotype among severe asthma patients in Brazil: The BRAEOS study. J. Bras. Pneumol. 48 , e20210367 (2022).
Wittig, H. J., Belloit, J., De Fillippi, l., Royal, G. & Gainesville, B. Age-Related Serum Immunoglobulin E Levels in Healthy Subjects and In Patients with Allergic Disease .
Wood, R. A., Phipatanakul, W., Hamilton, R. G. & Eggleston, P. A. A comparison of skin prick tests, intradermal skin tests, and RASTs in the diagnosis of cat allergy. J. Allergy Clin. Immunol. 703 , 773–779 (1999).
Duncan, C. J. A., Lawrie, A., Blaylock, M. G., Douglas, J. G. & Walsh, G. M. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur. Respir. J. 22 , 484–490 (2003).
Demarche, S. F. et al. Asthma control and sputum eosinophils: A longitudinal study in daily practice. J. Allergy Clin. Immunol. 5 , 1335–1343 (2017).
Pizzichini, M. M. M. et al. How does the GINA definition of control correlate with quality of life and sputum cellularity? ERJ Open Res. 5 , 1 (2019).
Simpson, J. L., McElduff, P. & Gibson, P. G. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration 79 , 147–151 (2009).
Download references
This research was supported by Fundo de Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), Protocol Number 1.139.117.
Author information
Authors and affiliations.
Programa de Pós-Graduação em Ciências Pneumológicas, Faculdade de Medicina, Universidade Federal do Rio Grande do Sul (UFRGS), Ramiro Barcelos, 2400, Porto Alegre, RS, 90035-003, Brazil
Vanessa Albano Barcellos, Vanessa Cristina Hartmann dos Santos & Paulo de Tarso Roth Dalcin
Serviço de Pneumologia, Hospital de Clínicas de Porto Alegre, Ramiro Barcelos, 2350, Porto Alegre, Brazil
Vanessa Albano Barcellos, Maria Ângela Fontoura Moreira & Paulo de Tarso Roth Dalcin
You can also search for this author in PubMed Google Scholar
Contributions
V.A.B.—Conception and design, data collection and analysis, data interpretation, writing of the main manuscript text, preparation of tables, critical review of the article for content, and final approval of the version to be published. V.C.H.S.—Conception and design, data collection and analysis, data interpretation, writing of the main manuscript text, preparation of tables, critical review of the article for content, and final approval of the version to be published. M.A.F.M.—Data collection and analysis, data interpretation, critical review of the article for content, and final approval of the version to be published. P.T.R.D.—Conception and design, analysis, data interpretation, writing of the main manuscript text, preparation of figures, critical review of the article for content, and final approval of the version to be published.
Corresponding author
Correspondence to Vanessa Albano Barcellos .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Barcellos, V.A., dos Santos, V.H., Moreira, M.F. et al. Asthma control and sputum eosinophils in adult patients: a cross-sectional study in southern Brazil. Sci Rep 13 , 21464 (2023). https://doi.org/10.1038/s41598-023-48381-1
Download citation
Received : 29 August 2023
Accepted : 25 November 2023
Published : 05 December 2023
DOI : https://doi.org/10.1038/s41598-023-48381-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Association of Sputum Eosinophilia With Easily Measured Type-2 Inflammatory Biomarkers in Untreated Mild Persistent Asthma.
BACKGROUND: A multicenter clinical trial in patients with mild persistent asthma indicated that response to inhaled corticosteroids (ICS) is limited to those with sputum eosinophilia. However, testing for sputum eosinophilia is impractical in most clinical settings. OBJECTIVE: We examined associations between sputum eosinophilia and type 2 inflammatory biomarkers in untreated mild persistent asthma. METHODS: Induced sputum, blood eosinophil count (BEC), fractional exhaled nitric oxide (FeNO), and serum periostin were obtained twice during the 6-week run-in period in a clinical trial that enrolled patients 12 years and older with symptomatic, mild persistent asthma without controller therapy. The optimal threshold for each biomarker was based on achieving 80% or greater sensitivity. Performance of biomarkers (area under the receiver operating characteristics curve [AUC], range 0.0-1.0) in predicting sputum eosinophilia 2% or greater was determined; AUCs of 0.8 to 0.9 and more than 0.9 define excellent and outstanding discrimination, respectively. RESULTS: Of 564 participants, 27% were sputum eosinophilic, 83% were atopic, 70% had BEC of 200/uL or higher or FeNO of 25 ppb or greater; 64% of participants without sputum eosinophilia had elevated BEC or FeNO. The AUCs for BEC, FeNO, and both together in predicting sputum eosinophilia were all below the threshold for excellent discrimination (AUC 0.75, 0.78, and 0.79, respectively). Periostin (in adults) had poor discrimination (AUC 0.59; P = .02). CONCLUSIONS: In untreated mild persistent asthma, there is substantial discordance between sputum eosinophilia, BEC, and FeNO. Until prospective trials test the ability of alternative biomarkers to predict ICS response, BEC or FeNO phenotyping may be an option to consider ICS through a shared decision-making process with consideration of other clinical features.
Duke Scholars
Altmetric Attention Stats
Dimensions citation stats, published in, publication date, related subject headings.
- 3204 Immunology

Official websites use .mass.gov
Secure websites use HTTPS certificate
A lock icon ( ) or https:// means you’ve safely connected to the official website. Share sensitive information only on official, secure websites.
- search across the entire site
- search in Massachusetts Department of Environmental Protection
- search in Executive Office of Energy and Environmental Affairs
- This page, Massachusetts Becomes First State to Require Analysis of Cumulative Impacts for Air Quality Permits near Environmental Justice Populations , is offered by
- Massachusetts Department of Environmental Protection
Press Release Massachusetts Becomes First State to Require Analysis of Cumulative Impacts for Air Quality Permits near Environmental Justice Populations
Media contact for massachusetts becomes first state to require analysis of cumulative impacts for air quality permits near environmental justice populations , edmund coletta, massdep – director of public affairs.
BOSTON — The Healey-Driscoll Administration today moved to address air quality issues in or near environmental justice areas by announcing amendments to state air pollution regulations. The changes require certain facilities seeking air emissions permits in or near communities with environmental justice populations to conduct a cumulative impact analysis, which evaluates existing local environmental and health conditions in a community. The regulations also require enhanced public outreach to, and meaningful involvement of, environmental justice populations in the permitting process. The new regulations are now in force and apply to permit applications filed with the Massachusetts Department of Environmental Protection (MassDEP) on or after July 1, 2024.
“Our administration is committed to addressing longstanding environmental injustice,” said Governor Maura Healey . “Massachusetts is proud to make history as the first state in the nation to launch a statewide program to require a detailed, site-specific evaluation of cumulative impacts to consider public health and other factors. We hope to set the standard for others to follow as we seek to right past wrongs and build healthier, more inclusive communities.”
“A cumulative impact analysis will offer a more complete picture of environmental and health conditions in affected communities and equip residents to be involved in the permitting process from the very beginning,” said Lieutenant Governor Kim Driscoll . “This is a significant development as our administration continues to prioritize participation, transparency, and protection of public health in permitting decisions.”
“This approach takes a holistic look at what existing conditions might be worsened by a new or increasing source of air pollution,” said Energy and Environmental Affairs Secretary Rebecca Tepper. “It provides opportunities for residents to meaningfully engage in the permitting process to help identify mitigation strategies and ensure that these facilities are a benefit to – not a further burden on – environmental justice communities.”
People are exposed to pollution through air, water, and land. Over time, these exposures can add up and interact with each other, and combined with existing health and socioeconomic conditions, can cause adverse health impacts. Communities that are home to numerous sources of pollution – such as highways or waste facilities – often have higher rates of asthma and other serious health conditions. This is especially true in communities with environmental justice areas, where residents have higher instances of health issues related to socioeconomic conditions. Current air pollution regulations do not require new facilities to assess potential pollution in light of existing environmental and health conditions or existing pollutant sources in the area. By requiring new and expanding facilities that will emit air pollution to conduct a cumulative impacts analysis prior to applying for an air permit, residents will be empowered with practical information about potential impacts to their health and community.
“The cumulative impact analysis gives our agency and the public a better basis to evaluate project proposals in real-world contexts,” said MassDEP Commissioner Bonnie Heiple. “MassDEP has developed innovative tools and compiled comprehensive datasets that can be used by permit applicants and interested residents to assess local impacts.”
“These new regulations will empower communities with information about the impacts of air pollution on their health,” said Undersecretary for Environmental Justice and Equity María Belén Power . “As new facilities seek to come into neighborhoods, environmental justice communities will have the data and transparency they deserve. This is an important step towards achieving justice for all people in Massachusetts.”
Community Engagement Early community engagement and advance notification are key requirements of the new rules.
The regulations apply to projects that would emit significant amounts of air pollutants in or near environmental justice communities – for example, certain power plants, large boilers, and manufacturing facilities. At least 60 days before applying for a new or modified air permit, the permit applicant must provide a notification and fact sheet about the proposed project to nearby environmental justice populations, local officials, and MassDEP. The applicant also must provide public involvement opportunities and document and respond to comments and concerns raised by the public.
Comprehensive Analysis The new rules require permit applicants to assess existing community conditions by evaluating 33 environmental, health, and socio-economic indicators . These indicators help characterize existing pollution sources, health vulnerabilities, and other stressors that could be worsened by increased air emissions from the proposed project. The updated rules require a more comprehensive analysis of the impacts of these projects to local communities, including consideration of:
- Existing air pollution and health conditions.
- Vulnerabilities in affected communities; and
- Socioeconomic and demographic indicators.
MassDEP has developed guidance and tools in support of the new regulations that can be used by permit applicants and environmental justice populations. These include guidance on how to conduct a cumulative impact analysis, including public outreach, assessment of existing community conditions, and analysis of cumulative impacts; a Mapping and Data Application for use in the assessment of existing community conditions; and a Massachusetts Air Toxics Risk Screening Tool (MATRiST) that can be used in the cumulative impact analysis to estimate cumulative air toxics risks from proposed projects. These resources are available online here.
MassDEP plans to review this program within two years of the effective date of the regulations, including by soliciting input and feedback from the public regarding potential updates.
The Healey-Driscoll Administration has made environmental justice central to its climate and environmental agenda. Under Governor Healey, the Massachusetts Executive Office of Energy and Environmental Affairs created the Office of Environmental Justice and Equity, led by Undersecretary María Belén Power and secured $7 million for environmental justice staffing and initiatives across agencies, including MassDEP. The Office of Environmental Justice and Equity also recently announced the state’s first Environmental Justice Strategy , which includes MassDEP’s department-specific strategy to meaningfully engage with environmental justice communities.
“The new Massachusetts air permit regulations take a bold step to protect overburdened and underserved communities in a new way, requiring consideration of cumulative impacts from environment, public health and socioeconomic conditions as part of the permitting process,” said EPA New England Regional Administrator David W. Cash. “The combination of considering cumulative impacts and enhanced community engagement to address community priorities early in the process is foundational to environmental justice. We hope that this will serve as a model for other states across the country and bring new air quality and public health improvements to Massachusetts residents.”
“For environmental justice communities and advocates, the concept of cumulative impact assessment – that some communities bear a greater collective burden than others – is fundamental. We applaud the Healey-Driscoll Administration and, in particular, the Department of Environmental Protection for moving the ball forward and incorporating cumulative impact assessment in the Commonwealth’s air permitting process,” said GreenRoots Executive Director Roseann Bongiovanni. “But we know that cumulative environmental impacts affect more than just the air we breathe; it affects our water and is reflected in the land use decisions we make. We look forward to continuing to work with the Administration to fully implement cumulative impact assessment in all aspects of the Commonwealth’s equitable protection of our environment and the health of its residents.”
“Cumulative Impact Assessments will vastly improve our ability to protect air quality by making fact-based, historically informed decisions about permitting in vulnerable communities. We applaud the Healey-Driscoll Administration and the Department of Environmental Protection for taking this first-in-the nation step to ensure cleaner air for all Massachusetts residents,” said Amy Boyd Rabin, Vice President of Policy for the Environmental League of Massachusetts.
“Safeguards for overburdened communities that already experience too much pollution and health damage are sorely needed and are critical to make sure we don’t deepen environmental injustices,” said Cindy Luppi, National Field Director, Clean Water Action. “We’re grateful that the Healey-Driscoll Administration wants to address this on-going pattern of injustice in some of the Commonwealth’s low-income and BIPOC communities. Meaningful regulations offer the promise of a healthier and more just tomorrow and we hope that many more states follow Massachusetts’ lead.”
More information on cumulative impact analysis requirements can be found on MassDEP’s website .
MassDEP’s mission is to protect and enhance the Commonwealth’s natural resources – air, water and land – to provide for the health, safety and welfare of all people, and to ensure a clean and safe environment for future generations. In carrying out this mission, MassDEP commits to address and advance environmental justice and equity for all people of the Commonwealth; to provide meaningful, inclusive opportunities for people to participate in agency decisions that affect their lives; and to ensure a diverse workforce that reflects the communities we serve.
Massachusetts Department of Environmental Protection
Help us improve mass.gov with your feedback.
The feedback will only be used for improving the website. If you need assistance, please visit the MassDEP Contacts & Service Center page . Please limit your input to 500 characters.
Thank you for your website feedback! We will use this information to improve this page.
If you need assistance, please visit the MassDEP Contacts & Service Center page .
If you would like to continue helping us improve Mass.gov, join our user panel to test new features for the site.

IMAGES
VIDEO
COMMENTS
Respiratory viruses have been tested in sputum specimens from patients with cystic fibrosis, asthma, and chronic obstructive pulmonary disease (COPD). ... Sputum analysis quality control is essential and directly affects the diagnostic results. From sputum specimens collection, samples transport, samples storage, diagnostic test procedures ...
use of this technique. This review assesses the extent to which induced sputum can distinguish between asthma phenotypes and guide treatment. Recent findings Although the short-term repeatability of sputum analysis is good, recent research has shown a great deal of variability in sputum inflammatory profiles over follow-up periods of several months to a few years. In particular, the ...
During the past 10 years, there have been an increasing number of publications concerning the diagnosis and treatment of asthma using sputum analysis. Analysis of induced sputum provides similar data to secretions obtained through bronchial wash, bronchoalveolar lavage, and, to some extent, bronchial biopsy. The techniques of cellular counting ...
Background: The debate as to whether asthma is a single or heterogeneous disease remains unresolved although pathological studies, mostly using fibreoptic bronchoscopy on small numbers of subjects, have emphasised the similarities between different clinical phenotypes. Methods: Lower airway inflammation was assessed non-invasively using induced sputum in 34 normal controls and 259 adults with ...
Background: The technique of induced sputum has allowed to subdivide asthma patients into inflammatory phenotypes according to their level of granulocyte airway infiltration. There are very few studies which looked at detailed sputum and blood cell counts in a large cohort of asthmatics divided into inflammatory phenotypes.
Reason. clear, white, or gray. usually indicates healthy lungs, but a lot of sputum may indicate a lung disease, allergy, or viral infection. dark yellow or green. can indicate a bacterial or ...
Serum IgE, sputum eosinophil percentages, FeNO, anxiety and depression scores were significantly increased in the three presentations of asthmatic patients as compared with healthy controls but there was no difference between the asthmatic groups. Comprehensive mass spectrometric analysis revealed more than a thousand proteins in the sputum ...
The technique of induced sputum has been pivotal in the emergence of the concept of inflammatory asthma phenotypes. Although it is technically demanding and time-consuming, several centers have applied the technique of induced sputum to characterize asthma inflammatory phenotypes in routine [1-7].It has been suggested that airway inflammation may be subdivided into four phenotypes according ...
Considering that asthma control is an important goal of asthma management and that its association with sputum eosinophilic inflammation has been less firmly established, in this exploratory study ...
The different colors of sputum (mucus) Clear. It's the normal color of mucus. 1. White. It may indicate airway inflammation. It's white because it contains white blood cells. These cells are recruited to the lungs as part of the asthma response. Asthma is an abnormal immune response. This means white blood cells are abnormally recruited to ...
During the past 10 years, there have been an increasing number of publications concerning the diagnosis and treatment of asthma using sputum analysis. Analysis of induced sputum provides similar data to secretions obtained through bronchial wash, bronchoalveolar lavage, and, to some extent, bronchial biopsy.
A recent study by Citation Green and colleagues (2002) demonstrated that the measurement of sputum eosinophilia can be used to manage symptoms in patients with moderate to severe asthma, which supports the use of induced sputum analysis in patient treatment. In fact, routine measurement of sputum eosinophil numbers in addition to management ...
A treatment strategy directed at normalisation of the induced sputum eosinophil count reduces asthma exacerbations and admissions without the need for additional anti-inflammatory treatment. ... in an exploratory post-hoc analysis, several patients had a sputum eosinophil count that was mostly within the normal range throughout the study. In ...
Clinical cluster analysis from the Severe Asthma Research Program (SARP) identified 5 asthma subphenotypes that represent the severity spectrum of early-onset allergic asthma, late-onset severe asthma, and severe asthma with chronic obstructive pulmonary disease characteristics. Analysis of induced sputum from a subset of SARP subjects showed 4 sputum inflammatory cellular patterns. Subjects ...
Rationale: The airway transcriptome includes genes that contribute to the pathophysiologic heterogeneity seen in individuals with asthma. Objectives: We analyzed sputum gene expression for transcriptomic endotypes of asthma (TEA), gene signatures that discriminate phenotypes of disease. Methods: Gene expression in the sputum and blood of patients with asthma was measured using Affymetrix ...
CONCLUSIONS: In untreated mild persistent asthma, there is substantial discordance between sputum eosinophilia, BEC, and FeNO. Until prospective trials test the ability of alternative biomarkers to predict ICS response, BEC or FeNO phenotyping may be an option to consider ICS through a shared decision-making process with consideration of other ...
IMProving Asthma Control Trial (IMPACT) m an inhaled corticosteroid oral leukotriene receptor antagonist taken regularly. DECEMBER 1, 2000 Study Protocol A study of patients with mild, persistent asthma comparing the effects of 18 onths of treatment with an inhaled corticosteroid taken only "as needed," with . taken regularl , y. and with an
BOSTON — The Healey-Driscoll Administration today moved to address air quality issues in or near environmental justice areas by announcing amendments to state air pollution regulations. The changes require certain facilities seeking air emissions permits in or near communities with environmental justice populations to conduct a cumulative impact analysis, which evaluates existing local ...